Abstract
The aim of this study was to compare both the behavioral and physiological effects of 2 drug regimens in children: chloral hydrate (CH), meperidine (M), and hydroxyzine (H) (regimen A) versus midazolam (MZ), M, and H (regimen B). Patients between 24 and 54 months of age were examined by crossover study design. Behavior was analyzed objectively by the North Carolina Behavior Rating System and subjectively through an operator and monitor success scale. Physiological data were recorded every 5 minutes and at critical points throughout the appointment. Sixteen patients completed this study. No significant differences in behavior were noted by the North Carolina Behavior Rating System or the operator and monitor success scale. A quiet or annoyed behavior was observed 93% and 90% of the time for regimen A and regimen B, respectively. Using the operator and monitor success scale, 63% of regimen A and 56% of regimen B sedations were successful. No statistically significant differences were noted in any of the physiological parameters between the 2 regimens. Ten episodes of hemoglobin desaturation were detected with regimen A sedations. There were no differences between the sedative drug regimens CH/M/H and MZ/M/H for behavioral outcomes or physiological parameters.
Keywords: Conscious sedation, Midazolam, Chloral hydrate, Children
Behavior management strategies for pediatric dental patients have evolved greatly over the past 2 decades, with adverse techniques such as hand-over-mouth exercise and hand-over-mouth with airway restriction having lost considerable popularity.1 In light of this trend, conscious sedation is a vital dimension of pediatric dentistry for those children who fail to cooperate for treatment in a conventional setting. Surveys of pediatric dentists in the mid to late 1980s found that 70 to 76% of respondents used conscious sedation.2,3 A recent survey of the American Association of Pediatric Dentistry members found an increased use of sedation by pediatric dentists in 2000.4 The popularity of conscious sedation among pediatric dentists is underscored by the safe and effective means by which sedative drugs can be used when practitioners follow the Guidelines for the Elective Use of Conscious Sedation, Deep Sedation, and General Anesthesia in Pediatric Dentistry.5,6
One popular drug regimen for the sedation of pediatric dental patients is a triple-dose cocktail that combines chloral hydrate (CH), meperidine (M), and hydroxyzine (H). Hasty et al7 reported excellent behavioral outcomes with this regimen and found this combination to be safe as assessed by pulse oximetery and capnography. A more recent and comprehensive retrospective review of 195 pediatric conscious sedations with the CH/M/H cocktail reported that 72% of the sedations were satisfactory and that adverse outcomes were few and minor under a strict protocol and use of oxygen supplementation.8 In one retrospective study,9 an 84% success rate was found when the regimen CH/M/H was used with N2O.
Despite the well-researched and documented safety and efficacy of the CH/M/H triple-dose cocktail,7–9 there are well-reasoned concerns with CH as a sedative agent for children. Laryngospasms, cardiac arrhythmias, cardiac arrest, and seizures are among the reported adverse reactions with CH.10–12 An extensive review of adverse events associated with CH administered during dental or medical procedures revealed that 5 of the 13 patients who sustained permanent neurological damage or death were dental patients.13 Incorrect dose calculations or inadequate communication among the dental staff have led to adverse reactions in the dental setting.14
One alternative to the use of CH in the triple-dose cocktail is midazolam (MZ). Midazolam is a short-acting benzodiazepine that has been praised for its sedative, hypnotic, anxiolytic, and anterograde amnesic effects.15–18 Other clinical advantages of MZ include water solubility, rapid onset, anticonvulsant action, clinically inactive metabolites, and high margin of safety.15–18 Marshall et al19 reported that the MZ oral syrup was accepted by 99% of all children studied. This formulation was found to be safe and effective over a fourfold dose range. Eighty-one percent of the children reached adequate sedation after only 30 minutes. Nathan and Vargas20 found that when MZ was combined with the narcotic M, there was an 80% success rate for children. Moreover, no episodes of persistent hemoglobin desaturation, loss of protective reflexes, respiratory depression, or emesis were reported.
Studies directly comparing CH and MZ provide support for the use of MZ as a good substitute for CH. Differences in behavior were negligible during sedation procedures comparing the 2 drugs.21 Haas et al22 concluded that patients receiving oral MZ had an increased level of sedation before the administration of local anesthetic.
Postsedation complications may be enhanced in CH sedations by the extended duration (4–8 hours) and beta elimination half-life of the active metabolite trichloroethanol (8–11 hours) as compared with the shorter duration (2–6 hours) and half-life (1–4 hours) of MZ.23,24 Midazolam offers additional advantages over CH with its anterograde amnesia and the availability of a reversal agent, flumazenil.
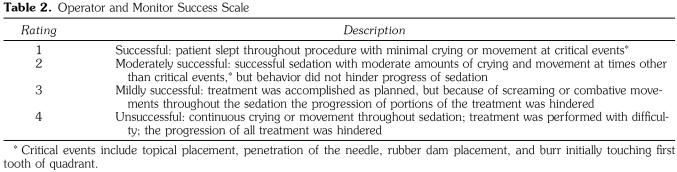
This prospective study had 2 specific aims. The first was to compare the behavioral differences resulting from the sedative effects of the triple-dose cocktail CH/M/H versus the regimen MZ/M/H. Behavior was assessed by the objective North Carolina Behavior Rating System (NCBRS) and a subjective scale that allows the operator and the monitor of the sedation to rate the success of the sedation by using the operator and monitor success scale (OMSS). The second aim was to compare the physiological effects of these regimens.
METHODS
Sample
Approval from the University of North Carolina (UNC) Institutional Review Board Committee on Investigations Involving Human Subjects was obtained. The sample consisted of 16 children referred for sedation after treatment was unsuccessful in a conventional setting. Patient age ranged between 24 and 54 months at the time of the first sedation. All children needed at least 2 sedation appointments of approximately equal length. All children included in the study had no previous operative dental experience under conscious sedation. Also, all children met the requirements of the American Society of Anesthesiologists Class I anesthesia risk. Any children with tonsils greater than +2 according to Brodsky's Tonsil Classification System25 were excluded from the study.
Sample size was calculated on a 2-sample comparison of proportions of the quiet and annoyed behaviors used in the NCBRS in a previous sedation study that assessed both physiological and behavioral parameters in 10 subjects.7 Power was set at 0.90 with β = 0.10 and α = 0.05, yielding an adequate sample size of 16.
Informed consent was obtained for the dental treatment; sedation; and use of physical restraint, videotaping, and participation in this investigation. Parents were instructed with preappointment procedures and postoperative instructions after the sedation appointment. The procedures, possible discomforts or risks, as well as possible benefits were explained fully to the legal guardians of the human subjects involved, and their informed consent was obtained before the start of the investigation.
Sedation Appointment
The conventional oral triple-dose cocktail (regimen A) included 50 mg/kg CH (Pharmaceutical Basics Inc, Morton Grove, Ill), 1.5 mg/kg M (Demerol, Winthrop- Breon, New York, NY), and 25 mg H (Vistaril, Pfizer Laboratories, New York, NY). The experimental group's triple cocktail (regimen B) included 1.0 mg/kg MZ (Versed, Roche Pharmaceuticals, Nutley, NJ; UNC Hospital's Pharmacy), 1.0 mg/kg M, and 25 mg H.20 The fixed dose of 25 mg H was used in accordance with the drug regimen followed in the Pediatric Sedation Clinic at UNC–Chapel Hill.7,8,26,27
A crossover study design was used wherein each patient served as his or her own control. Each patient was assigned randomly to receive 1 of the 2 drug regimens for the initial sedation appointment, with the other regimen administered at the second appointment. Regimens were administered by the principal investigator via medicine cup or oral syringe. The principal investigator was in the sedation room if the regimen administered needed to be revealed for emergency purposes. The operator, monitor, and assistant were blinded to the regimen being used. Monitors were board-certified pediatric dentists with extensive experience in conscious sedation for children.
One hour after regimen A was administered or 30 minutes after regimen B was administered, all patients were separated from their parents and transferred to the dental operatory and placed in a Papoose Board (Olympic Medical Group, Seattle, Wash). Because the purpose of this study was to compare these regimens at each individual patient's optimal sedative effect, the different start times were necessary because the optimal working time of MZ is much shorter than the working time of M. A shoulder roll was placed under the patient's neck and shoulders to aid in maintaining a patent airway. Supplemental oxygen (100%) was administered via nasal cannula (Salter Labs, Arvin, Calif) at 3.0 L/min throughout the sedation.
Behavioral Assessment
All sedation appointments were videotaped with a video camera (RCA VHS-C 3-in LCD AutoShot Camcorder, Indianapolis, Ind). Taping commenced from the time the patient entered the operatory until the time the patient left the operatory. Tapes were identified by a randomly assigned 3-unit code consisting of 1 letter and 2 numbers. A blinded, calibrated rater scored each tape by using the NCBRS (Table 1) as described by Chambers et al.28 Undesirable behaviors were defined as crying, screaming, head movement, torso movement, or foot movement. Behaviors were assessed throughout the appointment in 5 discrete time intervals: the preoperative period, the local anesthetic period, the rubber dam placement period, the operative period, and the postoperative period. The rater was trained and calibrated by assessing 5 videotaped sedations 3 times each to determine his or her success in recognizing each of the behaviors. After practice and calibration, the rater assessed the study videotapes in a random order.
Table 1.
North Carolina Behavior Rating System
The OMSS was created by the authors of this study to subjectively assess behavior (Table 2). This 4-point scale ranged from “successful” to “unsuccessful,” depending on how the patient's behavior affected the progression of treatment.
Table 2.
Operator and Monitor Success Scale
Physiological Assessment
All patients were monitored with a pulse oximeter, capnograph, and a precordial stethoscope. A precordial stethoscope was placed to the left of the sternum to monitor heart and breath sounds. A pulse oximeter probe BCI sensor (BCI International, Waukesha, Wis) was placed on the great toe of the left foot. Carbon dioxide monitoring was performed with the BCI International 9000 Capnograph/Oximeter Monitor (BCI International).
Heart rate (HR), respiratory rate, oxyhemoglobin saturation (SpO2), and end-tidal carbon dioxide (EtCO2) were recorded at 5-minute intervals throughout the entire sedation procedure with a time-based anesthesia record. Additionally, physiological data were recorded at critical events: the placement of topical anesthetic, penetration of the needle into mucosa, placement of the rubber dam, and initial burr contact with the first tooth in a quadrant.
The individual dedicated to monitor the patient alerted the operator to respiratory compromise when the pulse oximeter read below 90% SpO2 for longer than 15 seconds (true hemoglobin desaturation) or when the capnograph read 0 for respiratory rate and EtCO2 or no visual signs of breathing and no audible breath sounds via the precordial stethoscope were detected for longer than 15 seconds (true apnea). Any desaturation or apneic event accompanied by crying or movement was not recorded as a true event. If an apneic event or desaturation occurred, the operator was instructed by the monitor to reposition the child's head. Operative procedures were instructed to stop until the vitals returned to normal.
Data Analysis
As previously stated, 5 tapes of typical sedation appointments were viewed 3 times to evaluate the reliability of the behavior rater. For calibration and intraexaminer reliability determinations, the sedation appointment was not divided into discrete time blocks. Calibration sessions occurred at the beginning of the study, after viewing half of the tapes, and at the study's conclusion. The time each patient spent exhibiting 1 of the 4 behavior types was converted into percentages.
Reliability was based on a random effects model as described by Neter29 and utilized in the sedation study performed by Hasty et al.7 When the ratio is converted into percentages, perfect reliability is indicated by 100%. Reliability near 80% is considered good.
Because of the paired nature of the study design, the small sample size, and the data's nonnormal distribution, a Wilcoxon matched pairs signed-rank test was used to determine the differences in behavior and physiological readings between the 2 treatment groups. This test was used additionally to analyze differences in the OMSS because it is based on a scale rather than continuous data. Because the signed-rank test assumes no period effect, a Wilcoxon 2-sample test was used to determine if the order of the sedations significantly affected behavior or any of the physiological parameters. The level of significance was set at α = 0.05 for all statistical tests.
RESULTS
Thirteen girls and 3 boys completed the study. The average age of the patients at the time they received regimen A (CH/M/H) was 42.8 months (range 27–54 months), whereas the average age of the patients at the time they received regimen B (MZ/M/H) was 43.2 months (range 27–55 months). The average weight of the regimen A group was 15.4 kg (range 12.7–19.55 kg), whereas the average weight of the regimen B group was 15.4 kg (range 12.7–20.0 kg).
Behavioral Findings
The reliability at the beginning of the study was high (>95%) for all 4 behaviors. Within- and between-data set reliability remained greater than 95% throughout the study for the quiet, annoyed, and upset behaviors. Although within-data set reliability remained above 95%, the percentage reliability of recognizing a wild behavior in between-data sets fell to a low 74% throughout the course of the study. This lower reliability was attributed to the low prevalence of the wild behavior in the videotaped sedations.
To facilitate statistical analysis, all behaviors were converted from seconds of time to percentages of the total time. At the first sedation appointment, 9 patients received regimen A and 7 received regimen B. A. Wilcoxon 2-sample rank sum test confirmed that the sedation appointment order had no effect on any behavioral or physiological parameters (P > .05 ).
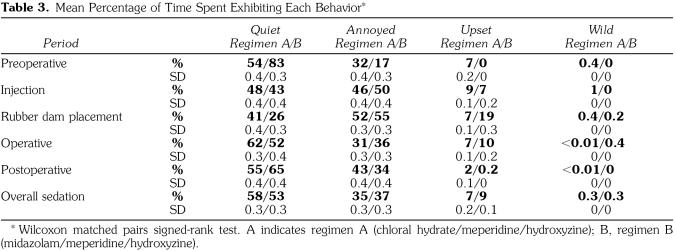
No significant difference in behavior was observed between regimen A and regimen B (P > .05) during any of the individual time blocks or for the overall sedation (Table 3). When administered regimen A, the patients spent an average of 93% of the overall sedation appointment exhibiting either a quiet or annoyed behavior, whereas with regimen B this percentage average was 90%. Because a rubber dam was not used in all operative procedures, only 13 pairs of patients could be evaluated during the rubber dam isolation time block (what procedures were to be performed and the operative protocol were dictated by the operator and attending faculty member, not the investigator). During 3 procedures, rubber dam isolation was attempted, but cotton roll isolation had to be used because of the state of the decay in the maxillary anterior.
Table 3.
Mean Percentage of Time Spent Exhibiting Each Behavior*
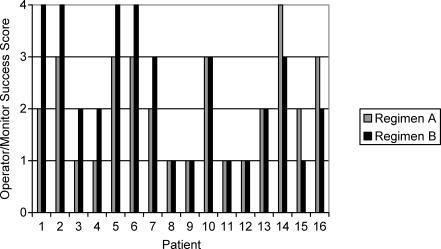
As illustrated in the Figure, 10 of the 16 patients who received regimen A received a score of 1 or 2 according to the OMSS. Nine of the 16 patients who received regimen B received a score of 1 or 2. In 7 patients, regimen A sedations received a better success score than the experimental group sedations. Regimen B sedations received a better success score than regimen A sedations in 3 patients. No difference was noted in the success of the 2 sedation combinations in 6 patients. These findings were not statistically significant (P > .05).
Physiological Findings
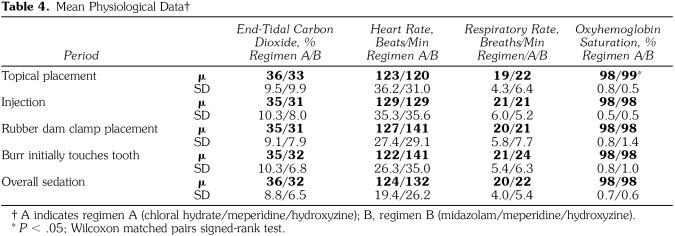
There were no significant differences (P > .05 ) in any physiological parameters for the overall sedation time between the 2 drug regimen groups (Table 4). Regimen B patients had a tendency to exhibit higher HRs during rubber dam clamp placement (140.68 beats/min) and at the moment of initial burr contact (140.76 beats/ min). These values were above a normal range for children 2–5 years of age (80–135 beats/min).30 Although the lower SpO2 level in patients who received regimen A was statistically significant (P = .01 ), this value had no clinical or practical significance.
Table 4.
Mean Physiological Data†
Ten episodes of SpO2 desaturation occurred in 2 patients who received regimen A, whereas no desaturations occurred with regimen B sedations. No apneic events occurred in patients receiving either regimen. No desaturation fell below 85%. After head repositioning by a chin lift, SpO2 immediately returned to above 90%. No desaturation event exceeded 25 seconds. No physiological signs of hypoxemia, such as blue skin, were observed.
DISCUSSION
Individual practitioners have a professional duty to know the effects of all drugs that are administered to patients. Many authors have chronicled the dangers of conscious sedation when proper monitoring, dosages, and strict procedural protocols are not followed.13,30 A review of adverse sedation events in pediatric medicine and dentistry performed by Cote et al13 points to a disproportionately high number of adverse events occurring in patients under the care of “dental specialists.” This review highlights concern with CH when administered outside its recommended dose range of 50–100 mg/kg, when given at home, when administered by a technician, and after premature discharge of the patient because of this drug's extended half-life.13
Many investigation endeavors have focused on parameters for minimizing the adverse physiological events associated with CH/M/H while maximizing the success of sedations.7–9,26,27 The thrust of this investigation was to explore an oral drug regimen that substituted MZ for CH in the triple-dose cocktail approach. This substitution was chosen because MZ provides patients with anterograde amnesia, a quicker recovery, and a reversal agent. The oral dosage of 1.0 mg/kg M, 1.0 mg/kg MZ, and 25 mg/kg H was based on a retrospective study by Nathan and Vargas,20 which found the combination of 1.0 mg/kg M and 1.0 mg/kg MZ to maximize effectiveness with minimal physiological adverse effects.
Behavioral Findings
This study design permitted an objective behavior assessment with a blinded, calibrated rater using the NCBRS. Patients sedated with regimen A (CH/M/H) and regimen B (MZ/M/H) exhibited either a quiet or an annoyed behavior during 93% or 90% of the overall sedation, respectively. These findings are comparable with those of Hasty et al,7 who found that a quiet or annoyed behavior ranged between 82 and 96% of the overall appointment time.
There was no difference in comparing the 2 regimens according to the subjective OMSS. Sixty-three percent (10/16) of the patients who were administered regimen A experienced a sedation that was considered successful or moderately successful. Fifty-six percent (9/16) of patients who were administered regimen B achieved the same level of success. Previous studies examining the drug combination CH/M/H report successes ranging from 72 to 100%, as assessed by the operators.7–9 The lower success noted in our study may be attributed to the specificity of the OMSS rather than limiting those involved in the sedation to chose a simplistic successful or unsuccessful rating for the sedation.
Although the OMSS represents a completely different method of assessing behavior from the NCBRS, there appears to be a discrepancy in how the operator and monitor perceived the sedations compared with the independent rater. This difference might have resulted from several factors. Because patients were restrained in a Papoose Board, movements of the torso may have been more difficult to visualize in a recorded videotape. Movements of the head may have been more difficult to visualize if uncooperative behaviors forced the operator to firmly hold the patient's head. Finally, if a patient was extremely vocal during the length of operative procedures, the operator and monitor may have rated the sedation in a more negative manner, even if the child remained still and operations proceeded with ease. In any case, the videotaped behavioral assessments were judged to be by far the most valid and reliable tool for behavior assessment.
Physiological Findings
No significant differences were found between the 2 drug regimens in any of the physiological parameters for the overall sedation time. Although regimen A had a significantly lower SpO2 during topical placement, this finding had no clinical or practical significance because an SpO2 value of 98% falls within acceptable limits. Ten episodes of hemoglobin desaturation occurred in 2 patients who received regimen A. These episodes did not exceed 25 seconds in either patient, and the oxygen saturation for the overall sedation remained within an acceptable range. This finding was not surprising because intraoperative desaturations are not uncommon events in conscious sedations involving CH/M/H.7,26,27 Factors involved in hemoglobin desaturation include airway control, drug selection, dosage, lidocaine administration, and tonsil size.7,31–34 No desaturation events were noted with regimen B, which mirrors a earlier study with the drug combination MZ/M.20
Despite the fact that intraoperative desaturations commonly occur during conscious sedations, desaturation events should not be overlooked as an unimportant finding. In our study, 2 of the 16 children who received the CH cocktail experienced desaturation events. During dental procedures, the operator may unknowingly apply pressure to the mandible, forcing the mandible to the child's chest and minimizing the available airway. In our study, simply repositioning the mandible quickly increased oxygen saturation levels. However, when an individual practitioner chooses to perform conscious sedation procedures, he or she must have proper advanced training in sedation and anesthesia and have the proper monitors and life-support training, such as the Pediatric Advanced Life Support course offered by the American Heart Association. During our study, the administrator of the drug regimens was in the sedation room in case the drug regimen that was given needed to be revealed to administer emergency medicines. Emergency equipment such as positive pressure masks and even intubation equipment was available if needed.
A tendency toward an increased HR was noted in regimen B patients when the rubber dam clamp and the burr first contacted a tooth. When MZ alone was used in other sedation studies, a clinically insignificant increased HR was noted.9,35 It is not possible to differentiate between an increased HR secondary to the drug regimen and an increase due to increased disruptive behaviors at the points in time in which the readings occurred. Although no significant differences were noted in behavior during the rubber dam placement or operative time blocks, behavior was rated over a course of time rather than at a specific point in time. Of critical importance was the average HR for the overall sedation, which reflects the average of multiple readings throughout the course of the sedation. This fell within the normal range of 85–135 beats/min for children between 2 and 5 years of age.36
Concerns With MZ
Although MZ has been administered orally for a long time, studies performed to determine the pharmacokinetics of a single dose of this drug yielded inconsistent results because the oral formulation was produced by individual pharmacies.15 Acidic conditions between 2.8 and 3.6 pH provide optimal conditions to solubilize MZ in solution. Under these conditions, MZ exists in equilibrium between the active closed-ring form and the inactive open-ring form of this drug. After ingestion, the pH rises above 5.0 and the equilibrium shifts, allowing the absorption of MZ in the active closed-ring form.37 The additions of sweeteners or juices by individual pharmacies may change the pH of the solution, leading to various degrees of MZ that are soluble in that formulation. Because of these differences, the pharmacokinetics would change from formulation to formulation.
In 1998, MZ became commercially available as an oral syrup, eliminating the guess work concerning multiple extemporaneous formulations. Roche Pharmaceuticals recalled this product in 2002 because of a manufacturing difficulty that produced a supernate in a few lots, rendering some doses subsaturated and other doses supersaturated (from a letter sent to health care professionals from Roche Laboratories, February 28, 2002). At that time, the MZ used in this study was obtained from UNC Hospital's pharmacy. The UNC Hospital's formulation is made from the injectable solution of MZ, Syrpalta (sucrose, water, and glycerin), and citric acid calibrated to a pH of 4.2 (UNC Hospital's Drug Information Center, oral communication, February 4, 2003). The Roche formulation of Versed syrup contained sorbitol, glycerin, citric acid, sodium citrate, sodium benzocaine, and other dyes and sweeteners calibrated to a pH of 3.0.37 Differences in pH may have resulted in differences in the solubility of MZ. Because of the small number of regimen B patients who received the commercial syrup versus the UNC formulation, we could not analyze behavioral or physiological differences that may have been secondary to the variation in formulations.
Future research efforts should focus on the efficacy of the MZ cocktail with lower doses of MZ.
CONCLUSIONS
In this study, regimen A included CH (50 mg/kg), H (25 mg), and M (1.5 mg/kg), and regimen B included MZ (1.0 mg/kg), H (25 mg), and M (1.0 mg/kg). Under the conditions of this study, (a) there were no differences in behavior between the 2 drug regimens as measured objectively by the NCBRS or subjectively by the OMSS; (b) there were no statistical differences during the overall sedation for any of the measured physiological parameters; and (c) 10 desaturation events occurred for patients who received regimen A, whereas no events occurred for patients who received regimen B.
Operator and monitor success scale (P = .27).
Acknowledgments
This investigation was supported by MCH grant 2 T17 MC0015-11 and by the AAPD Foundation and OMNII Pharmaceuticals through the OMNII Fellowship program. The authors express their appreciation to Mrs Jennifer Mann—our blinded, calibrated rater—for her hours of dedication to this study and to Doctoral Candidate Lan Kong and the UNC Biometrics Laboratory of the UNC School of Public Health, Chapel Hill, NC.
REFERENCES
- Carr KR, Wilson S, Nimer S, Thornton JB., Jr. Behavior management techniques among pediatric dentists practicing in the southeastern United States. Pediatr Dent. 1999;21:347–353. [PubMed] [Google Scholar]
- Davis MJ. Conscious sedation practices in pediatric dentistry: a survey of members of the American Board of Pediatric Dentistry College of Diplomates. Pediatr Dent. 1988;10:328–329. [PubMed] [Google Scholar]
- Houpt M. Project USAP the use of sedative agents in pediatric dentistry: 1991 update. Pediatr Dent. 1993;15:36–40. [PubMed] [Google Scholar]
- Houpt M. Project USAP 2000—use of sedative agents by pediatric dentists: a 15-year follow-up survey. Pediatr Dent. 2002;24:289–294. [PubMed] [Google Scholar]
- Saxen MA, Wilson S, Paravecchio R. Anesthesia for pediatric dentistry. Dent Clin North Am. 1999;43:231–245. [PubMed] [Google Scholar]
- Ad Hoc Committee on Sedation and Anesthesia. Guidelines for the elective use of conscious sedation, deep sedation and general anesthesia in pediatric dentistry. Pediatr Dent. 2002;24:74–80. [Google Scholar]
- Hasty MF, Vann WF, Jr, Dilley DC, Anderson JA. Conscious sedation of pediatric dental patients: an investigation of chloral hydrate, hydroxyzine pamoate, and meperidine vs. chloral hydrate, and hydroxyzine pamoate. Pediatr Dent. 1991;13:10–19. [PubMed] [Google Scholar]
- Leelataweewud P, Vann WF., Jr. Adverse events and outcomes of conscious sedation for pediatric patients: using chloral hydrate, meperidine, and hydroxyzine pamoate with O2 supplementation. J Am Dent Assoc. 2001;132:1531–1539. doi: 10.14219/jada.archive.2001.0086. [DOI] [PubMed] [Google Scholar]
- Wilson S, Easton J, Lamb K, Orchardson R, Cassamassimo P. A retrospective study of chloral hydrate, meperidine, hydroxyzine, and midazolam regimens used to sedate children for dental care. Pediatr Dent. 2000;22:107–113. [PubMed] [Google Scholar]
- Granoff DM, McDaniel DBL, Borkowf SP. Cardiorespiratory arrest following aspiration of chloral hydrate. Am J Dis Child. 1971;122:170–171. [PubMed] [Google Scholar]
- Rokicki W. Cardiac arrhythmia in a child after the usual dose of chloral hydrate. Pediatr Cardiol. 1996;17:419–420. doi: 10.1007/s002469900094. [DOI] [PubMed] [Google Scholar]
- Munoz M, Gomez A, Soult JA, et al. Seizures caused by chloral hydrate sedative doses. J Pediatr. 1997;131:787–788. doi: 10.1016/s0022-3476(97)70119-7. [DOI] [PubMed] [Google Scholar]
- Cote CJ, Karl HW, Notterman DA, Weinberg JA, McCloskey C. Adverse sedation events in pediatrics: analysis of medications used for sedation. Pediatrics. 2000;106:633–642. doi: 10.1542/peds.106.4.633. [DOI] [PubMed] [Google Scholar]
- Hayden J., Jr. Chloral hydrate as a sedative in dentistry. J Colo Dent Assoc. 1982;61:3–4. [PubMed] [Google Scholar]
- Payne K, Matheyse FJ, Liebenberg D, Dawes T. The pharmacokinetics of midazolam in paediatric patients. Eur J Clin Pharmacol. 1989;37:267–272. doi: 10.1007/BF00679782. [DOI] [PubMed] [Google Scholar]
- Singh N, Pandey RK, Saksena AK, Jaiswal JN. A comparative evaluation of oral midazolam with other sedatives as premedication in pediatric dentistry. J Clin Pediatr Dent. 2002;26:161–164. doi: 10.17796/jcpd.26.2.j714x4795474mr2p. [DOI] [PubMed] [Google Scholar]
- Kain ZN, Hafstadter MB, Mayes LC. Midazolam. Anesthiology. 2000;93:676–684. doi: 10.1097/00000542-200009000-00016. et al. [DOI] [PubMed] [Google Scholar]
- Kupietzky A, Houpt MI. Midazolam: a review of its use for conscious sedation of children. Pediatr Dent. 1993;15:237–242. [PubMed] [Google Scholar]
- Marshall J, Rodarte A, Blumer J, Khoo KC, Akbari B, Kearns G. Pediatric pharmacodynamics of midazolam oral syrup. Pediatrics. 2000;40:578–589. [PubMed] [Google Scholar]
- Nathan JE, Vargas KG. Oral midazolam with and without meperidine for the young pediatric dental patients. Pediatr Dent. 2002;24:129–138. [PubMed] [Google Scholar]
- Feld LH, Negus JB, White PF. Oral midazolam preanesthetic medication in pediatric outpatients. Anesthesiology. 1990;73:831–834. doi: 10.1097/00000542-199011000-00006. [DOI] [PubMed] [Google Scholar]
- Haas DA, Nenniger SA, Yacobi R. A pilot study of the efficacy of oral midazolam for sedation in pediatric dental patients. Anesth Prog. 1996;43:1–8. et al. [PMC free article] [PubMed] [Google Scholar]
- Oertel M, editor. Hudson, Ohio: Lexi-Comp Inc; 1998. UNC Drug Formulary 1998/1999; pp. 182–183. [Google Scholar]
- Oertel M, editor. Hudson, Ohio: Lexi-Comp Inc; 1998. UNC Drug Formulary 1998/1999; pp. 576–578. [Google Scholar]
- Brodsky L. Modern assessment of tonsils and adenoids. Pediatr Clin North Am. 1989;36:1551–1568. doi: 10.1016/s0031-3955(16)36806-7. [DOI] [PubMed] [Google Scholar]
- Leelataweewud P, Vann WF, Jr, Dilley DC, Lucas WJ. The physiological effects of supplemental oxygen versus nitrous oxide/oxygen during conscious sedation of pediatric dental patients. Pediatr Dent. 2000;22:125–133. [PubMed] [Google Scholar]
- Rohlfing GK, Dilley DC, Lucas WJ, Vann WF., Jr The effect of supplemental oxygen on apnea and oxygen saturation during pediatric conscious sedation. Pediatr Dent. 1998;20:8–16. [PubMed] [Google Scholar]
- Chambers WL, Fields HW, Machen JB. Measuring selected disruptive behaviors of the 36 to 60 month-old patient. Part 1: development and assessment of a rating scale. Pediatr Dent. 1981;3:251–256. [PubMed] [Google Scholar]
- Neter J. Applied Linear Statistical Models. 2nd ed. Homewood, Ill: Irwin Press; 1985. pp. 643–647. [Google Scholar]
- Goodson JM, Moore PA. Life-threatening reaction after pedodontic sedation: an assessment of narcotic, local anesthetic, and antiemetic drug interaction. J Am Dent Assoc. 1983;107:239–245. doi: 10.14219/jada.archive.1983.0225. [DOI] [PubMed] [Google Scholar]
- Moore PA, Mickey EA, Hargreaves JA, Needleman HL. Sedation in pediatric dentistry: a practical assessment procedure. J Am Dent Assoc. 1984;109:564–569. doi: 10.14219/jada.archive.1984.0148. [DOI] [PubMed] [Google Scholar]
- Moore PA. Local anesthesia and narcotic drug interaction in pediatric dentistry. Anesth Prog. 1988;35:17. [PMC free article] [PubMed] [Google Scholar]
- Allen GD. Diagnosis and treatment of respiratory problems in sedation and anesthesia for dentistry. Anesth Prog. 1992;39:150–156. [PMC free article] [PubMed] [Google Scholar]
- Verwest TM, Primosch RE, Courts FJ. Variables influencing hemoglobin oxygen desaturation in children during routine restorative dentistry. Pediatr Dent. 1993;15:25–29. [PubMed] [Google Scholar]
- Silver T, Wilson C, Webb M. Evaluation of two dosages of oral midazolam as a conscious sedation for physically and neurologically compromised pediatric dental patients. Pediatr Dent. 1994;16:350–359. [PubMed] [Google Scholar]
- Anderson J. Physiologic principles in pediatric dentistry. In: Pinkham JR, editor. Pediatric Dentistry: Infancy Through Adolescence. 2nd ed. Philadelphia, Pa: WB Saunders Co; 1994. p. 86. [Google Scholar]
- Roche Laboratories Inc. Versed—complete product information. Available at: http://www.rocheusa.com/products/versed/pi_syr.html. Accessed February 16, 2003.