Abstract
This review presents an imaging-centred approach to the diagnostic challenge of uncommon lung tumours in adults. Emphasis is placed on features that may be used to differentiate these tumours including fat content, tumour site, multifocality, calcification and predominant pattern of involvement.
Keywords: Lung neoplasms, bronchial neoplasms, tracheal neoplasms, computed tomography
Introduction
Squamous cell carcinoma, small cell carcinoma, adenocarcinoma and large cell carcinoma, collectively termed primary lung carcinoma, account for over 90% of all primary lung tumours . Other lung tumours vary in incidence from uncommon to exceptionally rare and may have epithelial, tracheobronchial gland, neuroendocrine, lymphopoietic, haematopoietic, mesenchymal or uncertain cell origin . With notable exceptions, the imaging appearances of these tumours are non-specific since most tumours demonstrate considerable overlap with their rare counterparts as well as with the more common primary lung carcinomas. Consequently the diagnosis of unusual lung tumour is usually made retrospectively from a biopsy or pathological specimen. Nevertheless, the presence of certain clinical and radiological features may alert the clinician and interpreting radiologist to the possibility of an uncommon lung tumour.
Fat-containing neoplasms
Although uncommon, the presence of fat within a pulmonary lesion is probably the single most important feature that can help determine cell type, since it effectively excludes the majority of lung tumours including primary lung carcinoma (Table 1).
Table 1.
Typical features of several uncommon lung tumours
| Hamar- | Carci- | Tracheo- | Lipoma | Papillo- | Solitary | Primary | Sclerosing | Kaposi’s | Leiomyoma/ | Angio- | Epithelioid | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| toma | noid | bronchial | matosis | papilloma | pulmonary | haeman- | sarcoma | myosarcoma, | sarcoma | haemangio- | ||
| gland | lymphoma | gioma | spindle cell | endothelioma | ||||||||
| carcinoma | sarcomas | |||||||||||
| Size (cm) | 4 | 1–3 | v | 1–7 | 1–2 | 0.5–1.2 | 2–8 | 1–5 | v | 1–24 | 1–2 | 0.3–3 |
| Nodule/mass | +++ | +++ | +++ | +++ | +++ | +++ | ++ | +++ | ++ | +++ | +++ | +++ |
| Air space | ||||||||||||
| involvement | — | — | — | — | — | — | ++ | — | + | — | — | + |
| Interstitial | ||||||||||||
| involvement | — | — | — | — | — | — | ++ | — | + | — | — | — |
| Solitary | ++ | +++ | +++ | +++ | — | +++ | ++ | +++ | +++ | ++ | — | |
| Multiple | (+) | — | — | — | +++ | — | ++ | — | ++ | + | + | +++ |
| + | ||||||||||||
| Tracheal | (+) | — | (+) | ++ | (+) | (+) | — | (+) | + | + | — | |
| Bronchial | (+) | ++ | — | +++ | ++ | +++ | (+) | — | (+) | + | ++ | ++ |
| Parenchymal | +++ | + | ++ | (+) | + | — | +++ | +++ | ++ | +++ | +++ | ++ |
| + | ||||||||||||
| Fat | ++ | — | — | ++ | — | — | — | — | — | — | — | — |
| Calcification | ++ | — | + | — | — | — | ++ | — | + | + | + | |
— = not a feature; (+) = exceptionally documented; + = uncommon; ++ = common; +++ = typical; v = variable.
Hamartomas are by far the commonest cause of a fat-containing lesion within the lungs. They account for approximately three-quarters of all benign lung neoplasms and have an overall incidence between 0.25% and 5.7% . The term pulmonary ‘hamartoma’ is nosologically incorrect since this lesion is probably a benign neoplasm, derived from bronchial wall mesenchyme . Over 90% of hamartomas have a peripheral location and are asymptomatic, typically presenting in the seventh decade as a solitary nodule or mass with a well-defined smooth or slightly lobulated margin . These are very slow-growing tumours with a mean size of 4 cm, although rapid growth and tumours of 10 cm diameter have been documented . Hamartomas have an endobronchial location in 1.5–8% of cases, appearing as a pedunculated polyploid mass attached to the bronchial mucosa, and only rarely are they continuous with the bronchial cartilage . Although hamartomas contain fibrous tissue, adipose and cartilage, the proportions of these constituents is variable. Calcification is identified on chest radiograph in less than 10% and may have irregular curvilinear, popcorn or stippled configurations . Popcorn calcification is uncommon although virtually diagnostic (Fig. 1(a)). Similarly, the demonstration of fat within hamartomas on radiographs, although almost diagnostic, is very rare. On high-resolution CT, fat attenuation is detectable in 34% of hamartomas, and fat and calcium in 19% . The presence of both fat and calcification within an intrapulmonary lesion is virtually diagnostic of hamartoma, especially in lesions less than 2.5 cm in diameter . While only 10% of lesions smaller than 2 cm contain calcification, this increases to 75% in lesions larger than 5 cm . Endobronchial hamartoma contains a greater proportion of fat than its peripheral counterpart . Since hamartomas are benign, they do not require removal unless symptomatic; consequently a correct radiological diagnosis may obviate biopsy or surgical resection. Using criteria for a combination of fat and calcification on thin section unenhanced CT, a definitive radiological diagnosis of hamartoma can be made in 60% of cases . It should be noted that thin CT sections are advocated to avoid false positives and negatives for fat arising as a result of partial volume averaging (Fig. 1(b)). In the remaining third of lesions in which no fat or calcium is demonstrable, termed indeterminate lesions, the differential diagnosis is extensive and includes bronchogenic carcinoma as well as carcinoid for central lesions. Under these circumstances histological or cytological diagnosis is usually required. The enhancement characteristics of hamartomas on CT are variable, but typically enhance heterogeneously, either peripherally or within septa traversing the lesion. However, homogenous enhancement or non-enhancement may be seen . Air within a hamartoma is exceptionally rare, and may be caused by an air bronchogram or cavitation . Multiple pulmonary hamartomas (chondromas) are rare, and are associated with gastric leiomyosarcoma and functioning extra-adrenal paraganglioma in the Carney triad .
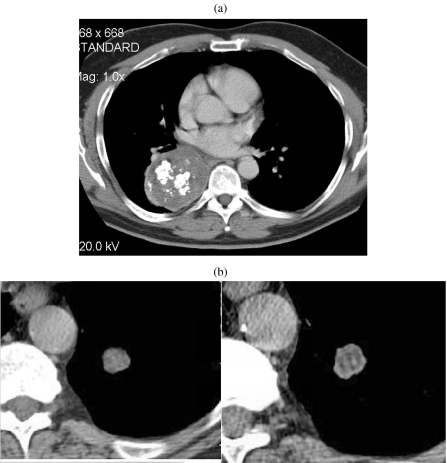
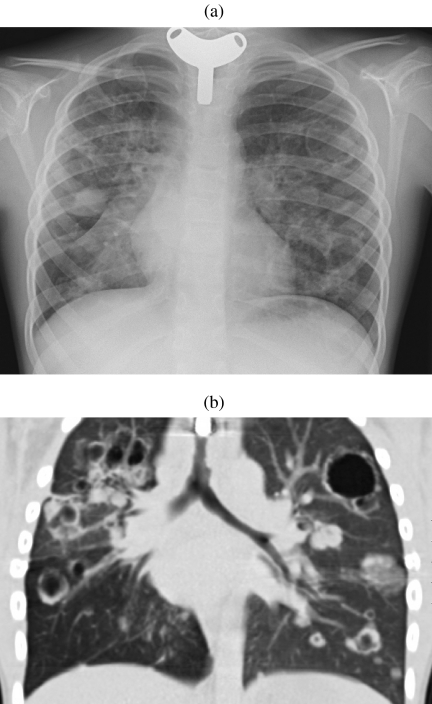
Figure 1.
Hamartoma. (a) Unusually large hamartoma with large chunks of ‘popcorn’ calcification. Initial diagnosis was made by biopsy and the mass was surgically excised. (b) A solitary nodule imaged on 5 mm (left) and 1.25 mm (right) slice thicknesses. The thinner section clearly demonstrates a significant amount of fat in this lesion indicating a hamartoma as the diagnosis. This could not be asserted with confidence on the 5 mm slice. The diagnosis was confirmed at surgery.
Pulmonary lipomas, pulmonary liposarcomas and exogenous lipoid pneumonia are much rarer causes of a fat-containing lung lesion. Pulmonary lipomas comprise 0.1% of all pulmonary tumours and, although morphologically distinct lipomas do occur, many lesions previously described as lipomas are probably hamartoma variants showing predominant adipose tissue expression . Unlike hamartomas, lipomas have a predilection for the airways in lobar or subsegmental location, appearing as a nodule or polypoid mass and may have areas of calcification . A dumb-bell-shaped lesion is also described, in which the tumour bridges the bronchial cartilage into the surrounding interstitium . Peripheral intrapulmonary lipomas have been documented but are exceptionally rare .
Pulmonary liposarcoma is one of the rarest forms of pulmonary sarcoma, with approximately a dozen documented cases . This malignancy has several histological subtypes, most of which contain no demonstrable fat on imaging. Consequently, on CT, most tumours have a non-specific appearance of a large well-defined soft tissue mass. Nevertheless, the lipoblastic subtype may have demonstrable areas of fat attenuation .
Exogenous lipoid pneumonia should be mentioned since it may appear as a focal area of consolidation with negative attenuation, and thus mimic a fat-containing neoplasm on imaging, due to lipid accumulation as a result of chronic aspiration of vegetable, mineral or animal oils . This uncommon condition occurs in people with neuromuscular disorders or anatomical abnormalities, as well as those regularly ingesting oils or taking oil-containing nasal drops . Although it is frequently asymptomatic, the CT appearances can be striking and commonly include multifocal areas of ground glass attenuation, consolidation and interstitial abnormality with a crazy-paving pattern, within which there may be areas of negative attenuation corresponding to phagocytosed lipid within macrophages. Pulmonary fibrosis is also a feature although this occurs to a variable degree .
Non fat-containing neoplasms
Tumour site
Airways
Within the trachea and major airways, the most common ‘unusual’ primary tumours are of salivary gland origin and termed tracheobronchial gland tumours. Adenoid cystic carcinoma and mucoepidermoid carcinoma are the two main forms of tracheobronchial gland tumour, and have a relative incidence of 4:1 . Adenoid cystic carcinoma is the second commonest primary tumour within the trachea after squamous cell carcinoma and so should be considered automatically in differential diagnosis at this location, particularly in younger adults . Less than 10% of primary tracheal neoplasms are of alternative histology including those of connective tissue, vascular, lymphoid, haematopoietic, epithelial and miscellaneous origin.
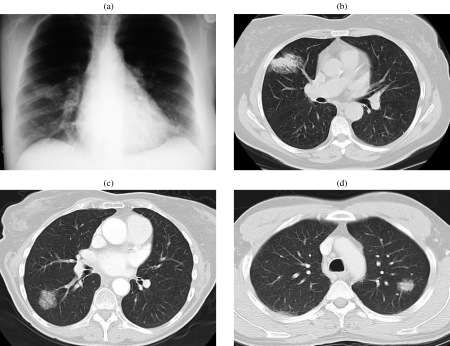
Tracheobronchial gland tumours usually have nonspecific appearances on CT including those of a circumferential or elevated soft tissue mass with either a smooth, lobulated or polypoid margin . Adenoid cystic carcinoma has a predilection for the more proximal airways (i.e. distal trachea to lobar bronchi), as well as a propensity for extratracheal extension (Fig. 2). This is in contrast to mucoepidermoid carcinoma which characteristically affects segmental bronchi and extends within the airways (Fig. 3(a, b)) . Tracheobronchial gland tumours may demonstrate mild enhancement and punctate calcification; the latter may be seen in up to 50% of mucoepidermoid carcinomas . They may present incidentally as a solitary nodule or mass, or commonly secondary to post obstructive collapse and pneumonitis, or they may be overlooked on initial imaging performed for symptoms otherwise attributed to refractory ‘late-onset asthma’. Occasionally, subtle ipsilateral oligaemia secondary to reflex vasoconstriction may be the only clue to a proximal airway tumour. It should be noted that submucosal spread is a feature of tracheobronchial gland tumours, particularly adenoid cystic carcinoma. Under these circumstances, the overlying bronchial epithelium may be deceptively intact on bronchoscopy . It is important to determine the longitudinal extent of tumour involvement since this has significant implications for both operability and planning of resection margins, and this may be achieved using reformatted fine section CT. These tumours may be classified as either low- or high-grade malignancies, although all types have a better overall prognosis than bronchogenic carcinoma .
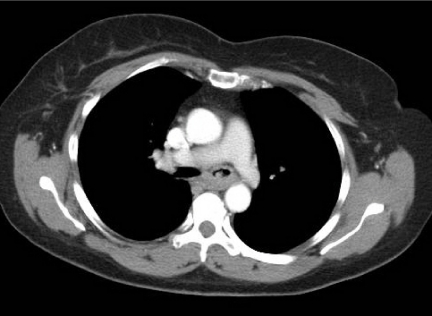
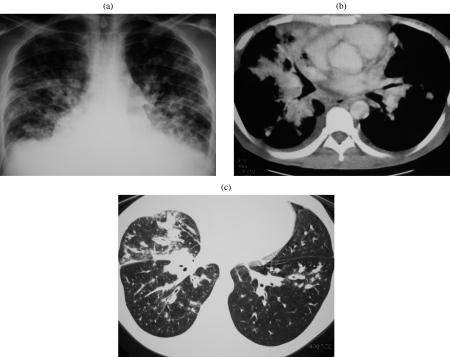
Figure 2.
Adenoid cystic carcinoma. Axial post-contrast CT image of a middle-aged female who presented with haemoptysis. There is abnormal soft tissue invading the left main bronchus and seen extending along the bronchus consistent with the submucosal spread often found in such tumours. Diagnosis was made at bronchoscopic biopsy.
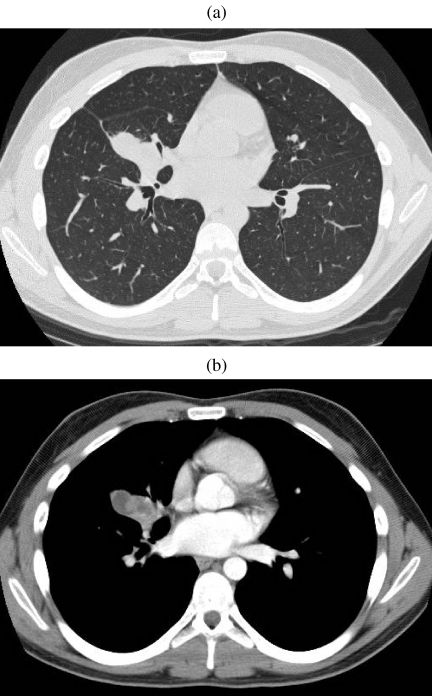
Figure 3.
Mucoepidermoid carcinoma. (a, b) CT on lung and soft tissue windows demonstrating a heterogeneous mass closely associated to the middle lobe bronchus. On bronchscopy, an expansile endobronchial tumour was seen. The diagnosis of mucoepidermoid carcinoma was established by bronchoscopic biopsy.
Endobronchial papillomatosis is a rare epithelial tumour that has a distinctive appearance and clinical presentation. Multiple tracheobronchial papillomas are an uncommon complication of childhood laryngeal papillomatosis and may occur with a latency of up to 10 years, i.e. may affect young adults . Tracheal and endobronchial papillomas are usually multiple, small, nodular and may be confluent in contrast to the majority of other airway neoplasms .
Unlike multiple papillomatosis, a solitary papilloma is seen in middle-aged adults and appears as a discrete polyploid nodule, typically within the lobar or segmental bronchus, and may have a filiform or finely corrugated appearance . Most of these lesions are histologically benign; however, a few cases have been documented which contain or are closely associated with carcinoma in situ and invasive squamous cell carcinoma .
Primary pulmonary carcinoid is a tumour of neuroendocrine origin and has a predilection for the airways . It accounts for 0.5–2.5% of all primary lung tumours, and is subdivided into typical (80%) and atypical (20%) types . It has a mean age of presentation of 45 years, younger than that for conventional bronchogenic carcinoma . Typical carcinoid usually appears as a solitary, well-defined 1–3 cm oval or lobulated soft tissue nodule . In approximately 85% of cases it arises from the airways, typically within the major to subsegmental bronchi, although rarely it has a tracheal origin . In 15% of cases it arises within the lung periphery, in which case it may not be directly related to an airway on gross inspection . Rarely, tumours are irregular or ill-defined . Most endobronchial carcinoids have a large extraluminal component, and for this reason are termed ‘iceberg’ lesions . Consolidation, atelectasis or mucoid impaction distal to an endobronchial carcinoid are common presentations (Fig. 4) . Approximately 30% of lesions demonstrate variable calcification or even ossification on CT , although this is usually not detectable radiographically . Tumour cavitation is not a feature . Local extension of tumour into the mediastinum may occur . Owing to their rich vascularity, carcinoid tumours characteristically, although not invariably, enhance intensely with intravenous contrast on CT and MRI . Atypical carcinoid represents a relatively high-grade, aggressive form of tumour that has histological features in common with small cell carcinoma of the lung . Atypical carcinoid is larger than typical carcinoid at presentation (mean size 3.9 cm) and is more commonly associated with adenopathy (40%) . This tumour has a higher incidence of malignant degeneration and local recurrence following resection . Pulmonary carcinoid tumours are seldom hormonally active, although they are the commonest cause of ectopic ACTH-dependent Cushing’s syndrome . Imaging has an important role in the detection of these lesions since biochemical and clinical distinction between different sources of ACTH may not be possible . Most hormonally active pulmonary carcinoids are small but detectable with meticulous technique using fine collimation CT . In addition, carcinoid tumours have a high expression of somatostatin receptors, which may be used in radionuclide imaging to detect hormonally active but otherwise radiographically occult tumours .
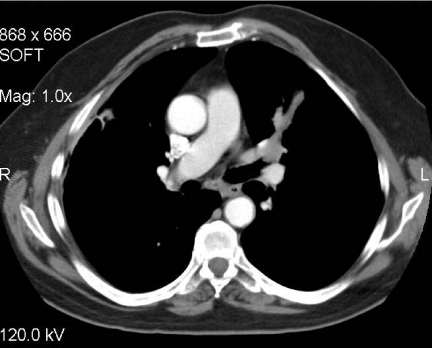
Figure 4.
Carcinoid tumour. Sited at the bifurcation of the lingular bronchus there is an endobronchial lesion that clearly occupies the lumen of the bronchus causing a mucocele distant to it. Biopsy confirmed the diagnosis of carcinoid.
Peripheral lesions
There is a wide differential diagnosis for non fat-containing nodules or masses located within the lung parenchyma, which are subsequently divided into predominant pattern of involvement.
Multiple nodules or masses.
Lung parenchymal involvement is an uncommon but severe manifestation of laryngotracheal papillomatosis, occurring in less than 1% of cases . It can occur with a latency of 10 years following onset of laryngeal disease, therefore the presentation is most common in older children and young adolescents . On CT, multiple small nodules are seen predominantly within the dependent (i.e. infero-posterior) portion of the lungs. These nodules may enlarge to several centimetres at which point they frequently cavitate and develop fluid levels (Fig. 5(a, b)) . The diagnosis of this condition is not usually in doubt as individuals may have concomitant or previously documented laryngeal disease . Pulmonary papillomatosis often carries a very poor prognosis due to extensive parenchymal infiltration and repeated infections. Furthermore, there is an increased risk of subsequent development of squamous cell carcinoma, particularly in patients with a smoking history or previous radiation exposure .
Figure 5.
Pulmonary papillomatosis. (a) Chest radiograph in a young adult with a previous tracheostomy for extensive laryngeal and pulmonary papillomatosis. Numerous nodules and thin-walled cysts are demonstrated bilaterally. (b) Coronally reconstructed CT of the same patient demonstrating tracheal plaques as well as numerous pulmonary nodules and cavitating masses. (Courtesy of Dr Catherine Owens, Great Ormond Street Hospital.)
Unlike other forms of primary pulmonary sarcoma, sarcomas of vascular origin may appear as multiple small nodules, usually between 1 and 2 cm in diameter . Primary pulmonary angiosarcoma is exceptionally rare and poorly documented with several previously described cases being subsequently attributed to metastatic angiosarcoma . This tumour may mimic pulmonary embolism clinically, i.e. presenting with haemoptysis, as well as radiologically, owing to the tumour originating within a pulmonary artery, although contrast-enhanced MR and CT may be used to differentiate these pathologies . Epithelioid haemangioendothelioma is a relatively indolent malignancy that usually presents incidentally in young adult females. Multiple nodules up to 2 cm are seen within the lung parenchyma that demonstrate either no growth or slow growth on serial imaging . These lesions rarely demonstrate calcification on CT, although hilar adenopathy and pleural effusions are seen in approximately 10% .
Pulmonary involvement with Kaposi’s sarcoma occurs in up to 35% of patients with AIDS and may involve the lung, pleura and tracheobronchial tree . In AIDS patients, the disease usually affects a younger age group than Kaposi’s sarcoma affecting otherwise immunocompetent, elderly adults. Furthermore, in AIDS patients, the pulmonary lesions represent one of many systemic manifestations of an aggressive disseminated malignancy . Nevertheless, the appearances of pulmonary Kaposi’s sarcoma are identical in AIDS and non-AIDS patients . Radiologically, this malignancy is characterised by peribronchovascular predominance of lesions which appear as multiple irregular, ill-defined nodular or linear opacities (Fig. 6(a–c)) . Areas of associated ground glass attenuation may also be seen, reflecting perinodular, intra-alveolar haemorrhage, although it should be noted that this feature is nonspecific and other causes for this appearance, including atypical infections and AIDS-related lymphoma, should be excluded . Other associated findings include interstitial thickening, endobronchial plaques, tumoral masses, fissural nodularity, adenopathy, pleural and pericardial effusions . Cavitation and calcification are not features of this condition .
Figure 6.
Kaposi’s sarcoma. (a, b) Chest radiograph and CT in a patient with AIDS demonstrating large pulmonary infiltrates with a predominant perihilar distribution. This was confirmed to be Kaposi’s sarcoma on previous transbronchial biopsy. (c) CT in another AIDS patient with less severe involvement illustrating the bronchovascular distribution of Kaposi’s sarcoma. (Courtesy of Prof Peter Armstrong, Barts and the London NHS Trust.)
Primary pulmonary lymphoma is rare, comprising less than 1% of primary pulmonary malignancies and only 3% of extranodal lymphomas . Although there are various defining criteria, one generally accepted definition is of a clonal lymphoid proliferation affecting the lung, with no other site of involvement for at least 3 months after diagnosis . The imaging appearances of lymphoma are protean and there is considerable overlap between phenotypes, although each type may tend to produce a typical radiological appearance. For instance, angiocentric lymphoma, a very rare phenotype that represents the malignant grade of tumour referred to as an angiocentric immunoproliferative lesion, typically appears as multiple bilateral small pulmonary nodular opacities that are more numerous in the lung periphery and lower zones . These opacities frequently enlarge to form large confluent masses. In addition, these masses can cavitate and occasionally result in pneumothorax. Air bronchograms are not a feature of this condition . Multiple peripheral parenchymal nodules and masses, with or without air bronchograms or cavitation, are also a typical feature of AIDS-related primary lymphoma of the lung . Other forms of lymphoma are described subsequently with respect to their typical imaging appearances.
Solitary nodules or masses.
Almost any cell type can present as a parenchymally based mass. Hamartomas and carcinoid tumours have been discussed previously. Of the remaining unusual tumours, primary connective tissue tumours are very rare and the majority of these are related to the chest wall although rarely they may arise within the lung . On imaging they typically appear as a nonspecific, solitary, well-circumscribed soft-tissue mass. Examples of tumours within this category include leiomyoma and sarcoma, rhabdomyosarcoma, pulmonary blastoma, glomus tumour, haemangiopericytoma, chondroma, chondrosarcoma, osteosarcoma, neural tumours, lipoma, liposarcoma, tumours of fibrous tissue including malignant fibrous histiocytoma and fibrosarcoma . These mesenchymal neoplasms range markedly in size from 0.6 to 25 cm and, not infrequently, demonstrate areas of necrosis, calcification or cavitation . It is important to note that a parenchymal tumour demonstrating sarcomatous features on needle aspiration or biopsy is more likely to be due to sarcomatoid degeneration within a carcinoma or a metastasis from an extrapulmonary sarcoma than to primary sarcoma . Furthermore, with the notable exception of hamartomas, determining whether a parenchymally based mesenchymal tumour is benign or malignant is usually not possible on imaging .
Tumours of smooth muscle origin, either leiomyoma or leiomyosarcoma, are among the most common primary mesenchymal lung tumours, and the lung parenchyma is the commonest location of this cell type . These tumours show considerable variation in aggressiveness although necrosis and cavitation are more typically associated with leiomyosarcoma . Primary pulmonary leiomyosarcomas are frequently asymptomatic and at presentation may appear as a large, heterogeneously enhancing well-circumscribed soft-tissue mass. Less commonly, leiomyosarcoma has an endobronchial, mediastinal, cardiac or pulmonary vascular origin .
Sclerosing haemangioma is a rare benign tumour of uncertain histogenesis, although current evidence suggests an epithelial origin . It predominantly affects young adult women and typically appears as a well-defined, round, intraparenchymal soft-tissue mass in a juxtapleural location . Other common features include calcification and intratumoral haemorrhage; consequently, this may result in areas of fluid attenuation and inhomogeneous enhancement . On CT, an uninterrupted ring of air surrounding the tumour is an uncommon but characteristic feature, and has been attributed to several mechanisms (Fig. 7) . More recently recognised CT features of this neoplasm include a prominent ‘feeding’ pulmonary artery as well as a tumour ‘tail-like’ projection .
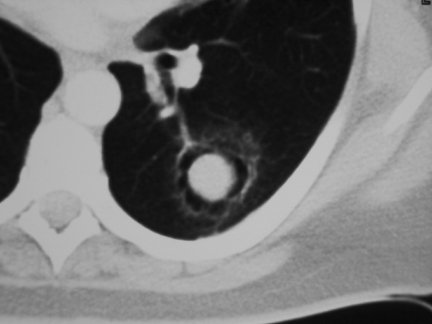
Figure 7.
Sclerosing haemangioma. These are the classical appearances of what is a rare lesion. Note the soft-tissue mass surrounded by an apparent air space giving the appearance of a mycetoma but without the cavity wall. Note also the tail-like projection.
Low-grade B cell lymphoma is the commonest phenotype of primary pulmonary lymphoma and is derived from mucosal associated lymphoid tissue . The typical radiological appearance of this neoplasm is of a solitary nodular opacity or mass ranging in size between 2 and 8 cm (Fig. 8(a, b)) . Although cavitation and air bronchograms may be present, calcification is not a feature .
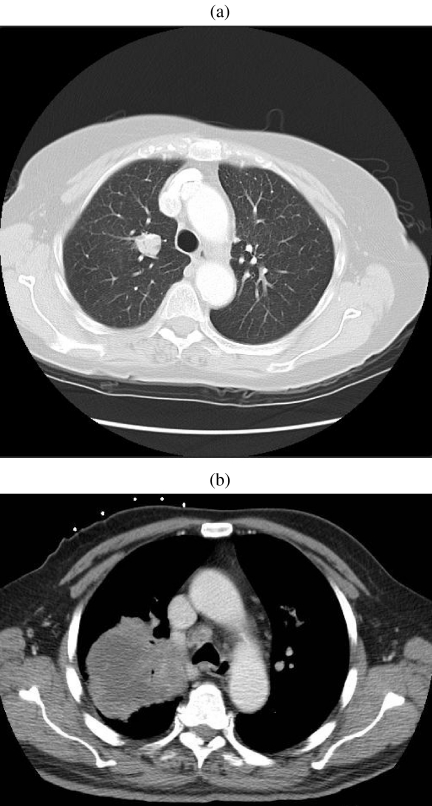
Figure 8.
Primary lymphoma. (a) A solitary 2 cm nodule diagnosed as primary lymphoma following surgical resection. (b) An 8 cm centrally necrotic mass eventually diagnosed as primary lymphoma after repeated biopsies.
Air space opacification.
Primary involvement of distal airspaces by tumour is a relatively infrequent but important cause of peripheral consolidation or ground glass appearance on CT. Bronchio-alveolar cell carcinoma (BAC), a subtype of adenocarcinoma, is the commonest neoplastic cause for this appearance (Fig. 9(a–d)). However, primary pulmonary lymphoma may manifest as focal areas of air space opacification on CT and this can vary markedly in size from subsegmental to confluent involvement of an entire lobe. Other features include air bronchograms, areas of cavitation as well as ground glass attenuation .
Figure 9.
Broncho-alveolar cell carcinoma (BAC). (a, b) Chest radiograph and CT demonstrating the classic appearance of BAC as a non-resolving area of consolidation. In this case a bronchoscopy was performed and the diagnosis of BAC established from washings and trans-bronchial biopsy. (c) The presence of a large ground glass nodule in this patient raised the possibility of alveolar cell carcinoma; the diagnosis was confirmed by bronchial washings. (d) The combination of solid and ground glass opacification in a lung lesion is more suggestive of malignancy than a nodule or ground glass alone. In this case the diagnosis was confirmed by percutaneous biopsy.
Kaposi’s sarcoma may demonstrate areas of ground glass and consolidation, although this occurs in conjunction with peribronchovascular nodules in patients with known immunosuppression .
Interstitial thickening.
Direct infiltration of the pulmonary interstitium by tumour cells is usually caused by metastatic involvement by extrapulmonary malignancies or by primary pulmonary carcinoma, especially small cell carcinoma and adenocarcinoma. Although uncommon, unusual lung primary neoplasms may also adopt a reticular appearance on radiographs, corresponding to nodular interlobular and intralobular interstitial thickening on CT. This manifestation may be dramatic in primary pulmonary lymphoma and Kaposi’s sarcoma, although it should be noted that other radiological manifestations, such as nodular opacities or areas of consolidation, may also be present . Benign pulmonary neoplasms and the majority of sarcomas, often referred to as spindle cell sarcomas, do not usually produce this radiological appearance .
Neoplasms arising within the pulmonary vasculature
Very rarely, a primary pulmonary neoplasm may arise within a pulmonary artery. Neoplasms arsing at this location are usually very aggressive and include angiosarcoma and leiomyosarcoma . Typically the correct diagnosis is not suspected initially because these tumours may mimic pulmonary thromboembolic disease both clinically and radiologically . Nevertheless, progressive enlargement of the affected pulmonary artery, seen on serial radiographs as an enlarging hilum, is an important characteristic feature, and may arise either as a result of direct expansion of the vessel by tumour or by pulmonary arterial hypertension secondary to distal embolism . On CT and MRI, the tumour may be seen to expand and extend along the pulmonary vasculature, penetrate the vessel wall into the lung parenchyma, and enhance, unlike bland thrombus .
Other features
Tumour size and growth
Tumour size at presentation is an unreliable indicator of the underlying pathology for both airway and parenchymal neoplasms. Clearly, the size and location of the airway involved by an endobronchial tumour will influence the maximum intraluminal dimension the tumour can achieve before becoming symptomatic. Conversely, neoplasms originating within the lung parenchyma have fewer physical constraints on their maximum size, thus may vary considerably in size at presentation. Of greater importance than the absolute size is the inherent growth of a neoplasm. Although there have been notable exceptions, a tumour volume doubling time, which equates to a 26% increase in diameter, of less than 20 days or greater than 2 years is generally accepted to indicate benignity . In practice, this feature is utilised more frequently in the surveillance of lesions that are suspected as being non-neoplastic such as pulmonary scars or granulomas rather than for follow-up of benign tumours.
Calcification
Calcification within neoplasms may have several aetiologies, including tumour engulfment of adjacent pre-existing adjacent calcification, dystrophic calcification within areas of tumour necrosis or as primary tumour calcification . Calcification may be homogeneous or heterogeneous, the latter being subdivided into dense punctate/stippled, central dense, laminated and popcorn patterns. Of these, all but the punctate patterns of heterogeneous calcification are indicative of benignity. Calcification has been documented in a range of uncommon lung tumours including carcinoid, hamartomas, sarcomas and sclerosing haemangioma . As described earlier, popcorn calcification is diagnostic of hamartoma, although this pattern is uncommon. Carcinoid is the most common tumour to calcify and this is observed in up to 39% of central lesions as opposed to 8% of peripheral tumours . Indeed, the presence of extensive calcification in a well-circumscribed centrally located mass that deforms or obstructs an airway should suggest this diagnosis . Furthermore, any nodule with extensive calcification is very likely to be benign since only approximately 6% of bronchogenic carcinomas demonstrate calcification on CT, typically stippled or eccentric, and the majority of these exceed 3 cm in diameter .
Conclusion
Unusual lung tumours constitute a broad range of histological types that, unsurprisingly, have a spectrum of imaging appearances. These neoplasms cannot usually be distinguished from the more common primary lung malignancies. Nevertheless, occasionally a single or combination of imaging features such as a tracheal location, the presence of fat, or pattern of calcification, can allude to a specific diagnosis of an unusual lung tumour. Furthermore, this may have practical implications for patient management, including obviating unnecessary surgery.
References
- 1.Fraser RS, Muller NL, Coleman N, Pare PD. 4th edn. Philadelphia, PA: W B Saunders; 1999. Diagnosis of Diseases of the Chest. [Google Scholar]
- 2.van den Bosch JM, Wagenaar SS, Corrin B, Elbers JR, Knaepen PJ, Westermann CJ. Mesenchymoma of the lung (so called hamartoma): a review of 154 parenchymal and endobronchial cases. Thorax. 1987;42:790–3. doi: 10.1136/thx.42.10.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poirier TJ, Van Ordstrand HS. Pulmonary chondromatous hamartomas: report of seventeen cases and review of the literature. Chest. 1971;59:50–5. doi: 10.1378/chest.59.1.50. [DOI] [PubMed] [Google Scholar]
- 4.Darke CS, Day P, Grainger RG, Smith GH. The bronchial circulation in a case of giant hamartoma of the lung. Br J Radiol. 1972;45:147–50. doi: 10.1259/0007-1285-45-530-147. [DOI] [PubMed] [Google Scholar]
- 5.Gjevre JA, Myers JL, Prakash UB. Pulmonary hamartomas. Mayo Clin Proc. 1996;71:14–20. doi: 10.4065/71.1.14. [DOI] [PubMed] [Google Scholar]
- 6.Tomashefski JF Jr. Benign endobronchial mesenchymal tumors: their relationship to parenchymal pulmonary hamartomas. Am J Surg Pathol. 1982;6:531–40. doi: 10.1097/00000478-198209000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Siegelman SS, Khouri NF, Scott WW Jr, Leo FP, Hamper UM, Fishman EK, et al. Pulmonary hamartoma: CT findings. Radiology. 1986;160:313–17. doi: 10.1148/radiology.160.2.3726106. [DOI] [PubMed] [Google Scholar]
- 8.Bateson EM, Abbott EK. Mixed tumors of the lung, or hamarto-chondromas: a review of the radiological appearances of cases published in the literature and a report of fifteen new cases. Clin Radiol. 1960;11:232–47. doi: 10.1016/s0009-9260(60)80048-7. [DOI] [PubMed] [Google Scholar]
- 9.Ahn JM, Im JG, Seo JW, Han HS, Yoon HK, Kim WS, et al. Endobronchial hamartoma: CT findings in three patients. AJR Am J Roentgenol. 1994;163:49–50. doi: 10.2214/ajr.163.1.8010245. [DOI] [PubMed] [Google Scholar]
- 10.Potente G, Macori F, Caimi M, Mingazzini P, Volpino P. Noncalcified pulmonary hamartomas: computed tomography enhancement patterns with histologic correlation. J Thorac Imaging. 1999;14:101–4. [PubMed] [Google Scholar]
- 11.Yamashita K, Matsunobe S, Tsuda T, Nemoto T, Matsumoto K, Miki H, et al. Solitary pulmonary nodule: preliminary study of evaluation with incremental dynamic CT. Radiology. 1995;194:399–405. doi: 10.1148/radiology.194.2.7824717. [DOI] [PubMed] [Google Scholar]
- 12.Demos TC, Armin A, Chandrasekhar AJ, Barron J. Cystic hamartoma of the lung. J Can Assoc Radiol. 1983;34:149–50. [PubMed] [Google Scholar]
- 13.Kiryu T, Kawaguchi S, Matsui E, Hoshi H, Kokubo M, Shimokawa K. Multiple chondromatous hamartomas of the lung: a case report and review of the literature with special reference to Carney syndrome. Cancer. 1999;85:2557–61. doi: 10.1002/(sici)1097-0142(19990615)85:12<2557::aid-cncr10>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Stey CA, Vogt P, Russi EW. Endobronchial lipomatous hamartoma: a rare cause of bronchial occlusion. Chest. 1998;113:254–5. doi: 10.1378/chest.113.1.254. [DOI] [PubMed] [Google Scholar]
- 15.Muraoka M, Oka T, Akamine S, Nagayasu T, Iseki M, Suyama N, et al. Endobronchial lipoma: review of 64 cases reported in Japan. Chest. 2003;123:293–6. doi: 10.1378/chest.123.1.293. [DOI] [PubMed] [Google Scholar]
- 16.Politis J, Funahashi A, Gehlsen JA, DeCock D, Stengel BF, Choi H. Intrathoracic lipomas: report of three cases and review of the literature with emphasis on endobronchial lipoma. J Thorac Cardiovasc Surg. 1979;77:550–6. [PubMed] [Google Scholar]
- 17.Wood J, Henderson RG. Peripheral intrapulmonary lipoma: a rare lung neoplasm. Br J Radiol. 2004;77:60–2. doi: 10.1259/bjr/94129286. [DOI] [PubMed] [Google Scholar]
- 18.Sawamura K, Hashimoto T, Nanjo S, Nakamura K, Iioka S, Mori T, et al. Primary liposarcoma of the lung: report of a case. J Surg Oncol. 1982;19:243–6. doi: 10.1002/jso.2930190414. [DOI] [PubMed] [Google Scholar]
- 19.Gimenez A, Franquet T, Prats R, Estrada P, Villalba J, Bague S. Unusual primary lung tumors: a radiologic-pathologic overview. Radiographics. 2002;22:601–19. doi: 10.1148/radiographics.22.3.g02ma25601. [DOI] [PubMed] [Google Scholar]
- 20.Wheeler PS, Stitik FP, Hutchins GM, Klinefelter HF, Siegelman SS. Diagnosis of lipoid pneumonia by computed tomography. JAMA. 1981;245:65–6. [PubMed] [Google Scholar]
- 21.Laurent F, Philippe JC, Vergier B, Granger-Veron B, Darpeix B, Vergeret J, et al. Exogenous lipoid pneumonia: HRCT, MR, and pathologic findings. Eur Radiol. 1999;9:1190–6. doi: 10.1007/s003300050815. [DOI] [PubMed] [Google Scholar]
- 22.Lee JS, Im JG, Song KS, Seo JB, Lim TH. Exogenous lipoid pneumonia: high-resolution CT findings. Eur Radiol. 1999;9:287–91. doi: 10.1007/s003300050669. [DOI] [PubMed] [Google Scholar]
- 23.Joshi RR, Cholankeril JV. Computed tomography in lipoid pneumonia. J Comput Assist Tomogr. 1985;9:211–13. [PubMed] [Google Scholar]
- 24.Kim TS, Lee KS, Han J, Im JG, Seo JB, Kim JS, et al. Mucoepidermoid carcinoma of the tracheobronchial tree: radiographic and CT findings in 12 patients. Radiology. 1999;212:643–8. doi: 10.1148/radiology.212.3.r99se09643. [DOI] [PubMed] [Google Scholar]
- 25.Spizarny DL, Shepard JA, McLoud TC, Grillo HC, Dedrick CG. CT of adenoid cystic carcinoma of the trachea. AJR Am J Roentgenol. 1986;146:1129–32. doi: 10.2214/ajr.146.6.1129. [DOI] [PubMed] [Google Scholar]
- 26.Smith L, Gooding CA. Pulmonary involvement in laryngeal papillomatosis. Pediatr Radiol. 1974;2:161–6. doi: 10.1007/BF00972728. [DOI] [PubMed] [Google Scholar]
- 27.Kramer SS, Wehunt WD, Stocker JT, Kashima H. Pulmonary manifestations of juvenile laryngotracheal papillomatosis. AJR Am J Roentgenol. 1985;144:687–94. doi: 10.2214/ajr.144.4.687. [DOI] [PubMed] [Google Scholar]
- 28.Blackledge FA, Anand VK. Tracheobronchial extension of recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol. 2000;109:812–18. doi: 10.1177/000348940010900905. [DOI] [PubMed] [Google Scholar]
- 29.McNamee CJ, Lien D, Puttagunta L, Conlan AA. Solitary squamous papillomas of the bronchus: a case report and literature review. J Thorac Cardiovasc Surg. 2003;126:861–3. doi: 10.1016/s0022-5223(03)00369-6. [DOI] [PubMed] [Google Scholar]
- 30.Maxwell RJ, Gibbons JR, O’Hara MD. Solitary squamous papilloma of the bronchus. Thorax. 1985;40:68–71. doi: 10.1136/thx.40.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue Y, Oka M, Ishii H, Kimino K, Kishikawa M, Ito M, et al. A solitary bronchial papilloma with malignant changes. Intern Med. 2001;40:56–60. doi: 10.2169/internalmedicine.40.56. [DOI] [PubMed] [Google Scholar]
- 32.McMullan DM, Wood DE. Pulmonary carcinoid tumors. Semin Thorac Cardiovasc Surg. 2003;15:289–300. doi: 10.1016/s1043-0679(03)70009-4. [DOI] [PubMed] [Google Scholar]
- 33.Jeung MY, Gasser B, Gangi A, Charneau D, Ducroq X, Kessler R, et al. Bronchial carcinoid tumors of the thorax: spectrum of radiologic findings. Radiographics. 2002;22:351–65. doi: 10.1148/radiographics.22.2.g02mr01351. [DOI] [PubMed] [Google Scholar]
- 34.Aronchick JM, Wexler JA, Christen B, Miller W, Epstein D, Gefter WB. Computed tomography of bronchial carcinoid. J Comput Assist Tomogr. 1986;10:71–4. doi: 10.1097/00004728-198601000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Zwiebel BR, Austin JH, Grimes MM. Bronchial carcinoid tumors: assessment with CT of location and intratumoral calcification in 31 patients. Radiology. 1991;179:483–6. doi: 10.1148/radiology.179.2.2014296. [DOI] [PubMed] [Google Scholar]
- 36.Choplin RH, Kawamoto EH, Dyer RB, Geisinger KR, Mills SE, Pope TL. Atypical carcinoid of the lung: radiographic features. AJR Am J Roentgenol. 1986;146:665–8. doi: 10.2214/ajr.146.4.665. [DOI] [PubMed] [Google Scholar]
- 37.Gould PM, Bonner JA, Sawyer TE, Deschamps C, Lange CM, Li H. Bronchial carcinoid tumors: importance of prognostic factors that influence patterns of recurrence and overall survival. Radiology. 1998;208:181–5. doi: 10.1148/radiology.208.1.9646811. [DOI] [PubMed] [Google Scholar]
- 38.Vincent JM, Trainer PJ, Reznek RH, Marcus AJ, Dacie JE, Armstrong P, et al. The radiological investigation of occult ectopic ACTH–dependent Cushing’s syndrome. Clin Radiol. 1993;48:11–17. doi: 10.1016/s0009-9260(05)80100-x. [DOI] [PubMed] [Google Scholar]
- 39.Kwong JS, Muller NL, Miller RR. Diseases of the trachea and main-stem bronchi: correlation of CT with pathologic findings. Radiographics. 1992;12:645–57. doi: 10.1148/radiographics.12.4.1636031. [DOI] [PubMed] [Google Scholar]
- 40.Kojima K, Okamoto I, Ushijima S, Yoshinaga T, Kitaoka M, Suga M, et al. Successful treatment of primary pulmonary angiosarcoma. Chest. 2003;124:2397–400. doi: 10.1378/chest.124.6.2397. [DOI] [PubMed] [Google Scholar]
- 41.Cronin P, Arenberg D. Pulmonary epithelioid hemangioendothelioma: an unusual case and a review of the literature. Chest. 2004;125:789–93. doi: 10.1378/chest.125.2.789. [DOI] [PubMed] [Google Scholar]
- 42.Gladish GW, Sabloff BM, Munden RF, Truong MT, Erasmus JJ, Chasen MH. Primary thoracic sarcomas. Radiographics. 2002;22:621–37. doi: 10.1148/radiographics.22.3.g02ma17621. [DOI] [PubMed] [Google Scholar]
- 43.Kacl GM, Bruder E, Pfammatter T, Follath F, Salomon F, Debatin JF. Primary angiosarcoma of the pulmonary arteries: dynamic contrast-enhanced MRI. J Comput Assist Tomogr. 1998;22:687–91. doi: 10.1097/00004728-199809000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Cox JE, Chiles C, Aquino SL, Savage P, Oaks T. Pulmonary artery sarcomas: a review of clinical and radiologic features. J Comput Assist Tomogr. 1997;21:750–5. doi: 10.1097/00004728-199709000-00018. [DOI] [PubMed] [Google Scholar]
- 45.Ross GJ, Violi L, Friedman AC, Edmonds PR, Unger E. Intravascular bronchioloalveolar tumor: CT and pathologic correlation. J Comput Assist Tomogr. 1989;13:240–3. doi: 10.1097/00004728-198903000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Khalil AM, Carette MF, Cadranel JL, Mayaud CM, Bigot JM. Intrathoracic Kaposi’s sarcoma. CT findings. Chest. 1995;108:1622–6. doi: 10.1378/chest.108.6.1622. [DOI] [PubMed] [Google Scholar]
- 47.Sivit CJ, Schwartz AM, Rockoff SD. Kaposi’s sarcoma of the lung in AIDS: radiologic-pathologic analysis. AJR Am J Roentgenol. 1987;148:25–8. doi: 10.2214/ajr.148.1.25. [DOI] [PubMed] [Google Scholar]
- 48.Edinburgh KJ, Jasmer RM, Huang L, Reddy GP, Chung MH, Thompson A, et al. Multiple pulmonary nodules in AIDS: usefulness of CT in distinguishing among potential causes. Radiology. 2000;214:427–32. doi: 10.1148/radiology.214.2.r00fe22427. [DOI] [PubMed] [Google Scholar]
- 49.Traill ZC, Miller RF, Shaw PJ. CT appearances of intrathoracic Kaposi’s sarcoma in patients with AIDS. Br J Radiol. 1996;69:1104–7. doi: 10.1259/0007-1285-69-828-1104. [DOI] [PubMed] [Google Scholar]
- 50.Cadranel J, Wislez M, Antoine M. Primary pulmonary lymphoma. Eur Respir J. 2002;20:750–62. doi: 10.1183/09031936.02.00404102. [DOI] [PubMed] [Google Scholar]
- 51.Bazot M, Cadranel J, Benayoun S, Tassart M, Bigot JM, Carette MF. Primary pulmonary AIDS-related lymphoma: radiographic and CT findings. Chest. 1999;116:1282–6. doi: 10.1378/chest.116.5.1282. [DOI] [PubMed] [Google Scholar]
- 52.Ray P, Antoine M, Mary-Krause M, Lebrette MG, Wislez M, Duvivier C, et al. AIDS-related primary pulmonary lymphoma. Am J Respir Crit Care Med. 1998;158:1221–9. doi: 10.1164/ajrccm.158.4.9801057. [DOI] [PubMed] [Google Scholar]
- 53.Gladish GW, Sabloff BM, Munden RF, Truong MT, Erasmus JJ, Chasen MH. Primary thoracic sarcomas. Radiographics. 2002;22:621–37. doi: 10.1148/radiographics.22.3.g02ma17621. [DOI] [PubMed] [Google Scholar]
- 54.Kim JH, Gutierrez FR, Lee EY, Semenkovich J, Bae KT, Ylagan LR. Primary leiomyosarcoma of the pulmonary artery: a diagnostic dilemma. Clin Imaging. 2003;27:206–11. doi: 10.1016/s0899-7071(02)00568-5. [DOI] [PubMed] [Google Scholar]
- 55.Nam JE, Ryu YH, Cho SH, Lee YJ, Kim HJ, Lee DY, et al. Air-trapping zone surrounding sclerosing hemangioma of the lung. J Comput Assist Tomogr. 2002;26:358–61. doi: 10.1097/00004728-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Im JG, Kim WH, Han MC, Han YM, Chung JW, Ahn JM, et al. Sclerosing hemangiomas of the lung and interlobar fissures: CT findings. J Comput Assist Tomogr. 1994;18:34–8. doi: 10.1097/00004728-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Cheung YC, Ng SH, Chang JW, Tan CF, Huang SF, Yu CT. Histopathological and CT features of pulmonary sclerosing haemangiomas. Clin Radiol. 2003;58:630–5. doi: 10.1016/s0009-9260(03)00177-6. [DOI] [PubMed] [Google Scholar]
- 58.Yankelevitz DF, Henschke CI. Does 2-year stability imply that pulmonary nodules are benign? AJR Am J Roentgenol. 1997;168:325–8. doi: 10.2214/ajr.168.2.9016198. [DOI] [PubMed] [Google Scholar]