Abstract
The mechanisms that allow the maintenance of immunological memory remain incompletely defined. Here we report that tumor necrosis factor receptor (TNFR)-associated factor (TRAF) 1, a protein recruited in response to several costimulatory TNFR family members, is required for maximal CD8 T cell responses to influenza virus in mice. Decreased recovery of CD8 T cells in vivo occurred under conditions where cell division was unimpaired. In vitro, TRAF1-deficient, antigen-activated T cells accumulated higher levels of the proapoptotic BH3-only family member Bim, particularly the most toxic isoform, BimS. In the presence of excess IL-15, memory phenotype T cells with similar surface phenotype and comparable levels of Bcl-2 family members could be generated from WT or TRAF1-deficient T cell receptor transgenic OT-I T cells. However, when the memory CD8 T cells were allowed to compete for survival signals in the absence of antigen in vivo, the TRAF1-deficient T cells showed decreased recovery compared with TRAF1-sufficient T cells. This defect in T cell recovery in vivo was alleviated by introduction of siRNA to down-modulate Bim in TRAF1-deficient memory T cells. These studies identify the TRAF1 signaling axis and Bim down-regulation as critical for CD8 memory T cell survival in vivo.
Keywords: Bcl-2 family, influenza virus, knockout/transgenic mice
The mechanisms that maintain the survival of memory T cells are not well understood but are critically important for protective immunity. Several members of the TNF receptor (TNFR) family have been implicated in regulating the survival of T lymphocytes and subsequent immunological memory (1).
TNFR family members involved in pro-survival signaling contain TNFR-associated factor (TRAF)-interacting motifs that function to recruit TRAFs (2) to their cytoplasmic tails. The prototypical TRAF family member, TRAF2, is an important mediator of survival signaling, and its overexpression can lead directly to NF-κB activation (2–4). TRAF1 is unique among TRAF proteins in lacking the N-terminal RING finger required for NF-κB activation (5, 6) and in having only 1 zinc finger. Thus, TRAF1 resembles a dominant-negative form of TRAF2 (3).
There are conflicting data on the role of TRAF1 in signaling downstream of TNFRs. TRAF1, as well as TRAF2, cellular inhibitor of apoptosis (cIAP) 1, and cIAP2, are all required to suppress TNF-induced apoptosis in NF-κB-deficient cell lines (7). TRAF1-deficient dendritic cells show stimulus-dependent TRAF2 degradation, increased apoptosis, and marked deficiency in NF-κB activation after CD40 stimulation (6), implicating TRAF1 in sustaining TRAF2-dependent signaling through CD40 (6). The overexpression of TRAF1 in many tumors of B cell origin is also consistent with a survival function for TRAF1 (8, 9). In contrast, overexpression of TRAF1 can lead to inhibition of TRAF2-mediated effects downstream of CD40 signaling (10). Additionally, a caspase-induced cleavage product of TRAF1 can interfere with TRAF2-mediated survival signaling (11–13). Thus, TRAF1 may be involved in the promotion as well as feedback inhibition of TRAF2-mediated signaling events.
In T cells, TRAF1 deficiency results in hyperresponsiveness to anti-CD3 and TNF stimulation in vitro, suggesting that TRAF1 is a negative regulator of T cell activation (14). In contrast, constitutive overexpression of a TRAF1 transgene in a T cell antigen receptor (TCR) transgenic model led to reduced antigen-induced cell death (15). However, the effect of TRAF1 deficiency on antigen-specific T cell responses in vivo has not yet been analyzed. Therefore, we examined the CD8 T cell response to influenza virus in wild-type (WT) and TRAF1−/− mice. In the absence of TRAF1, fewer antigen-specific T cells were recovered throughout the primary and secondary response to influenza virus. More strikingly, the absence of TRAF1 in T cells had a profound effect on the survival of memory T cells in the absence of antigen in vivo.
Results
Decreased Antigen-Specific T Cell Responses in TRAF1−/− Mice After Influenza Virus Infection.
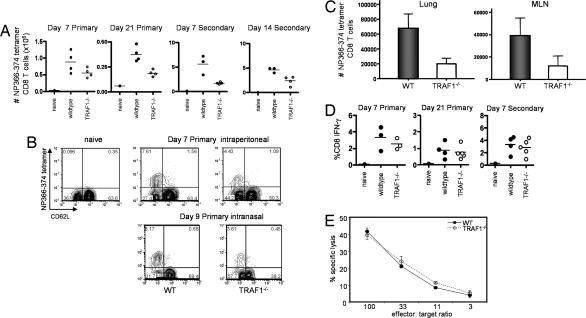
WT and TRAF1−/− mice were immunized i.p. with influenza A virus and T cells were enumerated by using Db/NP366-374 tetramers. TRAF1−/− mice elicited only 40–60% the frequency and total number of Db/NP366-374 tetramer-positive CD8 T cells in the spleen compared with WT mice throughout the primary and secondary response (Fig. 1A and B). After intranasal (i.n.) infection and secondary i.n. challenge with influenza virus at day 42 after priming, there was also a substantial decrease in antigen-specific CD8 T cell numbers in the lung and draining lymph nodes of TRAF1-deficient compared with TRAF1-sufficient mice (Fig. 1C). Thus, TRAF1 influences the number of antigen-specific CD8 T cells recovered after influenza virus infection during primary and memory T cell responses, regardless of the route of infection.
Fig. 1.
Effect of TRAF1 on the CD8 T cell response to influenza virus in mice. WT and TRAF1−/− mice were infected with influenza A/HK-X31. At day 21 after priming, some mice were challenged with influenza PR8, to assess the secondary response. (A) Total numbers of CD8 NP366-374-specific T cells per spleen. Differences between WT and TRAF1−/− groups were statistically significant, P < 0.05 at each time point. Results shown are from a single kinetic experiment with three or four mice per time point per group and are representative of three similar experiments. (B) Representative FACS plots for the data shown in A, gated on live CD8 cells. Numbers in each quadrant represent percentage of total CD8 T cell counts. (Lower) Day 9 (peak of primary response), after i.n. infection (representative of three mice). (C) WT or TRAF1−/− mice were immunized i.n. with 5 hemagglutinin units of influenza A/HK-X31, and, 42 days later, were challenged with the same dose of influenza A/PR8. Numbers of CD8 tetramer-positive cells were quantitated in the lung and draining mediastinal lymph nodes (MLN) of the mice. Lung lymphocytes and MLNs were pooled from two or three mice per group, and the results are the mean ± SD for three groups of each. (D) Splenocytes were restimulated with NP366-374 peptide and analyzed by intracellular staining for IFN-γ. Similar results were obtained when the data were converted to total number of IFN-γ-producing cells per spleen (data not shown). Plots are representative of at least three experiments per time point with n ≥ 3 per group in each experiment. (E) Antigen-specific cytotoxicity of adherent cell-depleted splenocytes from WT and TRAF1−/− mice was measured directly ex vivo as described in Methods. Data are averages of duplicates and representative of three similar experiments.
Although antigen-specific T cell numbers were decreased in TRAF1−/− mice, after restimulation with peptide there was no decrease in the frequency of CD8 T cells producing IFN-γ in WT versus TRAF1−/− mice during the primary or secondary response (Fig. 1D). Similarly, the direct ex vivo killing functions of WT and TRAF1-deficient T cells were indistinguishable (Fig. 1E).
Role of TRAF1 in CD8 T Cells Versus Host Cells in CD8 T Cell Expansion.
In T cells, TRAF1 can associate with TNFR2, CD30, HVEM, GITR, and 4-1BB and in non-T cells, for example dendritic cells, with CD40 and RANK (2). Adoptive transfer experiments using T cells purified from influenza-primed WT or TRAF1−/− mice transferred into WT or TRAF1-deficient hosts, where the cells were rechallenged, were consistent with both T cell intrinsic and extrinsic effects of TRAF1 (data not shown). To pinpoint the TRAF1 effects to T cells versus host cells, we turned to an adoptive transfer model in which the antigen-specific CD8 T cells selectively lacked or expressed TRAF1.
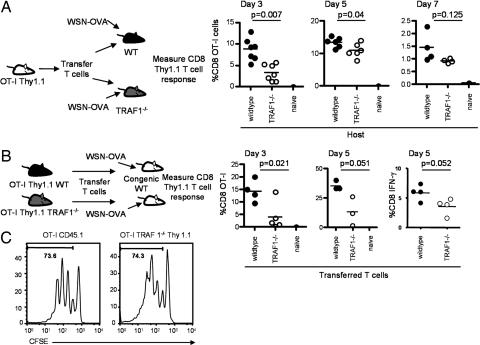
To analyze effects of TRAF1 in host cells, congenically marked OT-I CD8 TCR transgenic T cells were transferred into naive WT or TRAF1−/− recipients and, 24 h later, mice were challenged with a recombinant influenza virus, WSN-OVA, expressing the ovalbumin 257–264 epitope (16). Decreased primary expansion of the WT OT-I T cells was observed in TRAF1−/− recipients compared with the WT recipients, with the largest impact of TRAF1 observed at day 3 after infection (Fig. 2A). By day 7, there was no significant difference in CD8 T cell numbers between WT and TRAF1−/− recipients, suggesting that TRAF1 in the host plays a role in accelerating the initial expansion of antigen-specific CD8 T cells. Furthermore, transfer of WT memory OT-I T cells into WT or TRAF1-deficient hosts showed similar recovery of the memory T cells at 3 weeks after transfer (data not shown).
Fig. 2.
T cell extrinsic and intrinsic effects of TRAF1 on T cell expansion. OT-I T cells were transferred into congenic hosts, and 24 h later, mice were infected with influenza virus WSN-OVA and donor T cell expansion was followed as indicated. (A) Expansion of adoptively transferred OT-I T cells after immunization in WT and TRAF1−/− hosts is reported as percentage of CD8 T cells that are Thy1.1 or CD45.1 positive. Data are representative of two similar experiments. (B) Equal numbers of purified T cells from naive WT or TRAF1−/− OT-I mice were transferred into WT congenic hosts. Plots show the percentage of CD8 Thy1.1+ (donor) T cell expansion. Data are representative of three similar experiments. At day 5, in some experiments, IFN-γ-producing cells were analyzed after peptide restimulation. (C) Purified OT-I and OT-I TRAF1−/− T cells were labeled with CFSE before transfer. Mice were killed 48 h after infection with influenza WSN-OVA. Similar results were obtained in two independent experiments with three and four mice per group, and a representative histogram from one experiment, representing the median from each group in terms of percentage in the divided gate, is shown. Carboxyfluorescein diacetate succinimidyl ester (CFSE) staining is shown after gating on the CD8 live cell population and the CD45.1 or Thy1.1 marker.
To address T cell intrinsic effects of TRAF1 on CD8 T cell responses, purified Thy1.1 naive OT-I WT or OT-I TRAF1−/− T cells were transferred into naive Thy1.2 WT recipients, and 24 h later, mice were challenged with WSN-OVA. Again, the TRAF1-deficient OT-I T cells showed decreased CD8 T cell numbers compared with the TRAF1-sufficient T cells (Fig. 2B) as well as a decrease in the number of IFN-γ-producing cells, after peptide restimulation (Fig. 2B Right, day 5). In a separate experiment, using CFSE-labeled cells, the proportion of cells that had undergone one, two, three, or four divisions in each mouse showed some variability, but there was no consistent difference between the WT and TRAF1−/− OT-I T cells (Fig. 2C). Thus decreased recovery of antigen-specific T cells in TRAF1−/− mice is not explained by a proliferative defect of the TRAF1-deficient cells.
Analysis of Pro- and Antiapoptotic Bcl-2 Family Members in the Presence and Absence of TRAF1.
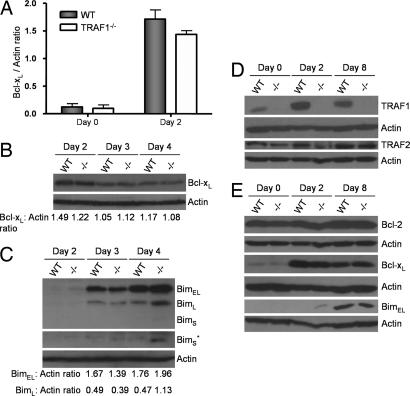
Decreased numbers of antigen-specific cells under conditions of equal division (Fig. 2) could be due to a survival advantage of the TRAF1-containing T cells. The ratio between antiapoptotic mediators Bcl-2 and Bcl-xL to the proapoptotic molecule Bim is critical in determining the survival of activated T cells (17). Bim is a BH3-only Bcl-2 family member that acts upstream of Bax/Bak, controlling their ability to induce apoptosis (18–21). In T cells, Bim is sequestered on the mitochondrial membrane by Bcl-2 and Bcl-xL (22) and antigen-activated T cells are particularly sensitive to the ratio of Bcl-2 and Bcl-xL to Bim (17, 21). Thus we analyzed Bcl-2, Bcl-xL, and Bim levels in TRAF1-deficient and WT OT-I CD8 T cells after activation of purified live populations (>80% annexin V−). The levels of Bcl-xL are greatly increased upon activation in both WT and TRAF1−/− OT-I cells (Fig. 3A, B, and E). Although we consistently observed marginally lower (−15%) levels of Bcl-xL protein in day 2 activated TRAF1-deficient CD8 T cells in comparison with WT T cells (Fig. 3A), this difference was transient and was no longer apparent by day 4 (Fig. 3B).
Fig. 3.
Analysis of pro- and antiapoptotic Bcl-2 family members in antigen-activated WT or TRAF1−/− T cells. (A) Bcl-xL expression levels in purified TRAF1-sufficient (WT) or deficient (TRAF1−/−) CD8 OT-I cells stimulated for 2 days with SIINFEKL (n = 3). (B and C) WT and TRAF1−/− CD8 OT-I cells were stimulated for 2, 3, or 4 days with SIINFEKL and the levels of Bcl-xL (B) and BimEL, BimL, and BimS (C) in purified live CD8 T cells were analyzed by Western blotting. The ratios of Bcl-xL to actin (B) and BimEL/BimL to actin (C) are shown. ∗, Longer exposure for the BimS isoform. (D and E) WT or TRAF1−/− OT-I splenocytes were stimulated with SIINFEKL for 48 h (day 2) and rested for 24 h, and viable cells were cultured with 20 ng/ml recombinant human IL-15 for an additional 5 days (day 8). TRAF1 and TRAF2 (D) and Bcl-2, Bcl-xL, and BimEL (E) expression in purified CD8 OT-I cells at different stages of activation was analyzed by Western blotting. Shown are representative blots of three independent experiments. Bim isoforms: extra long (EL), long (L), and short (S).
By 2 days after activation, low levels of the BimEL isoform appeared in TRAF1-deficient T cells but this isoform was undetectable in WT T cells (Fig. 3 C and E). BimL levels increased substantially between day 3 and 4, and the BimS form appeared only in the TRAF1−/− T cells at this time point (Fig. 3C). Furthermore, BimL levels were 2.4–fold higher by day 4 in TRAF1−/− T cells compared with WT T cells. In contrast, Bcl-2 protein levels were unaltered with activation of either TRAF1-sufficient or -deficient CD8 T cells (Fig. 3E). These data show that TRAF1-sufficient cells maintain lower levels of Bim in activated T cells, consistent with a prosurvival effect of TRAF1.
Generation of TRAF1-Deficient Memory T Cells.
TRAF1-deficient mice challenged with influenza virus at day 42 after priming show a substantial defect in their recall CD8 T cell response (Fig. 1). Thus TRAF1 appears to play a role in the generation or maintenance of CD8 T cell memory. To examine the effects of TRAF1 on long-term CD8 memory T cell survival, we used a modified version (23) of a previously published method to develop memory phenotype T cells in vitro (24). In the WT T cells, TRAF1 levels increased with activation and persisted at an intermediate level in memory T cells (Fig. 3D). After conversion to memory T cells, by using antigen followed by IL-15 treatment, the cultures contained 99% CD8 T cells and the WT and TRAF1-deficient OT-I T cells were indistinguishable with respect to levels of Bcl-2, Bcl-xL, and Bim (Fig. 3E) as well as in their surface phenotype, which resembles that of central memory T cells (CD62Lhi, CD44hi, and CD69lo; data not shown). These experiments demonstrate that memory phenotype T cells can be generated from WT or TRAF1-deficient T cells and that after 5 days in culture with IL-15 the ratios of Bcl-2 and Bcl-xL to Bim are comparable.
Role of TRAF1 in Antigen-Independent Survival of CD8 Memory T Cells in Vivo.
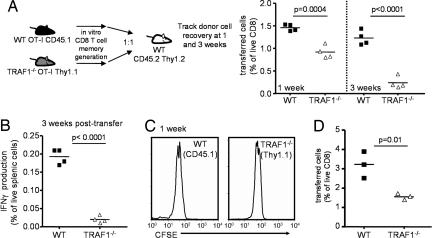
To assess the role of TRAF1 in memory T cell survival independently of the continued presence of antigen, a 1:1 mixture of WT CD45.1 and TRAF1−/− Thy1.1 memory phenotype OT-I T cells was transferred into naive CD45.2/Thy1.2 C57BL/6 mice without further antigenic stimulation (Fig. 4A). At 24 h after transfer, there were approximately equal numbers of the WT and TRAF1−/− OT-I T cells in the mice (data not shown). However, by 1 week after transfer there was a decrease in the recovery of TRAF1-deficient compared with TRAF1-sufficient T cells, and this defect increased from a 2-fold to a 7-fold defect over the next 2 weeks (Fig. 4A). By 3 weeks after transfer, the ratio of WT to TRAF1−/− OT-I T cells within each mouse was 7.29 ± 1.28, 8.5 ± 2.66, and 7.83 ± 2.03 in three independent experiments with three or four mice in each experiment. This decreased cell recovery of TRAF1-deficient T cells was also reflected in the decreased recovery of TRAF1-deficient IFN-γ-producing cells, after restimulation of the splenocytes with peptide (Fig. 4B).
Fig. 4.
Effect of TRAF1 on antigen-independent memory T cell survival in vivo. Memory-like T cells were generated in vitro from WT CD45.1 and TRAF1−/− Thy1.1 OT-I mice (see Fig. 3). A 1:1 mixture of cells (4 × 106 total) was transferred into a congenically distinct WT host (CD45.2 and Thy1.2). (A) Kinetic analysis of WT and TRAF1−/− OT-I T cell recovery at 1 and 3 weeks after transfer. Data are representative of three similar experiments. (B) Splenocytes were restimulated with peptide for 6 h and stained for intracellular cytokine production by the CD45.1 and Thy1.1 (transferred) T cells. (C) Memory-like donor T cells from WT and TRAF1−/− mice were stained with CFSE and transferred into separate WT hosts. CFSE staining was analyzed at 1 week after transfer. Data are representative of two similar experiments. (D) Secondary expansion of memory OT-I T cells after antigenic challenge in vivo. WSN-OVA was injected at 24 h after transfer and cell numbers were monitored 5 days later (representative of two similar experiments).
We used CFSE labeling to monitor cell division at 1 week after transfer, because there was already a significant decrease in the number of TRAF1−/− T cells recovered by this time point. In the absence of antigen, there was little or no cell division of both WT and TRAF1−/− T cells by 1 week after transfer and there was no difference in CFSE distribution between the WT and TRAF1−/− T cells (Fig. 4C). Thus, differences in the number of memory T cells recovered after transfer are not due to differences in the rates of division. Consistent with the ratio of proapoptotic to antiapoptotic protein observed in Fig. 3, these findings imply a role for TRAF1 in the survival of CD8 memory T cells.
We also immunized a subset of the mice with WSN-OVA, 24 h after transfer of memory T cells to determine their capacity for secondary expansion immediately after transfer. There was an ≈2-fold lowering in CD8 T cell numbers at day 5 after secondary antigenic challenge (Fig. 4D). These data show that TRAF1 is required for maximal recovery of CD8 memory T cells in vivo.
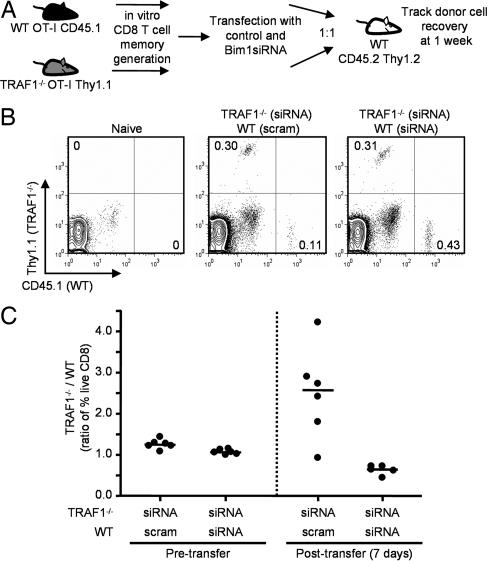
Bim Down-Regulation by siRNA Leads to Increased Memory T Cell Survival in Vivo.
To determine whether increased Bim levels contributed to decreased recovery of TRAF1-deficient T cells in vivo, we used siRNA to knock down Bim in the memory T cells before adoptive transfer. When WT OT-I memory T cells were transfected with a control scrambled duplex RNA and TRAF1-deficient OT-I memory T cells were transfected with a siRNA that targets all three Bim isoforms and the cells were transferred in a 1:1 ratio into a congenic host (CD45.2 Thy1.2) (Fig. 5A), we observed an increased ratio of TRAF1−/− to WT cells 1 week after transfer (Fig. 5 B and C). In contrast, we recovered higher numbers of WT memory T cells when TRAF1-deficient cells did not express the siRNA for Bim (see Fig. 4A). Furthermore, when Bim was knocked down in both WT and TRAF1−/− memory T cells, similar numbers of the two populations were recovered in vivo at 1 week after transfer (Fig. 5 B and C). These findings suggest that the increased levels of Bim protein in TRAF1-deficient T cells contribute to decreased survival of the memory T cells in vivo.
Fig. 5.
Enhanced survival of OT-I TRAF1−/− memory T cells in vivo after Bim down-modulation. (A) Memory-like T cells were generated in vitro from WT CD45.1 and TRAF1−/− Thy1.1 OT-I mice and transfected with either a control scrambled duplex RNA (scram) or a Bim-specific siRNA (siRNA). A 1:1 mixture of cells (4 × 106 total) was transferred into a congenically distinct WT host (CD45.2 and Thy1.2). Recovery of cells was determined at 1 week after transfer. (B) Representative FACS plots of recovered T cells 1 week after transfer following transfection with duplex RNA. (C) The ratios of recovery of live TRAF1−/− to WT transferred OT-I memory T cells are shown 1 week after transfer. The data are representative of two similar experiments with five or six independent transfections and transfers per experiment.
Discussion
The mechanisms regulating the survival of activated and memory T cells have not been fully defined. Here we show that TRAF1 expression in T cells is required for maximal recovery of CD8 T cells both in antigen-specific responses in vivo and in the weeks after adoptive transfer of memory CD8 T cells into a naive host. The effects of TRAF1 on T cell recovery could be due to effects on survival, trafficking, or cell division. We found similar defects in TRAF1-deficient antigen-specific CD8 T cell numbers in the spleen, mediastinal lymph nodes, and lung of influenza virus-infected mice after secondary immunization, arguing against a selective trafficking defect. Because TRAF1-sufficient and deficient T cells showed similar rates of division in vivo, these results imply an effect of TRAF1 on CD8 T cell survival. In support of this possibility, we observed that activated TRAF1-deficient antigen-specific CD8 T cells had increased levels of Bim as well as a trend toward lower Bcl-xL, relative to TRAF1-sufficient T cells. Furthermore, siRNA-mediated Bim down-modulation resulted in increased recovery of TRAF1-deficient memory T cells in vivo. Taken together, these data point to the TRAF1 signaling axis and Bim down-modulation as critical for memory CD8 T cell survival.
In addition to the T cell intrinsic effects of TRAF1 on CD8 T cell numbers, we found that TRAF1 in cells other than the antigen-specific CD8 T cells contributed to initial expansion of CD8 T cells. CD8 T cell extrinsic effects of TRAF1 could be due to TRAF1 effects on RANK or CD40 signaling (25, 26), which in turn can have an impact on CD8 T cells through antigen-presenting cells (27–29). However, this was largely a kinetic effect, as by day 7 after transfer, there was no longer a significant difference in antigen-specific CD8 T cell numbers between WT and TRAF1−/− hosts. Furthermore, transfer of OT-I memory T cells into WT or TRAF1-deficient hosts showed no defect in cell recovery at 3 weeks after transfer (data not shown). TRAF1 is also dispensable for CD40-dependent antibody production, because TRAF1−/− mice exhibited normal influenza-specific antibody responses, including class switch to IgG2a (data not shown), confirming previous reports that TRAF1 does not influence antibody responses (14).
The decreased number of TRAF1-deficient compared with TRAF1-sufficient OT-I T cells recovered was also reflected in the decreased numbers of IFN-γ-producing cells. In contrast, with influenza infection of TRAF1-deficient mice, there was no decrease in antigen-specific effector function, under conditions where the numbers of antigen-specific T cells were decreased. This lack of impairment in the effector function under conditions where T cell numbers are decreased may reflect increased sensitivity of TRAF1-deficient T cells to antigen receptor stimulation in the influenza model. Indeed, a previous study showed that TRAF1-deficient T cells are hyperresponsive to anti-CD3-induced signaling, suggesting a possible negative role for TRAF1 downstream of the TCR (14).
An apparently negative role for TRAF1 in TNFR2- and CD3-mediated signaling pathways (30) might be due to receptor-specific effects of TRAF1. On the other hand, a number of gene-targeted mice, including TRAF2-deficient mice (31), show altered basal levels of T cell response to anti-CD3. This change might be due to altered TCR signaling thresholds in mature T cells that have developed in the absence of particular TRAF molecules. In the present report with both influenza and OVA-specific CD8 T cell responses, it is clear that the net effect of TRAF1 on antigen-specific CD8 T cells is to increase their overall numbers in vivo.
The most dramatic effect of TRAF1 deficiency is on long-term survival of CD8 memory T cells. TRAF1-sufficient CD8 memory T cells showed a 7- to 8-fold survival advantage over TRAF1-deficient T cells when they were allowed to compete for survival signals after transfer into a naive host. Decreased T cell recovery after antigen stimulation correlates with increased Bim expression in activated TRAF1-deficient T cells, consistent with the role of Bim in promoting death of activated T cells by antagonizing the effects of Bcl-2/Bcl-xL (17–19, 21). This was most evident at day 4 of activation, when the TRAF1-deficient T cells show significantly higher levels of the most potent proapoptotic isoforms (BimL and BimS) (18). However, WT and TRAF1-deficient memory T cells had comparable levels of Bcl-XL and Bim at the end of the 5-day culture in IL-15, suggesting that excess IL-15 can compensate for the survival defects in TRAF1-deficient T cells in vitro. However, these defects in survival become apparent in the weeks after transfer into a naive host. Bim down-modulation in the memory T cells with siRNA rescued the OT-I TRAF1-deficient T cells, suggesting a link between the presence of TRAF1 and Bim down-regulation in T cell survival. The link between TRAF1 and Bim protein levels remains unknown. However, in other models, Erk signaling has been shown to down-regulate Bim expression (32–34).
The particular TRAF1-linked receptors involved in memory T cell maintenance in this study have not been identified. IL-15 can induce 4-1BB on CD8 memory T cells, and in the absence of antigen the presence of 4-1BBL in the host results in a 2 to 3-fold survival advantage of the CD8 memory T cells over 3 weeks (23). 4-1BB recruits TRAF1 during T cell activation (31), and recent data from our laboratory show that TRAF1 is required for Bim down-modulation and T cell survival downstream of 4–1BB signaling (G.P., L.P., and T.H.W., unpublished work). These data suggest that after transfer into a naive host, memory T cells may encounter IL-15, up-regulate 4-1BB, and receive TRAF1-dependent survival signals leading to Bim down-regulation. TRAF1-deficient T cells show a larger defect in survival (this report) compared with the 2- to 3-fold defect observed in 4-1BBL-deficient hosts (23), suggesting that more than one TRAF1-binding TNFR family member contributes to survival of the CD8 memory T cells.
In summary, TRAF1 in T cells, as well as in other cells, enhances antigen-specific CD8 T cell numbers in vivo. The presence of TRAF1 in the T cells has minimal effects on cell division but rather appears to influence T cell survival, at least in part through down-regulation of Bim. More dramatic effects of TRAF1 were observed on long-term antigen-independent survival of CD8 memory T cells in vivo, suggesting that TRAF1 and Bim down-modulation are critical mediators of CD8 memory T cell survival.
Methods
Mice.
C57BL/6 WT mice were obtained from Charles River Laboratories (St. Constant, PQ, Canada). CD45.1 and Thy1.1 congenic mice were obtained from Taconic Laboratories (Germantown, NY). TRAF1−/− gene-targeted mice, backcrossed onto the C57BL/6 background (n = 8) have been described (14). OT-I mice (35) were provided by Pamela Ohashi (Ontario Cancer Institute, Toronto, ON, Canada). Mice were crossed to generate OT-I TRAF1−/− Thy1.1, and OT-I WT CD45.1 congenic mice. OT-I transgene expression was assayed by using anti-Vα2 and anti-Vβ5.1 (eBiosciences, San Diego, CA). Mice were maintained under specific pathogen-free conditions in sterile microisolator caging. Animal studies were approved by the University of Toronto animal care committee in accordance with the regulations of the Canadian Council on Animal Care.
Influenza Virus Infection.
Seven- to 10-week-old mice were infected i.p. with 200 hemagglutinin units (HAU) of influenza A/HK-X31 (H3N2) or with 5 HAU i.n. At 3 weeks after infection, some mice were challenged with the influenza A/PR8/34 (H1N1), which shares the immunodominant nucleoprotein (NP) epitope of the HK-X31 strain but differs in the major neutralizing antibody epitopes of the hemagglutinin (H) and neuraminidase (N) proteins, thereby minimizing viral neutralization that would limit secondary CD8 T cell responses. Lungs were perfused with 5 ml of PBS, and lymphocytes from pooled lung samples (n = 2) were enriched by isolation over an 80/40% Percoll gradient. For immunization after adoptive transfer of OT-I cells, mice were infected with influenza A/WSN/33 (WSN)-OVA expressing the immunodominant Kb restricted ovalbumin 257–264 epitope, SIINFEKL, incorporated into the viral neuraminidase stalk (16) provided by Peter Doherty (St. Jude Children's Research Hospital, Memphis, TN).
Flow Cytometry.
MHC class I tetramers (36) were obtained from the National Institute for Allergy and Infectious Diseases tetramer facility (Emory University, Atlanta, GA). Cells were surface-stained with anti-mouse CD8α-PE, anti-mouse CD62L-FITC, and Db/NP366–374-APC-labeled tetramers. Adoptively transferred T cells were detected by using anti-Thy1.1 or anti-CD45.1. For intracellular IFN-γ staining, splenocytes were restimulated for 6 h at 37°C with 1 μM NP366–374 peptide (with Golgi stop, BD Biosciences, Mountain View, CA) followed by surface-staining with anti-CD8 and anti-CD62L, fixation, and staining with anti-mouse IFN-γ (BD PharMingen, San Diego, CA). Samples were analyzed by using a FACSCalibur and Cell Quest (BD Biosciences) or FloJo software (TreeStar, San Carlos, CA). CFSE staining was done at a T cell concentration of 5 × 107 per ml in prewarmed PBS with 2–2.5 μM CFSE for 15 min at 37°C.
Analysis of Cytotoxicity.
T cell cytotoxicity was analyzed immediately ex vivo at day 7 after infection. Cells were plated for 1 h at 37°C, and the nonadherent cells were counted and serially diluted preparations were analyzed for cytotoxic activity against 51Cr-labeled EL4 cells that had been prepulsed with 50 μM NP366–374 peptide during the chromium labeling. After 5 h, 70 μl of supernatant was harvested onto 96-well harvest plates (Canberra Packard, Mississauga, ON, Canada) and counted on a Topcount scintillation counter (Canberra Packard). The percentage of specific lysis was determined as described in ref. 37.
T cells, Memory T Cells, and Adoptive Transfers.
T cells were purified from the spleen and lymph nodes of mice by negative selection using T Cell Immunocolumns (Cedarlane, Hornby, ON, Canada). Purified OT-I transgenic WT or TRAF1−/− T cells (106) were transferred. Twenty-four hours later recipients were challenged with WSN-OVA and donor T cell expansion was analyzed.
Memory OT-I T cells were generated by stimulation with SIINFEKL peptide followed by culture in IL-15 (20 ng/ml) as described in ref. 23. Total culture time was 8–9 days and cells were analyzed for various activation and memory phenotype surface markers at days 0, 2, and 8 of culture. At the end of culture, >95% of all viable cells were CD8+, Vα2+, Vβ5+, and CD45.1+. WT and TRAF1−/− OT-I cells were mixed at a 1:1 ratio, confirmed by FACS analysis. A total of 4 × 106 cells were adoptively transferred by tail vein injection into CD45.2, Thy1.2 WT hosts.
Western Blot Analysis.
Cell lysates of OT-I CD8 T cells (>85%), purified by negative selection using a mouse CD8 T cell enrichment kit (StemCell Technologies, Vancouver, BC, Canada), were prepared from naive or 2-day-activated OT-I cultures, or at day 8, after IL-15 stimulation as described above for memory T cell generation. Cells were washed in cold PBS and lysed in Tris-buffered saline containing 1 mM EDTA, 0.2% Triton X-100, 0.1% SDS, and the complete protease inhibitors mixture (Roche, Laval, QC, Canada). Thirty micrograms of proteins was subjected to SDS/PAGE and then transferred to poly(vinylidene difluoride) (PVDF) membranes (Pall Life Sciences, Mississauga, ON, Canada). Membranes were probed with antibodies specific for TRAF1 (Santa Cruz Biotechnology, Santa Cruz, CA), TRAF2 (Cell Signaling Technology, Pickering, ON, Canada), Bcl-2 (Santa Cruz Biotechnology), Bcl-xL (BD Biosciences), Bim (Cell Signaling Technology), or β-actin (Sigma, St. Louis, MO) and incubated with horseradish peroxidase-conjugated anti–rabbit Ig antibody (Sigma). Signals were revealed by chemiluminescence (Amersham Pharmacia, Baie d'Urfé, QC, Canada) and visualized by autoradiography.
Knockdown of Bim by siRNA Transfection.
Day 7 OT-I memory T cells (3 × 106 cells) were transfected, using the mouse T cell Nucleofector kit (Amaxa, Gaithersburg, MD), with 100 μM either the control scrambled duplex RNA or an siRNA that targets all three isoforms of Bim (Integrated DNA Technologies, Coralville, IA). Cells were kept in culture overnight before i.v. transfer into a naive host.
Statistical Analysis.
Where indicated, P values were obtained by using the Student t test (unpaired, two-tailed, 95% confidence interval).
Acknowledgments
We thank Jennifer Gommerman for critical reading of the manuscript. We gratefully acknowledge the National Institutes of Health tetramer facility for providing MHC I/peptide tetramers. This work was supported by a grant from the National Cancer Institute of Canada with funds from the Canadian Cancer Society (to T.H.W.) and by National Institutes of Health Grant CA095127 (to E.N.T.). L.S. is the recipient of a postdoctoral fellowship from the Fonds de la Recherche en Santé du Québec.
Abbreviations
- Bim
Bcl-2-interacting mediator of cell death
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- i.n.
intranasal
- TCR
T cell antigen receptor
- TNFR
TNF receptor
- TRAF
TNFR-associated factor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Watts TH. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal BB. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 3.Rothe M, Sarma V, Dixit VM, Goeddel DV. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 4.Dempsey PW, Doyle SE, He JQ, Cheng G. Cytokine Growth Factor Rev. 2003;14:193–209. doi: 10.1016/s1359-6101(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 5.Hostager BS, Catlett IM, Bishop GA. J Biol Chem. 2000;275:15392–15398. doi: 10.1074/jbc.M909520199. [DOI] [PubMed] [Google Scholar]
- 6.Arron JR, Pewzner-Jung Y, Walsh MC, Kobayashi T, Choi Y. J Exp Med. 2002;196:923–934. doi: 10.1084/jem.20020774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 8.Zapata JM, Krajewska M, Krajewski S, Kitada S, Welsh K, Monks A, McCloskey N, Gordon J, Kipps TJ, Gascoyne RD, et al. J Immunol. 2000;165:5084–5096. doi: 10.4049/jimmunol.165.9.5084. [DOI] [PubMed] [Google Scholar]
- 9.Munzert G, Kirchner D, Stobbe H, Bergmann L, Schmid RM, Dohner H, Heimpel H. Blood. 2002;100:3749–3756. doi: 10.1182/blood.V100.10.3749. [DOI] [PubMed] [Google Scholar]
- 10.Fotin-Mleczek M, Henkler F, Hausser A, Glauner H, Samel D, Graness A, Scheurich P, Mauri D, Wajant H. J Biol Chem. 2004;279:677–685. doi: 10.1074/jbc.M310969200. [DOI] [PubMed] [Google Scholar]
- 11.Leo E, Deveraux QL, Buchholtz C, Welsh K, Matsuzawa S, Stennicke HR, Salvesen GS, Reed JC. J Biol Chem. 2001;276:8087–8093. doi: 10.1074/jbc.M009450200. [DOI] [PubMed] [Google Scholar]
- 12.Jang HD, Chung YM, Baik JH, Choi YG, Park IS, Jung YK, Lee SY. Biochem Biophys Res Commun. 2001;281:499–505. doi: 10.1006/bbrc.2001.4369. [DOI] [PubMed] [Google Scholar]
- 13.Henkler F, Baumann B, Fotin-Mleczek M, Weingartner M, Schwenzer R, Peters N, Graness A, Wirth T, Scheurich P, Schmid JA, Wajant H. J Biol Chem. 2003;278:29216–29230. doi: 10.1074/jbc.M211090200. [DOI] [PubMed] [Google Scholar]
- 14.Tsitsikov EN, Laouini D, Dunn IF, Sannikova TY, Davidson L, Alt FW, Geha RS. Immunity. 2001;15:647–657. doi: 10.1016/s1074-7613(01)00207-2. [DOI] [PubMed] [Google Scholar]
- 15.Speiser DE, Lee SY, Wong B, Arron J, Santana A, Kong YY, Ohashi PS, Choi Y. J Exp Med. 1997;185:1777–1783. doi: 10.1084/jem.185.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Topham DJ, Castrucci MR, Wingo FS, Belz GT, Doherty PC. J Immunol. 2001;167:6983–6990. doi: 10.4049/jimmunol.167.12.6983. [DOI] [PubMed] [Google Scholar]
- 17.Marrack P, Kappler J. Annu Rev Immunol. 2004;22:765–787. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S, Huang DC. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 20.Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 21.Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, Marrack P. Immunity. 2002;16:759–767. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Y, Swanson BJ, Wang M, Hildeman DA, Schaefer BC, Liu X, Suzuki H, Mihara K, Kappler J, Marrack P. Proc Natl Acad Sci USA. 2004;101:7681–7686. doi: 10.1073/pnas.0402293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pulle G, Vidric M, Watts TH. J Immunol. 2006;176:2739–2748. doi: 10.4049/jimmunol.176.5.2739. [DOI] [PubMed] [Google Scholar]
- 24.Manjunath N, Shankar P, Wan J, Weninger W, Crowley MA, Hieshima K, Springer TA, Fan X, Shen H, Lieberman J, von Andrian UH. J Clin Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong BR, Josien R, Lee SY, Vologodskaia M, Steinman RM, Choi Y. J Biol Chem. 1998;273:28355–28359. doi: 10.1074/jbc.273.43.28355. [DOI] [PubMed] [Google Scholar]
- 26.Pullen SS, Miller HG, Everdeen DS, Dang TT, Crute JJ, Kehry MR. Biochemistry. 1998;37:11836–11845. doi: 10.1021/bi981067q. [DOI] [PubMed] [Google Scholar]
- 27.Borrow P, Tishon A, Lee S, Xu J, Grewal IS, Oldstone MB, Flavell RA. J Exp Med. 1996;183:2129–2142. doi: 10.1084/jem.183.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee BO, Hartson L, Randall TD. J Exp Med. 2003;198:1759–1764. doi: 10.1084/jem.20031440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Josien R, Li HL, Ingulli E, Sarma S, Wong BR, Vologodskaia M, Steinman RM, Choi Y. J Exp Med. 2000;191:495–502. doi: 10.1084/jem.191.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsitsikov EN, Laouini D, Dunn IF, Sannikova TY, Davidson L, Alt FW, Geha RS. Immunity. 2001;15:647–657. doi: 10.1016/s1074-7613(01)00207-2. [DOI] [PubMed] [Google Scholar]
- 31.Saoulli K, Lee SY, Cannons JL, Yeh WC, Santana A, Goldstein MD, Bangia N, DeBenedette MA, Mak TW, Choi Y, Watts TH. J Exp Med. 1998;187:1849–1862. doi: 10.1084/jem.187.11.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weston CR, Balmanno K, Chalmers C, Hadfield K, Molton SA, Ley R, Wagner EF, Cook SJ. Oncogene. 2003;22:1281–1293. doi: 10.1038/sj.onc.1206261. [DOI] [PubMed] [Google Scholar]
- 33.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. J Biol Chem. 2003;278:18811–18816. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 34.Craxton A, Draves KE, Gruppi A, Clark EA. J Exp Med. 2005;202:1363–1374. doi: 10.1084/jem.20051283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 36.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 37.Bertram EM, Lau P, Watts TH. J Immunol. 2002;168:3777–3785. doi: 10.4049/jimmunol.168.8.3777. [DOI] [PubMed] [Google Scholar]