Abstract
A novel pharmacology paradigm has been developed which quickly and efficiently moves prospective anticancer drugs from the discovery phase through pharmacology testing and into therapeutic trial assessment. Following discovery, the drug is first assessed in a clonogenic assay which determines the cytotoxic effect of different concentrations of the drug at 3 different exposure durations: 2h, 24h and continuous (168 h). Second, pharmacokinetic information is obtained in both plasma and tumor for the drug administered at the maximum tolerated dose given intravenously. The first study defines the time-concentration profile required to obtain a specific cell survival for the tumor cells; the second study determines the concentration-time profile that can be obtained in both plasma and tumor at the maximum tolerated dose of the drug. The integration of this information determines whether a successful therapeutic trial is possible. Only when a drug shows therapeutic efficacy is a proteomics-based mechanism of action study initiated. Two drugs have been assessed in this paradigm: salicortin and fascaplysin A.
Keywords: drug development, anticancer, natural products, salicortin, fascaplysin A
INTRODUCTION
Screening of organisms in nature for medicines to counter human diseases has had a long and bountiful past from Ayurvedic medicine (Swerdlow, 2000), to modern ethical drugs, and over–the-counter treatments. Indeed, the majority of our prescription drugs, particularly in the case of cancer (Newman et al., 2003), and the vast majority of our nutraceuticals, have their origin in nature’s products. While bacteria and plants have been the major contributors of the past contributing drugs like adriamycin and taxol, respectively; marine organisms including sponges, tunicates and marine microorganisms (including sponge-associated microorganisms) have to date contributed a number of experimental drugs including ecteinascidin 743, bryostatin-1, and dolastatin analogs (Newman and Cragg, 2004), and have a bright potential for anticancer drug discovery.
The natural product chemistry philosophy dominant for many years was “grind-and-find”; that is, fractionate and identify any and all chemicals in a given organism. This approach was particularly strong in academia with its student-based work force, and has indeed been productive in finding many new classes of cytotoxic compounds, including active anticancer drugs, some of which are presently in clinical development. Within the last decade, however, funding agencies have demanded that the search for new drugs be carried out in conjunction with function-based, bioassays. These assays have been either traditional ones such as antimicrobial and cytostatic assays; or, focused on functional, cellular or biochemical assays associated with specific disease states. The majority of present day assays tend to focus on targets such as apoptosis, mitosis, or specific proteins involved in cell cycle control or signal transduction. During the past decade, pharmaceutical companies have led the search for compounds effective against individual proteins, mostly those in signal transduction pathways that are associated with either cancer or other human disease states. They have attempted to satisfy their need for new anticancer drugs in their pipelines by establishing assays that are mechanism-based, high-throughput, molecular-target-oriented and combinatorial chemistry-linked (Jones & Fitzpatrick, 1999). However, the massive infusion of funds into this effort has not been equaled by a large number of clinically active compounds. Leaving aside the antibody targeted therapies such as herceptin, the compounds All-trans retinoic acid, gleevec and iressa are the few FDA-approved small molecules with only iressa showing any activity against solid tumors, the major oncologic diseases (Ross et al, 2004).
One of the problems with target-oriented development can be seen with the farnesyltransferase inhibitors (FTI’s)(Brunner et al., 2003). They were developed based on the expected interference with ras activity and indeed demonstrated such activity in tumor cells with mutated ras. Surprisingly, FTI’s not only had activity in tumor cells without mutated ras, but also inhibited ras in normal cells, without leading to cell death. The mechanism of action of FTI’s remains complex and unknown at present and yet this class of compounds is still being extensively studied not only in preclinical models but also in clinical trials. Further, FTI’s have shown little inherent anticancer activity in clinical trials whether alone or in combination with other drugs.
Despite the scientific research, financial resources and public relations efforts that have been expended on their in-house drug discovery efforts, many pharmaceutical companies presently find that they have seriously depleted anticancer drug development pipelines (Agres, 2003). This dire situation is compounded by the financial squeeze created by the loss of well-known drugs coming off patent protection that previously provided the financial resources for maintaining their molecular target-oriented research and development effort (Harris, 2002).
This has led the pharmaceutical industry increasingly to turn to both academia and biotech companies where early stage discovery and development is generating promising leads. This move to decentralize the anticancer discovery and development process represents a significant opportunity both for the expansion of natural product investigators into these potentially large, untapped sources, and for wider access to nature’s chemical inventory. The unique and novel approach described here holds significant promise for discovering new anticancer drugs from natural products.
METHODS
Our paradigm
During the past decade, we have been involved in searching for and discovering new anticancer agents effective against the major human solid tumors, including breast, lung, colon, pancreas and prostate (Corbett et al, 1987, 1992; Carmelli et al, 1990; Valeriote et al, 1992, 1994, 1995; Amagata et al, 2003). The preclinical paradigm that we presently use has undergone significant conceptual development over the years and its present stage is illustrated in Figure 1.
Figure 1.
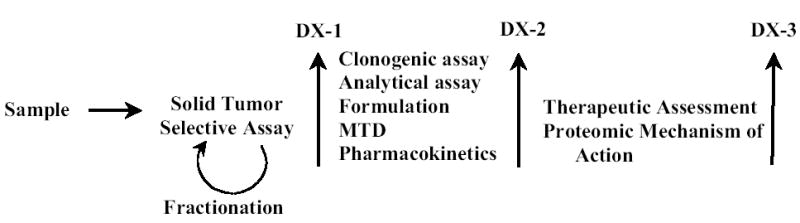
Drug discovery and development paradigm.
In our quest to discover anticancer leads, we have taken a different and, we believe, more rational approach in which assessment of anticancer activity is first defined at the cellular level, and, definition of the molecular mechanism of drug action is much later, following the demonstration of in vivo therapeutic efficacy. Our approach is the antithesis of the presently relatively unsuccessful molecular target approach in which the expenditure of valuable resources on mechanism of action is done only on those leads that are known to be effective in vivo.
This multi-faceted cellular, clonogenic, pharmacologic, therapeutic, and proteomics approach is the heart of our preclinical discovery/development enterprise. The first critical component of our paradigm is the discovery assay. To be successful, the assay needs a wide range of potential chemotypes. Consequently, we continue to expand our source of samples to encompass an increasingly diverse collection of natural products, including plants, cyanophytes, sponges and other marine organisms. The natural product sample is supplied as either an organic or aqueous extract and first tested in our in vitro, clonogenic-based, solid tumor selective assay. If the sample is selective, and is a pure compound, it proceeds past decision point 1 (DX-1) for further studies. If the sample is a natural product extract, it goes through a series of bioassay-directed fractionation steps, each time followed by the solid tumor selective assay, until a pure, active compound is defined, which then proceeds past DX-1.
Solid tumor selective assay
We have developed a unique in vitro disk diffusion assay, which assesses differential, anti-clonogenic activity among solid tumor cells, leukemia cells and normal, bone marrow committed progenitor cells. The “target” here is the proliferating solid tumor cell. This strategy consequently targets all biochemical and molecular elements or alterations characteristic of solid tumors rather than specific single targets which may bear little relevance to in vivo drug efficacy. The difference between the killing of solid tumor and either leukemia or normal cells determines whether a sample is solid tumor selective (Valeriote et al, 2002; Subramanian et al, 2002). By first selecting for samples with greater cytotoxic effects on solid tumor cells as compared to leukemia cells, we select for those compounds which have the greatest potential to target critical cancer-related activities more specific to the solid tumor cell. By also selecting for samples with greater cytotoxic effects on solid tumor cells compared to normal cells, we attempt to further enhance for compounds which target the solid tumor cell while at the same time dereplicating those targets whose modulation would lead to general cell cytotoxicity. By employing normal bone marrow committed progenitor cells, we attempt to minimize drug toxicity to a major clinically sensitive tissue.
Natural product extracts demonstrating solid tumor selectivity are followed up with rounds of bioassay-directed fractionation eventually leading to pure, active compound(s). This assay has been well described and, during the past decade has been highly effective in generating numerous leads (Corbett et al, 1994, 1997a; Golakoti et al, 1995; Moore et al, 1996; Valeriote et al, 1994, 1995, 2002).
Two examples used here are salicortin and fascaplysin A (Figure 2). Fascaplysin (Figure 2A) was purified from the sponge Fascaplysinopsis reticulata obtained from a Papua-New Guinea collection. The initial extract demonstrated significant selectivity in the in vitro assay; and, subsequent rounds of bioassay-directed fractionation led to its purification and consequent structural determination. A number of fascaplysin-alkaloid cation salts have been reported as well as a number of analogues from Fascaplysinopsis (Jimenez et al, 1991a,b, Seagraves et al, 2003a, b, Seagraves et al, 2004).
Figure 2.
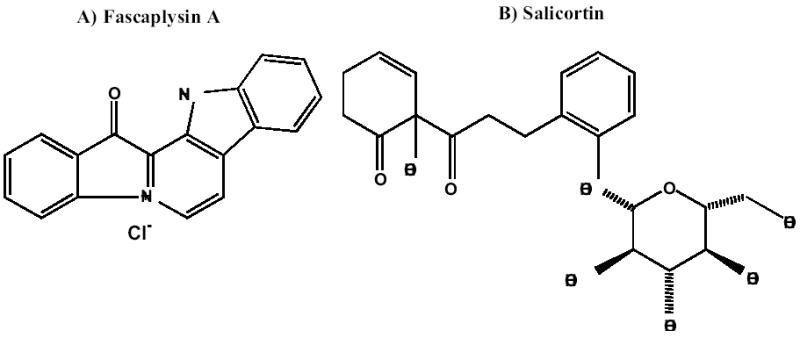
Chemical Structure of solid tumor selective compounds.
Salicortin, first reported from the bark of the willow, Salix purpurea (Thieme, 1964) is an abundant phenolic glycoside in most members of the Salicaceae plant family (Figure 2B). Salicortin and its derivative, tremulacin, are known to act as defensive substances in quaking aspen (Populus tremuloides) against a variety of insect herbivores (Clausen et al, 1989).
As is the case for many natural product discoveries, the chemical structure was known but anticancer activity was not defined (Roll et al, 1988).
Compounds demonstrating selectivity, such as salicortin and fascaplysin, pass DX-1 and are described thereafter as drugs since they move to the second, developmental stage of the discovery/developmental paradigm.
Clonogenic cell assay
The drug development process begins with the generation of the concentration-clonogenic cell survival characteristics of the drug in vitro at selected times. Survival is determined against that solid tumor cell type which demonstrated solid tumor selectivity in the in vitro assay; and which, later will be the tumor of choice for pharmacokinetic, therapeutic efficacy and mechanism studies. Cells are exposed to different concentrations of the drug for either 2 h, 24 h or 7 days (continuous exposure), following which the exposure is terminated, and the cells assessed for their clonogenic potential. The clonogenic assay defines cell survival with data plotted as a concentration-survival curve (Figure 3A).
Figure 3.
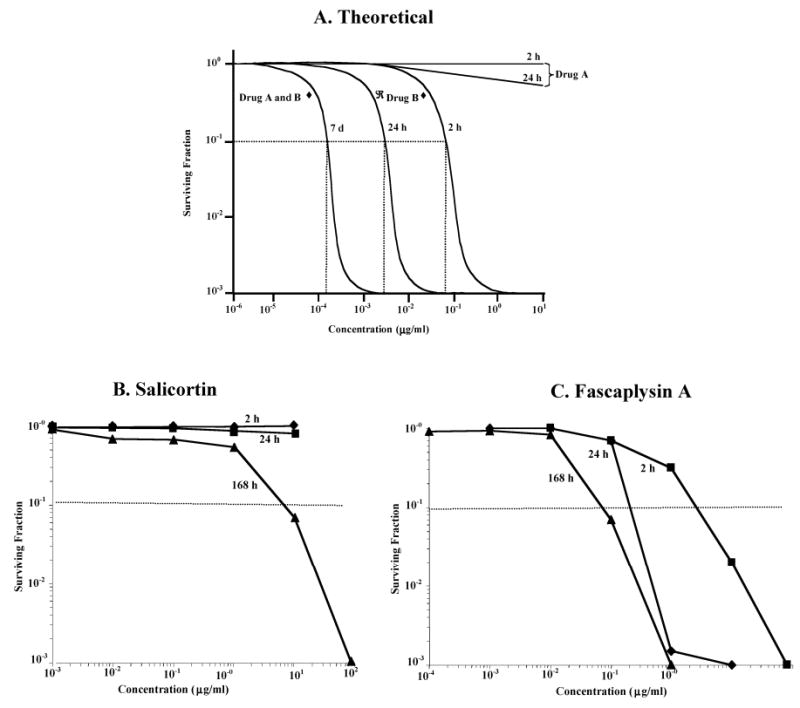
Clonogenic cell survival-concentration relationship following exposure to either A) theoretical drugs A and B, B) salicortin, and C) fascaplysin A to HCT-116 colon carcinoma cells.
The results of this assay are used to define the drug exposures that should be necessary to achieve a therapeutic effect in vivo. We propose that a therapeutic effect would be noted in tumor-bearing mice if 90% or greater of the tumor cells were destroyed by exposure to the drug in vivo. This minimal targeted concentration is shown in Fig. 3A as that required to yield a survival of 10−1 for any exposure duration. While tumor cells would, in fact, be exposed to a decreasing concentration of drug with time after an intravenous administration, these defined minimal targeted concentrations must be achieved in vivo for the durations indicated. This should predict the cytotoxic effect achieved in vivo, as a first approximation, and be on the conservative side of our response prediction.
We have found two widely different patterns as shown in Figure 3A. The first pattern is illustrated by the hypothetical, Drug A. Here, even at high concentrations, there is little if any cytotoxic effect following either a 2 h or a 24 h exposure at the maximum concentration of 10 μg/ml. However, the drug is cytotoxic for the 7 day (continuous) exposure (10−1 survival at 1.5×10−4 μg/ml or 150 pg/ml). The therapeutic implication of these results is that for a drug having a pattern similar to drug A, a chronic in vivo dosing schedule would be required to maintain an effective cytotoxic level (150 pg/ml or higher in this case) in the tumor-bearing animal. A second pattern is illustrated by the hypothetical, Drug B where a therapeutic effect could result from either a short, 2 h exposure [at a concentration of 8×10−2 μg/ml (80 ng/ml) or higher], a longer, 24 h exposure [at 3×10−3 mg/ml (3 ng/ml) or higher], or a longer term exposure for 7 days [at 1.5×10−4 μ g/ml (150 pg/ml) or higher]. The therapeutic implication of these results is that for a drug having a pattern similar to drug B, either an acute (single bolus) or a chronic (daily) in vivo dosing schedule could maintain an effective cytotoxic level (150 pg/ml or higher in this case) in the tumor-bearing animal. The results obtained for salicortin against HCT-116 colon cancer cells are shown in Fig. 3B, and show a pattern similar to drug A in Fig. 3A. Both the 2 h exposure and the 24 h exposure produce little if any cell killing up to an exposure concentration of 100 ug/ml. However, a continuous exposure (7 days; 168 h) results in a surviving fraction of 10−1 at 500 ng/ml. The therapeutic implication of these results is that a chronic (daily) in vivo dosing schedule is required for salicortin to produce an effective cytotoxic level in the tumor-bearing animal.
The results obtained for fascaplysin A against HCT-116 colon cancer cells are shown in Fig. 3C, and show a pattern similar to drug B in Fig. 3A. A 2 h exposure results in a surviving fraction of 10−1 at 2 μg/ml; a 24 h exposure requires 300 ng/ml for a similar survival, and a continuous exposure (7 days; 168 h) requires 70 ng/ml. The therapeutic implication of these results is that either an acute (single bolus) or a chronic (daily) in vivo dosing schedule for fascaplysin A could produce an effective cytotoxic level in the tumor-bearing animal.
Analytical assay
Concurrent with the clonogenic assay, we develop an HPLC-based analytical assay for each drug. The analytical method must quantify the drug both alone and after extraction from both plasma and tumor tissue. The method is then applied to measuring the drug in in vivo pharmacologic studies (Workman, 1993; Garrett & Workman, 1999). While most natural products are amenable to UV-visible spectroscopic detection, sufficient sensitivity may require other detection procedures such as fluorescent spectroscopy, evaporative light scatter or mass spectrometry, particularly for drugs lacking a chromophore or for those that are very potent.
For salicortin, a C18 reverse phase column with a mobile phase of 46% acetonitrile in 0.1% formic acid/64% formic acid (1%) using a step gradient of 90:10 for 3 min., and 55:45 for 17 minutes, with a detector set at 271 nm resulted in a retention time of 10 minutes and a limit of detection in plasma of 125 ng/ml. For fascaplysin A, a C18 reverse phase column with a mobile phase of 20% ethanol/80% phosphoric acid (0.1% v/v) and a detector set at 300 nm yielded a retention time of 14 minutes and a limit of detection in plasma of 50 ng/ml.
Formulation
A major problem with natural products is their general lack of aqueous solubility, as might be expected particularly for compounds derived from marine organisms. For preclinical therapeutic assessment, many investigators believe that they “resolve” this problem by simply preparing a suspension of the drug and injecting it subcutaneously. This likely results in the majority of the drug remaining at the injection site and never getting to the tumor. It seems to us that it is paramount for the successful development of a drug to obtain an intravenous-compatible preparation in order to maximize the concentration of drug delivered to the tumor cells. Formulation development is critical to ensure solubility of the new compound in acceptable and appropriate vehicles for intravenous administration. While most natural products that have anticancer activity are not very soluble in water, nearly all can be dissolved in either ethanol or dmso at concentrations in the tens of milligrams per ml. Subsequent addition of surfactant and amphiphilic compounds such as Cremaphor, polyethylene glycol or propylene glycol (or a mixture thereof) will stabilize the drug so that it remains in solution upon further dilution with an aqueous diluent such as saline. After dilution, the stabilizers should not exceed 5% v/v to limit any solvent toxicity.
Maximum Tolerated Dose (MTD)
The formulated drug is given intravenously for the determination of the single-bolus MTD. Two-fold dilutions of the drug are administered to groups of 5 mice per dose and both weight and survival of the mice are followed for 30 days. If no mice die, higher doses are studied; if all mice die, lower doses are studied. Eventually, some mice will die at one dose and no mice die at the next lower dose. The MTD is that dose above which none of the 5 mice die and at least one of the mice die in the next higher dose. With 5 mice per group, this could also be defined as less than an LD20 (below the lethal dose for 20% of the mice). While this MTD is first determined as a single, bolus, intravenous dose, two other schedules may also be studied by us. If the drug is toxic within minutes after administration of a bolus dose, the toxicity can be attributed to high peak plasma levels of the drug and a 1 h infusion is tested in an attempt to decrease the high drug level. By use of the infusion schedule, a higher MTD may be attained and effective concentrations may then be achieved at the tumor site. The second schedule studied is a daily-times-five (daily for 5 days) bolus (or infusional) schedule. This schedule allows one both to administer as much drug as possible over a short period of time and to maintain effective concentrations when a chronic exposure is projected to be the most effective as defined by the clonogenic cell killing studies (as was the case for salicortin, Fig. 3B).
For fascaplysin A, a maximum concentration of 8.2 mg/ml was soluble in ethanol and the maximum injectable dose of 100 μg/mouse (about 5 mg/kg) was not toxic to the mice. Since an MTD could not be determined, the subsequent pharmacokinetics was done at this maximum achievable dose.
Pharmacokinetics
The pharmacokinetic behavior of the drug in both plasma and tumor is next determined at the MTD. The drug is injected intravenously into tumor-bearing mice and both blood and tumor tissues are assayed for drug concentration at different times thereafter. Three different plasma pharmacokinetic profiles are shown in Figure 4A. Drug X shows a rapid disappearance of drug to plasma concentrations 0.1% or lower within 2 h after administration.
Figure 4.
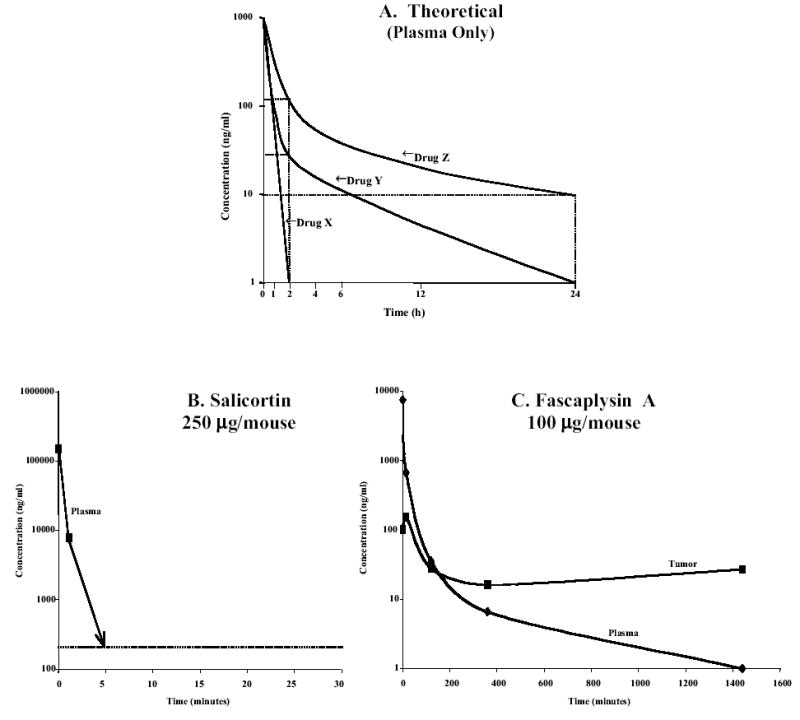
Pharmacokinetic profile for A) three hypothetical drugs (X, Y, X) in plasma; B) Salicortin administered at 250 μg/mouse (10mg/kg) intravenously; and C) Fascaplysin A administered at 100 μg/mouse (4mg/kg) intravenously. Data points shown are for 1min, 15min, 2h, 6h an 24h.
Drug Y has plasma drug levels between 10% and 1% of the initial level after 2 h, however, the elimination phase half-life is sufficiently short that the drug level is at 0.1% of the initial concentration at 24 h. Drug Z has plasma drug levels of about 10% of the initial level at 2 h but has an elimination phase half-life sufficiently long that drug levels of 1% of the initial concentration remain in the plasma by 24 h.
In our paradigm, pharmacokinetic studies are carried out in tumor-bearing mice so that pharmacokinetic profiles for drug levels are also defined in the tumors. Theoretical examples are not shown here because they can be much more varied than those for plasma. Consequent to uptake, intracellular metabolism, binding and elimination from the tumor cell, a large number of possible cellular pharmacokinetic profiles can results with either limited concentrations of drug appearing in the tumor or the drug being concentrated within the tumor cell.
The pharmacokinetic profile shown in Fig. 4B for salicortin is similar to that for Drug X in Fig. 4A. It rapidly disappears from the plasma and by 5 min. is below the limit of detection for the HPLC assay. The initial plasma concentration, indicated at “0” time is calculated as if the drug was distributed within the total mouse plasma (250 μg/ml). By 1 min. the level is at 9 μg/ml, and by 5 min. is below the 250 ng/ml level of detection. From 1 min. through the 24 h tumor assay, there was no detectable drug in the tumor.
The pharmacokinetic profile shown in Fig. 4C for fascaplysin A is similar to that for Drug Y in Fig. 4A. It demonstrates a biphasic curve with an initial plasma halflife of 5 min. and a long terminal halflife of 350 min. The initial plasma concentration (at 1 minute) following the administration of 100 μg/mouse is about 9 μg/ml. There is a rapid decrease to less than 1% of this initial level by 2 h (to 30 ng/ml), and then to approximately 1 ng/ml by 24 h. Fascaplysin A concentration in the tumor increased rapidly to 200 ng/ml by 15 min. and then decreased to 30 ng/ml by 2 h where it remained at that level throughout the 24 h sampling period.
Integration of Data from Clonogenic, MTD, and Pharmacokinetic Assays
Before proceeding to therapeutic efficacy studies, a comparative analysis of the clonogenic and pharmacokinetic profiles, the latter at the MTD, is done in order to determine whether expensive therapeutic studies have a reasonable probability of success. Given a pharmacokinetic profile like drug X (Fig. 4A), there is little likelihood that the parent drug would be active in vivo since by 2 h the levels have decreased to less than 1 ng/ml and we would not proceed to a therapeutic trial. There could, however, be sufficient parent drug in the tumor tissue, or, one or more metabolites with anticancer activity which could modify this conclusion. Given a pharmacokinetic profile like drug Y, a plasma concentration of 30 ng/ml at 2 h, and 1 ng/ml at 24 h would require a concomitant clonogenic pattern shown by drug B in Fig. 3A to have a chance of yielding a therapeutic effect. In this case, no therapeutic activity would be expected for a bolus exposure. However, if repeated doses of drug Y were administrable for 7 days, without an increase in host toxicity, then a plasma concentration could be maintained above 1 ng/ml which is above that predicted by clonogenic pattern B to be therapeutically effective. We would then move drug Y forward, past Decision Point 2 (DX-2; Fig.1) into an in vivo therapeutic trial with the defined chronic schedule. Finally, for the plasma pharmacokinetic profile of drug Z in Fig. 4A, levels at 2 h, 24 h and 7 days are above that required by the clonogenic pattern for drug B shown in Fig. 3A so that either a single bolus or a daily chronic schedule would be expected to be therapeutically effective. In this case, we would move drug Z forward, past DX-2, into an in vivo therapeutic trial.
For salicortin, the plasma or tumor level needed to be maintained at 500 ng/ml or above for up to 7 days exposure (Fig. 3B). The pharmacokinetic data (Fig. 4B) indicated that the plasma level was below this value within 5 min. of drug injection, and did not reach this level in the tumor over the subsequent 24 h. From these results, salicortin would not be moved forward to therapeutic efficacy trials.
For fascaplysin A, comparing the pharmacokinetic profile (Fig. 4C) with the clonogenic pattern (Fig. 3C) predicts a therapeutic effect for either bolus or chronic drug treatment. The clonogenic pattern for 2h, 24h and continuous concentrations exposures predict a therapeutic effect if the plasma or tumor concentrations are above 80 ng/ml, 3 ng/ml and 150 pg/ml, respectively. The pharmacokinetic profile for Fascaplysin A (Fig. 4C) demonstrates a concentration at 30 ng/ml for the 2 h point for both tumor and plasma which is below the 80 ng/ml required for efficacy; thus a therapeutic effect would not be predicted on this basis for a bolus injection. However, the 24 h tumor concentration (30 ng/ml) is above that required by the clonogenic data (3 ng/ml) for a therapeutic effect. Therefore, a single dose (and also daily doses) of fascaplysin A at this dose level would be expected to have a therapeutic effect in mice bearing HCT-116 tumors.
Therapeutic Efficacy
The pharmacologic and clonogenic information described above is incorporated into a schedule and dose protocol for a given drug. This is tested for the determination of therapeutic efficacy in the next stage of our preclinical paradigm. Different doses, up to the MTD, are administered to tumor-bearing mice and tumor volume is measured every 2 to 3 days. The tumor volumes of the treated mice are compared to those of the untreated controls and, if a sufficient difference is found, the drug is declared to have therapeutic efficacy (Corbett et al 1995, 1997b).
For the therapeutic study with fascaplysin A, 2×106 HCT-116 cells were injected subcutaneously into two groups of 6 scid mice. One group served as the untreated control (C) and about 2 weeks later, when the tumors were 250 mm3, the second group (T) received 100 μg/mouse (4 mg/kg) fascaplysin A daily for 5 days. No toxicity was observed over the subsequent 30 days. The controls reached 4 times their original volume at day 13 while the treated group reached 1000 mm3 at day 23. The %T/C value was 42% at day 15. Thus, even at this less than optimal dose, because an MTD could not be obtained for fascaplysin A, a therapeutic effect was observed.
Proteomic Mechanism of Action
Our approach to defining the molecular mechanism of action at the proteome level reflects the importance of proteins as functional gene products, “drugable” targets and the role they play in molecular pathways (Nakeff & Subramanian, 2002; Nakeff et al, 2002). The proteomics-based analysis utilizes 2D liquid protein separation/mass mapping (Yan et al, 2003). This innovative platform is ideally suited for the rapid, reproducible, quantitative and relatively inexpensive detection and ultimate identification of intact proteins (both known and unique) from whole tumor cell lysates and their drug-induced post-translational modifications. This approach combines first dimension protein fractionation by the isoelectric point (pI) of proteins of drug-treated and untreated whole cell lysates using chromatofocusing with analytical columns followed by second dimension separation of proteins in each pI fraction by non-porous reverse-phase HPLC. This second dimension procedure generates complex proteome maps of differential protein expression. Since the entire 2D fractionation is performed in a liquid phase, many of the problems inherent in the use of 2D gels and protein arrays are circumvented. This greatly simplifies the use of mass spectrometry to both identify and fully quantify known and unique proteins by their signature molecular weights and determine drug-induced post-translational drug modifications.
We propose that the therapeutic efficacy of drugs demonstrating solid tumor selectivity results from the drug-induced modulation of the expression of multiple proteins, many of which are resident on different regulatory pathways. Furthermore, the extent of modulation must be maintained for periods of time that are long enough to prevent the drug-exposed clonogenic tumor cells from either repairing the drug-induced block of the pathway or switching to alternate regulatory pathways to circumvent the effects of this block. It is not unreasonable to expect that the maintenance of these multiple drug-induced changes for up to 24h (approximating one cell cycle time of the tumor cell’s growth phase) is sufficient to lead to the proliferative death of the target tumor cells, resulting in an in vivo response. The importance of using our paradigm to generate drug-modulated molecular pathway information is that it reflects the action of a drug with demonstrated activity in vivo. This provides in vivo validation of both identified drug-modulated pathways and proteins as potential biomarkers to measure the efficacy of drug action in the tumors of patients in Phase I/II clinical trials. Our paradigm ends once we have demonstrated a mechanism of action for the in vivo-active drug since we expect that the information portfolio obtained is of sufficient value for licensing of our drugs into the pipelines of the pharmaceutical industry for subsequent INDA-directed studies and eventual clinical trials.
For fascaplysin A, recent studies have shown that it is both a DNA intercalator (Hormann et al, 2001) and also selectively inhibits cyclin dependent kinase 4 (cdk4)/cyclin D1 complex (Soni et al, 2000). This demonstration of multiple sites of action fits our hypothesis for therapeutically effective drugs. Having already demonstrated two such sites for fascaplysin A, together with its known therapeutic efficacy, this drug is an excellent candidate for a more complete proteomics analysis to reveal the pathways involved in the drug’s cytotoxic effect.
CONCLUSION
Mining of both terrestrial and marine organisms for effective cancer treatment is a focus of our drug discovery program. It is an obvious approach since history bears out the success of such an endeavor with regard to present clinically effective anticancer drugs. Nature’s inventory of novel and effective drugs has been but surface-mined and significant deposits most likely still exist. Integration of natural product chemistry with modern fractionation and identification technologies, together with a streamlined cellular and pharmacologic approach, such as that described above, with the mechanism of action defined after therapeutic efficacy has been shown, is a prescription for success. Our paradigm is not only logical but also effectively optimizes the use of the limited amount of compound usually available from natural sources. Accordingly, only a few milligrams are used prior to DX-1, 10 to 20 mg between DX-1 and DX-2 and 100 mg needed after DX-2. In this way, over 98% of the potential samples are eliminated before DX-1 and another 90% are eliminated because of a lack of an appropriate clonogenic/pharmacologic profile. This leaves about 0.2% of the input compounds with the highest potential to proceed to the development stage that requires amounts of additional compound prepared from a reasonably-sized biomass collection. If not, then synthetic chemistry is incorporated at this stage. This not only prepares the drug needed for the therapeutic trials but also provides a head start on the process chemistry that likely will be needed prior to INDA-directed studies. Given the depleted state of pharmaceutical anticancer drug development pipelines, there would seem to be an excellent opportunity for a renewed emphasis on natural product drug discovery and development from Nature’s medicine chest.
Acknowledgments
Supported by USPHS grants CA47135 and CA92143 awarded by the National Cancer Institute, Department of Health and Human Services.
Footnotes
Sponsored by Fred Valeriote
References
- Agres T. Alliances eye early-stage drugs. Drug Disc Develop. 2003;6:17–18. [Google Scholar]
- Amagata T, Rath C, Rigot JF, Tarlov N, Tenney K, Valeriote FA, Crews P. Structures and cytotoxic properties of trichoverroids and their macrolide analogs produced by saltwater culture of Myrothecium verrucaria. J Med Chem. 2003;46:4342–4350. doi: 10.1021/jm030090t. [DOI] [PubMed] [Google Scholar]
- Brunner TB, Hahn SM, Gupta AK, Muschel RJ, McKenna WG, Bernhard EJ. Farnesyltransferase inhibitors: An overview of the results of preclinical and clinical investigations. Cancer Res. 2003;63:5656–5668. [PubMed] [Google Scholar]
- Carmeli S, Moore RE, Patterson GML, Corbett TH, Valeriote FA. Tantazoles: Unusual cytotoxic alkaloids from the blue-green alga Scytonema mirabile. JACS. 1990;112:8195–8197. [Google Scholar]
- Corbett TH, Valeriote FA, Baker LH. Is the P388 murine tumor no longer adequate as a drug discovery model? Invest New Drugs. 1987;5:3–20. doi: 10.1007/BF00217664. [DOI] [PubMed] [Google Scholar]
- Corbett T, Valeriote FA, Polin L, Panchapor C, Pugh S, White K, Lowichik N, Knight J, Bissery MC, Wozniak W, LoRusso P, Biernat L, Polin D, Knight L, Biggar S, Looney D, Demchik L, Jones J, Jones L, Blair S, Palmer K, Essenmacher S, Lisow L, Mattes KC, Cavanaugh PF, Rake JB, Baker LH. Discovery of solid tumor active agents using a soft-agar-colony-formation disk-diffusion-assay. In: Valeriote FA, Corbett TH, Baker LH, editors. Cytotoxic Anticancer Drugs: Models and Concepts For Drug Discovery and Development. Kluwer Academic Publishers.; [Google Scholar]
- Corbett T, Lowchik N, Pugh S, Polin L, Panchapor C, White K, Knight J, Demchik L, Jones J, Jones L, Biernat L, LoRusso P, Foster B, Heilbrun L, Rake J, Mattes KC, Perni R, Powles RG, Hlavac AG, Wentland MP, Coughlin SA, Baker L, Valeriote F. Antitumor activity of N-[[1-[[2-(diethylamino)ethyl]amino]-9-oxo-9H-thioxanthen-4-yl]methyl]methanesulfonamide ( WIN33377) and analogues. Exp Opin Invest Drugs. 1994;3:1281–1292. [Google Scholar]
- Corbett T, Valeriote F, LoRusso P, Polin L, Panchapor C, Pugh S, White K, Knight J, Demchik L, Jones J, Jones L, Lowichik N, Biernat L, Foster B, Wozniak A, Lisow L, Valdivieso M, Baker L, Leopold W, Sebolt J, Bissery MC, Mattes K, Dzubow J, Rake J, Perni R, Wentland M, Coughlin S, Shaw JM, Liversidge G, Liversidge E, Bruno J, Sarpotdar P, Moore R, Patterson G. Tumor models and the discovery and secondary evaluation of solid tumor active agents. Int J Pharmacognosy. 1995;33 (Supplement):102–122. [Google Scholar]
- Corbett TH, Valeriote FA, Demchik L, Lowichik N, Polin L, Pancahpor C, Pugh S, White K, Kushner J, Rake J, Wentland M, Golakoti T, Hetzel C, Ogino J, Patterson G, Moore R. Discovery of cryptophycin-1 and BCN-183577: Examples of strategies and problems in the detection of antitumor activity in mice. Invest New Drugs. 1997a;15:207–218. doi: 10.1023/a:1005875015011. [DOI] [PubMed] [Google Scholar]
- Corbett T, Valeriote F, LoRusso P, Polin L, Panchapor C, Pugh S, White K, Knight J, Demchik L, Jones J, Jones L, Lisow L, Teicher BA. Cancer Drug Discovery and Development. Part 2. Humana Press; Totowa, NJ: 1997b. In vivo methods for screening and preclinical testing: Use of rodent solid tumors for drug discovery; pp. 75–100. [Google Scholar]
- Garrett MD, Workman P. Discovering novel chemotherapeutic drugs for the third millennium. Eur J Cancer. 1999;35:2010–2030. doi: 10.1016/s0959-8049(99)00280-4. [DOI] [PubMed] [Google Scholar]
- Golakoti T, Ogino J, Heltzel CE, Le Husebo T, Craig MJ, Larsen LK, Patterson GKL, Moore RE, Mooberry SL, Corbett TH, Valeriote FA. Structure determination, conformational analysis, chemical stability studies and antitumor evaluation of the cryptophycins Isolation of eighteen new analogs from Nostoc sp Strain GSV 224. J Amer Chem Soc. 1995;117:12030–12049. [Google Scholar]
- Harris G. For drug makers, good times yield to a new profit crunch. Wall Street Journal. 2002 April 18;239(76) [Google Scholar]
- Hormann A, Bhabatosh C, Fretz H. DNA binding properties of the marine sponge pigment fascaplysin. Bioorganic & Medicinal Chem. 2001;9:917–921. doi: 10.1016/s0968-0896(00)00313-8. [DOI] [PubMed] [Google Scholar]
- Jiminez C, Quinoa E, Adamczeski M, Hunter LM, Crews P. Novel sponge-derived amino acids. 12 Tryptophan-derived pigments and accompanying sesterpenes from Fascaplysinopsis reticulata. J Org Chem. 1991a;56:3403–3410. [Google Scholar]
- Jiminez C, Quinoa E, Crews P. Novel marine sponge alkaloids 3. ?carbolinium salts from Fascaplysinopsis reticulata. Tetrahedron Letters. 1991b;32:1843–1846. [Google Scholar]
- Jones DA, Fitzpatrick FA. Genomics and the discovery of new drug targets. Curr Opin Chem Biol. 1999;3:171–176. doi: 10.1016/s1367-5931(99)80013-1. [DOI] [PubMed] [Google Scholar]
- Moore RE, Corbett TH, Patterson GML, Valeriote FA. The search for new antitumor drugs from blue-green algae. Current Pharm Design. 1996;2:317–330. [Google Scholar]
- Nakeff A, Subramanian B. Proceedings of the 3rd Samuel A. Latt/Motown Microarray Meeting: Cytometry. 2002;47:44–45. [Google Scholar]
- Nakeff A, Sahay N, Pisano M, Subramanian B. Painting with a molecular brush: Genomic/proteomic interfacing to define the drug action profile of novel solid-tumor anticancer agents. Cytometry. 2002;47:72–79. [PubMed] [Google Scholar]
- Newman DJ, Cragg CM, Snader KM. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg CM. Marine natural products and related compounds in clinical and advanced preclinical trials. J Nat Prod. 2004;67:1216–1238. doi: 10.1021/np040031y. [DOI] [PubMed] [Google Scholar]
- Roll DM, Ireland CM, Lu HSM, Clardy J. Fascaplysin, an unusual antimicrobial pigment from the marine sponge Fascaplysinopsis sp . J Organic Chem. 1988;53:3276–3278. [Google Scholar]
- Ross JS, Schemkein DP, Pietrusko R, Rolfe M, Linette GP, Stec J, Stagliano NE, Ginsburg GS, Symmans F, Pusztai L, Hortobagyi GN. Targeted therapies for cancer 2004. Amer J Clin Pathol. 2004;122:598–609. doi: 10.1309/5CWP-U41A-FR1V-YM3F. [DOI] [PubMed] [Google Scholar]
- Segraves NL, Lopez S, Johnson TA, Said SA, Fu X, Schmitz FJ, Pietraszkiewicz H, Valeriote FA, Crews P. Structures and cytotoxicities of fascaplysin and related alkaloids from two marine phyla-Fascaplysinopsis sponges and Didemnum tunicates. Tetrahedron Letters. 2003;44:3471–3475. [Google Scholar]
- Segraves NL, Robinson SJ, Garcia D, Said SA, Fu X, Schmitz FJ, Pietraszkiewicz H, Valeriote FA, Crews P. Comparison of fascaplysin and related alkaloids – A study of structures, cytotoxicities, and sources. J Nat Prod. 2004;67:783–792. doi: 10.1021/np049935+. [DOI] [PubMed] [Google Scholar]
- Soni R, Muller L, Furet P, Schoepfer J, Stephan C, Zumstein-Mecker S, Fretz H, Chaudhuri B. Inhibition of cyclin-dependent kinase 4 (cdk4) by fascaplysin, a marine natural product. Biochem Biophys Res Comm. 2000;275:877–884. doi: 10.1006/bbrc.2000.3349. [DOI] [PubMed] [Google Scholar]
- Subramanian B, Nakeff A, Media J, Valeriote F. Cellular drug action profile paradigm applied to XK469. J Exptl Therap Oncol. 2002;2:1–11. doi: 10.1046/j.1359-4117.2002.01040.x. [DOI] [PubMed] [Google Scholar]
- Swerdlow JL. Nature’s Medicine. Plants That Heal. National Geographic; Washington, D.C.: 2000. [Google Scholar]
- Valeriote FA, Corbett TH, Baker LH. Drug Discovery -1990. In: Valeriote FA, Corbett TH, Baker LH, editors. Cytotoxic Anticancer Drugs: Models and Concepts for Drug Discovery and Development. Kluwer Academic Publishers; Norwell, MA: 1992. pp. 1–9. [Google Scholar]
- Valeriote F, Moore RE, Patterson GML, Paul VJ, Scheuer PJ, Corbett T. Discovery of natural products from microalgae and marine organisms. In: Valeriote FA, Corbett TH, Baker LH, editors. Anticancer Drug Discovery and Development: Natural Products and New Molecular Models. Kluwer Academic Publishers; Boston/Dordrecht/London: 1994. pp. 1–25. [Google Scholar]
- Valeriote F, Corbett T, LoRusso P, Moore RE, Scheuer P, Patterson G, Paul V, Grindey G, Bonjouklian R, Pearce H, Suffness M. Discovery of anticancer agents from natural products. Int J Pharmacognosy, Supp. 1995;33:59–66. [Google Scholar]
- Valeriote F, Grieshaber CK, Media J, Pietraszkiewicz H, Hoffmann J, Pan M, McLaughlin S. Discovery and development of anticancer agents from plants. J Expl Therap Oncol. 2002;2:228–236. doi: 10.1046/j.1359-4117.2002.01038.x. [DOI] [PubMed] [Google Scholar]
- Workman P. Pharmacokinetics and cancer: successes, failures and future prospects. Cancer Surv. 1993;17:1–26. [PubMed] [Google Scholar]
- Yan F, Subramanian B, Nakeff A, Barder TJ, Parus SJ, Lubman DM. A comparison of drug-treated and untreated HCT-116 human colon adenocarcinoma cells using a 2-D liquid separation mapping method based upon chromatofocusing pI fractionation. Anal Chem. 2003;75:2299–2308. doi: 10.1021/ac020678s. [DOI] [PubMed] [Google Scholar]


