The epidemic of type 2 diabetes imposes an enormous and growing burden on health care worldwide. The number of people with type 2 diabetes around the world is estimated to rise from 151 million in 2000 to 300 million by 2025.1 The recognition that strict glycaemic control can reduce microvascular complications has made the effective treatment of hyperglycaemia a priority.2 3 4 5 6 Recently, the diabetes control and complications trial reported that intensive therapy aimed at normoglycaemia has beneficial effects on cardiovascular disease in type 1 diabetes.7 In type 2 diabetes, epidemiological data from the UK prospective diabetes study suggest that lowering glycaemia will reduce the risk of cardiovascular disease.8 The treatment of hyperglycaemia in type 2 diabetes is complex; combinations of glucose lowering drugs are often needed to achieve and maintain blood glucose at target values. The development of new classes of drugs to lower blood glucose has increased the treatment options for type 2 diabetes and has contributed to the uncertainty surrounding these new therapeutic approaches. Here we present a management guideline that may help healthcare providers treat patients with type 2 diabetes.
Sources and selection criteria
This review is largely based on the recently published American Diabetes Association/European Association for the Study of Diabetes treatment guideline for type 2 diabetes.9 We also searched the Cochrane Library for evidence based guidelines using the keywords “type 2 diabetes”, “blood glucose lowering agents”, “glucose monitoring”, “lifestyle”, and “exercise and diet”
What level of glycaemic control should we aim for?
Studies have shown that the development of microvascular disease is reduced when glycaemic control is improved and have helped establish treatment targets for glycaemia in type 2 diabetes.4 6 Ideally glycated haemoglobin (HbA1C) should be as close to normal as possible without imposing a high risk of severe hypoglycaemia. The upper limit of normal of the diabetes control and complications trial standardised assay is 6.1%. The consensus guideline of the American Diabetes Association/European Association for the Study of Diabetes for the management of hyperglycaemia states that “an HbA1C of 7% or greater should serve as a call to action to initiate or change therapy with the goal of achieving a level as close to the non-diabetic range as possible.”9 This goal is similar to that used in the clinical trials that established the benefits of intensive therapy.4 5 7
How do I establish and sustain glycaemic control?
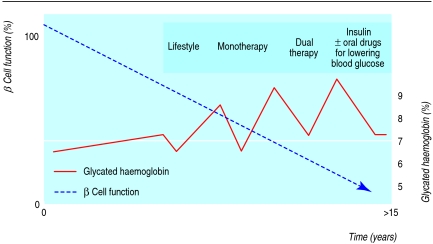
The clinical course of type 2 diabetes is characterised by a gradual decline in β cell function so that treatment needs to be adjusted regularly. The traditional approach to lowering blood glucose in type 2 diabetes consists of an ordered sequence of lifestyle modification, oral monotherapy, oral combination therapy, and finally treatment with insulin (with or without oral drugs for lowering blood glucose). This strategy usually results in recurrent failure because patients are allowed to become hyperglycaemic before the next step is considered. The aim should be to keep glycaemic levels as near to normal as possible (fig 1).
Fig 1 Traditional treatment strategy for type 2 diabetes and its consequences. In type 2 diabetes β cell function declines over the years, irrespective of treatment with metformin, sulfonylurea (as monotherapy or dual therapy), or insulin. Treatment therefore has to be adjusted at regular intervals according to the level of glycaemia. Because doctor and patient need to agree about adding another drug at each step, glycated haemoglobin values will recurrently fail to reach target
The main aim should be to choose interventions that effectively lower hyperglycaemia and sustain acceptable control. Other drug specific beneficial effects that might contribute to long term outcome are also important. Safety, cost, patient acceptance, and availability are obvious considerations.
Summary points
Initial treatment should consist of lifestyle intervention and metformin
Treatment should aim to keep blood glucose concentrations as close to the non-diabetic range as possible
The relentless decline of β cell function requires early intervention, regular monitoring of glycaemia, and prompt adjustment of the (combination of) blood glucose lowering drugs, including insulin
Good glycaemic control will reduce the occurrence of microvascular and perhaps cardiovascular complications of type 2 diabetes
Scientific evidence for any algorithm is largely lacking
Lifestyle change: an option?
The potentially most effective (but most difficult) step is to change patients' lifestyles and to achieve a clinically meaningful and long lasting weight loss. Despite the demonstrated efficacy, evidence for a successful and durable lifestyle modification in diabetes is lacking. A recent Cochrane analysis found no high quality data to support the effectiveness of dietary treatment of type 2 diabetes, although some studies suggest that exercise might improve HbA1c values.10 Weight loss with bariatric surgery is the most effective and durable treatment for type 2 diabetes.11
Well designed and conducted studies are needed, as are new ideas on changing lifestyles. Despite disappointing long term results, patients should be encouraged to take more exercise and reduce energy intake, especially as relatively modest targets may be clinically effective. Moreover, drugs that are subsequently prescribed may be more effective if a lower body mass index is maintained.
Is metformin still the first line drug?
Metformin, a biguanide, is widely accepted as the first line drug. Metformin is relatively effective, safe, and cheap; experience with this drug is extensive; and preliminary data indicate that it may lower cardiovascular disease in obese patients with type 2 diabetes.5 12 13 In addition, unlike many other blood glucose lowering drugs, metformin does not cause weight gain and may be associated with weight loss, so it will not adversely affect patients' attempts to lose weight. Lifestyle counselling and metformin should thus be started at the same time, if no contraindications are present. This advice goes against the usual approach, where metformin is added if lifestyle changes do not control glucose satisfactorily. Other drugs will be needed if target values of blood glucose are not maintained despite titration of the metformin dose over an eight to 12 week period.
In patients with intolerance to metformin, or when insulin deficiency may be the primary defect, which might be the case in lean patients with more severe hyperglycaemia, sulfonylurea or even insulin may be considered as first line treatment.
Which drugs after metformin?
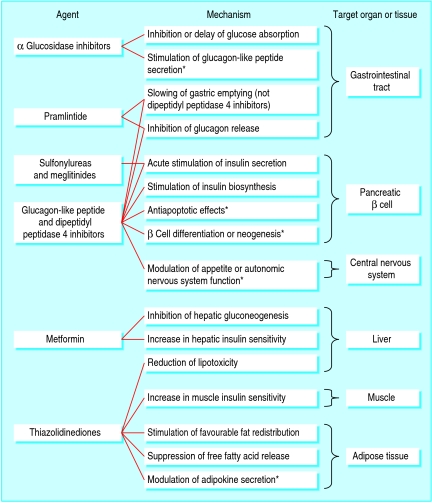
Tables 1 and 2 list the main characteristics of currently available drugs for second line treatment, and fig 2 shows their known and putative mechanisms of action. Sulfonylureas, thiazolidinediones, and insulin are the most widely used agents.
Table 1.
Main characteristics of currently available oral blood glucose lowering drugs
| Drug(s) | Reduction in glycated haemoglobin (%) | Main mode of action | Benefits | Side effects and limitations |
|---|---|---|---|---|
| Metformin | 1.5 | Lowers production of hepatic glucose | No weight gain; cheap | Gastrointestinal complaints; lactic acidosis (very rare) |
| Sulfonylureas | 1.5 | Stimulates insulin secretion | Cheap | Hypoglycaemia, sometimes severe and of long duration; weight gain |
| Thiazoldinediones | 0.5-1.5 | Improve insulin sensitivity | Improve lipid profile and may reduce risk of cardiovascular disease | Fluid retention, which can cause heart failure (rare); weight gain; expensive |
| α Glucosidase inhibitors* | 0.5-0.8 | Retard intestinal absorption of glucose | No weight gain | Gastrointestinal side effects; multiple daily dosing required; expensive |
| Meglitinides* | 1-1.5 | Stimulate insulin secretion | Short acting, less risk of hypoglycaemia | Need to be taken at meal time; expensive |
| Dipeptidylpeptidase 4 inhibitors* | 0.5-10 | Stimulate insulin secretion | Low risk of hypoglycaemia | Experience limited; expensive |
*Not yet licensed for use in the United Kingdom.
Table 2.
Main characteristics of parenteral blood glucose lowering agents and inhaled insulin
| Drug | Reduction in glycated haemoglobin (%) | Main mode of action | Benefits | Side effects and limitations |
|---|---|---|---|---|
| Pramlintide* | 0.5-1 | Retards gastric emptying and reduces energy intake | Weight loss | Needs to be injected before meals; gastrointestinal side effects; expensive; experience limited |
| Exenatide* | 0.5-1.0 | Stimulates insulin secretion; suppresses glucagons; retards gastric emptying and reduces energy intake | Weight loss | Needs multiple injections; gastrointestinal side effects; expensive; experience limited |
| Subcutaneous insulin | >2 | Stimulates peripheral glucose uptake and inhibits hepatic glucose output | Reduces severe hyperglycaemia; cheap; much experience | Weight gain; hypoglycaemia; needs to be injected; blood glucose must be monitored |
| Inhaled insulin* | 0.5-1.0 | Stimulates peripheral glucose uptake and inhibits hepatic glucose output | No injections needed | Needs multiple daily dosing; blood glucose must be monitored; long term pulmonary effects unknown; expensive and experience limited |
Not yet licensed for use in the United Kingdom.
Fig 2 Established and *putative mechanisms of action of blood glucose lowering drugs in humans
Sulfonylureas are potent stimulators of insulin secretion; their glucose lowering potency is similar to that of metformin.14 The main side effects are weight gain and severe hypoglycaemia, which can lead to seizure and coma, particularly in elderly people treated with long acting sulfonylureas such as glibenclamide and chlorpropamide.15 Thiazolidinediones sensitise adipose tissue, liver, and muscle to insulin, but they are less effective at lowering glycaemia than metformin and the sulfonylureas.16 Long term trials are currently studying whether thiazolidinediones can sustain glycaemic target values for a longer period of time than metformin and sulfonylureas.17 Apart from their glucose lowering action, their effects on lipids and inflammation may contribute to the putative reduction in risk of cardiovascular disease. The PROactive trial of the thiazolidinedione, pioglitazone, found no benefit with regard to its primary aggregate cardiovascular outcome, but it did find a 16% decrease in its secondary end point—death, myocardial infarction, and stroke—after three years of treatment.18 Thiazolidinediones are more expensive than insulin and generic sulfonylureas and metformin. The main adverse effect is fluid retention, which can cause heart failure.
The α glucosidase inhibitors, the meglitinides, and pramlintide are less widely prescribed, possibly because of limited efficacy, gastrointestinal side effects (α glucosidase inhibitors and pramlintide), high costs, and the need for multiple daily administrations.19 20
Sulfonylureas, thiazolidinediones, or insulin?
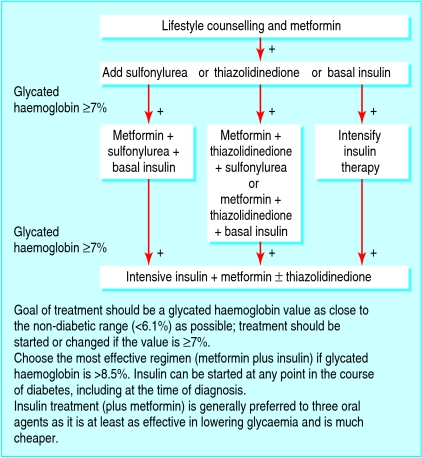
A sulfonylurea, a thiazolidinedione, or insulin is the preferred option to add to metformin (fig 3). The choice should be based on the degree of hyperglycaemia; the most effective glucose lowering agent, insulin, should be used when HbA1c values are high (>8.5%). Any accompanying comorbidity or other factor that may affect the patient's ability to monitor their blood glucose and patient preference must be taken into account. Drug intolerance or existing contraindications for any of the oral glucose lowering agents are also indications for insulin therapy. Insulin therapy can be started at any time in the course of type 2 diabetes, even at the time of diagnosis. Insulin can correct almost any degree of hyperglycaemia, provided that adequate doses are used. This can only be done safely when guided by self monitoring of blood glucose. When HbA1c is more than 1.5% above target, insulin or a sulfonylurea is the best choice. A thiazolidinedione may be prescribed when glucose values are nearer to target and no contraindications exist.
Fig 3 Management of hyperglycaemia in type 2 diabetes
And then? Triple oral insulin as add-on or insulin alone?
A combination of three oral agents for lowering blood glucose (metformin, a sulfonylurea, and a thiazolidinedione) should be considered only when patients are already close to target and when circumstances make it difficult to use insulin. The combination of three oral agents is more expensive than using insulin plus metformin, and no benefit has been shown.
If target HbA1c values are not achieved by dual oral therapy, the next step is to start basal insulin therapy. Intermediate acting or long acting insulin taken at bedtime is a good first choice because of the ease of use.21 22 When treatment needs to be intensified by adding injections of short acting insulin before main meals, the sulfonylurea should be gradually stopped, as preprandial insulin and sulfonylurea do not work well together.
Inhaled insulin was recently approved for use in the United States. Inhaled insulin is suitable for taking before meals because it is more rapidly absorbed than regular human insulin.23 Experience so far is limited, and the (very) long term potential consequences for lung function and structure need careful monitoring. Inhaled insulin may be more acceptable than injected insulin to patients who fail to achieve target glycaemia on oral blood glucose lowering agents. However, whether inhaled insulin can obtain HbA1c values less than 7% needs to be determined.
In resource limited settings, the aim should be to control plasma glucose with the least expensive but effective blood glucose lowering drugs. Nevertheless, even in these settings, the management algorithms proposed above may be widely applicable. Patients must be educated and empowered to manage their diabetes.
What is the evidence for the proposed algorithm?
Little direct trial evidence is available on the superiority of any specific regimen over others. Such studies are badly needed, especially with new drugs on the market. Newer compounds are not included in the algorithm, partly because of limited evidence and high costs.
Will new drugs be able to halt the decline of β cell function?
The algorithm described here will probably not slow down the clinical course of type 2 diabetes.21 However, with timely use of oral blood glucose lowering agents and insulin it should be possible to sustain acceptable glycaemic control.
Resources for healthcare professionals
Morral N. Novel targets and therapeutic strategies for type 2 diabetes. Trends Endocrinol Metab 2003;54:169-75
American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2005;28(suppl 1):S4-36
Canadian Diabetes Association. Clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2003;27(suppl 2):S1-152
Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, et al. Management of hyperglycaemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2006;49:1711-21
Information resources for patients
American Diabetes Association (www.diabetes.org)—provides information for patients and healthcare professionals
Diabetes UK (www.diabetes.org.uk)—large UK organisation that works for people with diabetes, funding research, campaigning, and helping people live with the condition
Canadian Diabetes Association (www.diabetes.ca)—provides information for patients as well as clinical practice guidelines
International Diabetes Federation (www.idf.org)—aims to promote diabetes care and prevention worldwide
Newer drugs such as glucagon-like peptide receptor agonists or inhibitors of the degrading enzyme dipeptidyl peptidase 4 may alter the clinical course of diabetes (see appendix on bmj.com). Glucagon-like peptide, produced by the L cells of the small intestine after food intake, potentiates glucose stimulated insulin secretion, inhibits glucagon secretion, retards gastric emptying, and reduces appetite (fig 2). In animal studies, this peptide stimulates proliferation of β cells and inhibits their apoptosis,24 a finding that must be confirmed in human type 2 diabetes. Exenatide is a synthetic analogue of the Gila monster salivary gland product exendin-4, which has similar characteristics to, but a much longer half life than, glucagon-like peptide.25 26 It lowers body weight but has a high frequency of gastrointestinal side effects and must be injected several times a day. Exenatide was approved in the US in 2005. A slow release form of exenatide has recently been developed and is undergoing a clinical development programme.
Several long term studies are assessing whether thiazolidinediones, dipeptidyl peptidase 4 inhibitors, and glucagon-like peptide receptor agonists can sustain good glycaemic control. These studies may have a major effect on the treatment of type 2 diabetes. The algorithm may then target the maintenance of β cell function, not just the management of hyperglycaemia.
Some outstanding research questions
Is it possible to modify the clinical course of the disease—that is, halt or retard the gradual decline of β cell function?
Which lifestyle intervention or drugs (or both) can favourably affect the clinical course?
Does it matter how normoglycaemia is sustained for long term outcome?
Which affordable and available treatment regimen will result in the best long term outcome in terms of microvascular and macrovascular disease?
What are the advantages of new drugs compared with cheaper older ones?
Conclusions
Much of the burden of type 2 diabetes can be prevented with stringent control of hyperglycaemia and other cardiovascular disease risk factors. Treatment needs to focus on maintaining blood glucose values as close to the non-diabetic range as possible, with early initiation of effective interventions such as (combinations of) blood glucose lowering drugs and insulin, and prompt adjustment of treatment when HbA1c is above the target value.
Supplementary Material
Contributors: RJH and DMN wrote the report, with considerable input from MD. J-CM provided input and oversaw the writing of the paper. RJH is guarantor.
Competing interests: RJH receives research support from Amylin, Eli-Lilly, Glaxo Smith Kline, Novartis, and NovoNordisk, and serves on advisory boards of Amylin, Bristol-Myers Squibb, Merck, Novartis, NovoNordisk, Pfizer, and Sanofi-Aventis. MD receives research support from Novartis and Glaxo-Smith Kline and serves on the advisory board of Sanofi-Aventis. DMN receives research grants from Sanofi-Aventis and NovoNordisk.
References
- 1.Zimmet P, Alberti KGGM, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001;414:782-7. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Standards of medical care of diabetes. Diabetes Care 2005;28(suppl 1):S15-35. [DOI] [PubMed] [Google Scholar]
- 3.European Diabetes Policy Group. A desk-top guide to type 2 diabetes mellitus. Diabet Med 1999;16:716-30. [PubMed] [Google Scholar]
- 4.United Kingdom Prospective Diabetes Study (UKPDS) Group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complication in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53. [PubMed] [Google Scholar]
- 5.United Kingdom Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood glucose control with metformin on complication in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854-65. [PubMed] [Google Scholar]
- 6.Ohkubo Y, Kishikawa H, Araki E, Takao M, Isami S, Motoyoshi S, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with NIDDM: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103-17. [DOI] [PubMed] [Google Scholar]
- 7.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, et al. Management of hyperglycaemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2006;49:1711-21. [DOI] [PubMed] [Google Scholar]
- 10.Moore H, Summerbell C, Hooper L, Cruickshank K, Vyas A, Johnstone P, et al. Dietary advice for treatment of type 2 diabetes in adults. Cochrane Database Syst Rev 2004;(2):CD004097. [DOI] [PubMed]
- 11.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al; Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683-93. [DOI] [PubMed] [Google Scholar]
- 12.DeFronzo R, Goodman A, Multicenter Metformin Study Group. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. N Engl J Med 1995;333:541. [DOI] [PubMed] [Google Scholar]
- 13.Salpeter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 2006;(1):CD002967. [DOI] [PubMed]
- 14.Groop L. Sulfonylureas in NIDDM. Diabetes Care 1992;15:737-47. [DOI] [PubMed] [Google Scholar]
- 15.Holstein A, Plaschke A, Egberts E-H. Lower incidence of severe hypoglycemia in patients with type 2 diabetes treated with glimepiride versus glibenclamide. Diabetes Metab Res Rev 2001;17:467-73. [DOI] [PubMed] [Google Scholar]
- 16.Yki-Jarvinen H. Drug therapy: thiazolidinediones. N Engl J Med 2004;351:1106. [DOI] [PubMed] [Google Scholar]
- 17.Viberti G, Kahn SE, Greene DA, Herman WH, Zinman B, Holman RR, et al. A diabetes outcome progression trial (ADOPT): an international multicenter study of the comparative efficacy of rosiglitazone, glyburide, and metformin in recently diagnosed type 2 diabetes. Diabetes Care 2002;25:1737-43. [DOI] [PubMed] [Google Scholar]
- 18.Dormandy JA, Charbonnel B, Eckland DJA, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive (prospective pioglitazone clinical trial in macrovascular events): a randomized controlled trial. Lancet 2005;366:1279-89. [DOI] [PubMed] [Google Scholar]
- 19.Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2005;(2):CD003639. [DOI] [PMC free article] [PubMed]
- 20.Malaisse WJ. Pharmacology of the meglitinide analogs: new treatment options for type 2 diabetes mellitus. Treat Endocrinol 2003;2:401-14. [DOI] [PubMed] [Google Scholar]
- 21.Cook MN, Girman CJ, Stein PP, Alexander CM, Holman RR. Glycemic control continues to deteriorate after sulfonylureas are added to metformin among patients with type 2 diabetes. Diabetes Care 2005;28:995-1000. [DOI] [PubMed] [Google Scholar]
- 22.Yki-Jarvinen H, Kauppinen-Makelin R, Tiikkainen M, Vahatalo M, Virtamo H, Nikkila K, et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia 2006;49:442-51. [DOI] [PubMed] [Google Scholar]
- 23.Rosenstock J, Zinman B, Murphy LJ, Clement SC, Moore P, Bowering CK, et al. Inhaled insulin improves glycemic control when substituted for or added to oral combination therapy in type 2 diabetes. Ann Intern Med 2005;143:549-58. [DOI] [PubMed] [Google Scholar]
- 24.Drucker DJ. Biologic actions and therapeutic potential of the proglucagon-derived peptides. Nat Endocrinol Metab 2005;1:22-31. [DOI] [PubMed] [Google Scholar]
- 25.Holst JJ. Glucagon-like peptide-1: from extract to agent. The Claude Bernard lecture. Diabetologia 2006;49:253-60. [DOI] [PubMed] [Google Scholar]
- 26.Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes. Ann Intern Med 2005;143:559-69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.