Abstract
When used in the context of multidisciplinary team discussion, image guided biopsy using ultrasound (US) or computed tomography (CT) guidance is of value in planning management of women with suspected ovarian cancer and peritoneal carcinomatosis (PC) of uncertain aetiology. It is essential in women believed to have ovarian cancer but with poor performance status or with advanced disease believed beyond the scope of primary cytoreductive surgery for whom staging surgical pathology will not be obtained. It provides a site-specific primary tumour diagnosis in 93% of cases and it should replace diagnostic laparoscopy or laparotomy for this purpose. It allows provision of primary (neoadjuvant) chemotherapy based on a firm histological diagnosis. It is mandatory in women with a history of cancer whose metastases may mimic ovarian cancer (e.g. breast, GI tract, melanoma). More women with prior breast cancer who re-present with peritoneal cancer will have a new gynaecological primary than recurrence of their original primary tumour; the two options require radically different therapies. Finally it is a valuable problem solving tool in situations of diagnostic uncertainty, e.g. unusual imaging patterns of disease such as PC with bilateral solid ovarian masses or non-enlarged ovaries and with an unusual tumour marker profiles suggesting primary tumours outwith the ovary. The technique is simple, safe and effective and can be combined with palliative drainage of ascites at the same procedure.
Keywords: Peritoneum, neoplasms; peritoneum, biopsy; ovary, neoplasms; ovary, metastases
Introduction
For most common and intermediate incidence cancers a firm histological diagnosis is available prior to staging investigations. With cancer of the ovary a diagnosis is uncommon before major surgery and is presumed from a characteristic clinical presentation of elevated levels of the tumour marker CA-125 and a typical pattern of imaging showing a complex pelvic mass with or without evidence of peritoneal spread. Unfortunately there is overlap between the imaging appearances of ovarian cancer, benign ovarian masses and metastasis to the ovary and peritoneum from other primary sites. Further peritoneal carcinomatosis can be mimicked by curable diseases such as tuberculosis and even lymphoma.
The size of the problem is illustrated by the Radiology Diagnostic Oncology Group (RDOG) major multicentre diagnostic imaging study of women prior to ovarian cancer surgery [1–3]. These studies compared US, CT and MR imaging in 280 women, evaluating these modalities for cancer diagnosis and staging. In the study 189 women had unilateral masses and 91 had bilateral masses. Only 114 of the 280 women had ovarian cancer and of these 27 were not primary ovarian cancer but other malignancies metastatic to the ovary, some of which demand entirely different cancer management. Women with a history of malignancy were excluded so the risk of confusion between primary ovarian cancer and other cancers metastatic to the ovaries was less than in routine clinical practice where women with previously treated breast and GI tract cancer are encountered.
The RDOG studies also showed that the CT appearance of ovarian metastases may be indistinguishable from that of primary ovarian cancer [3]. Both primary ovarian cancer and metastases may produce the bilateral solid masses considered typical of Krukenberg tumours. The only factor favouring primary ovarian cancer in the RDOG studies was multilocularity. This was a judgement that could only be made using US or MR imaging and was not a significant feature for CT [3]. Conversely CT well illustrates the stomach, colon, appendix and pancreas and these should be inspected as potential primary cancer sites within the abdomen.
Thus, in this study of women with complex ovarian masses, the majority did not have primary ovarian cancer. Indeed, in 58 women their masses were not even neoplastic. Although we are not told whether cytoreductive cancer surgery was carried out in these women or was more limited by findings at surgery, the data re-emphasise the challenge faced by physicians and radiologists in defining appropriate management strategies for women with ovarian masses on a case by case basis.
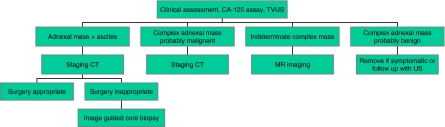
Two clear messages emerge from these and other recent data. A rational and evidenced based imaging pathway for women suspected to have ovarian cancer is suggested (Fig. 1). The first is that MR imaging is a powerful tool to investigate the woman with an indeterminate mass and no signs of peritoneal spread—i.e. women who may have benign disease. The second is that a firm histological diagnosis should be sought in all cases when there is uncertainty that the diagnosis of malignancy actually represents primary ovarian cancer.
Figure 1.
Pathway of imaging in investigation of suspected ovarian cancer.
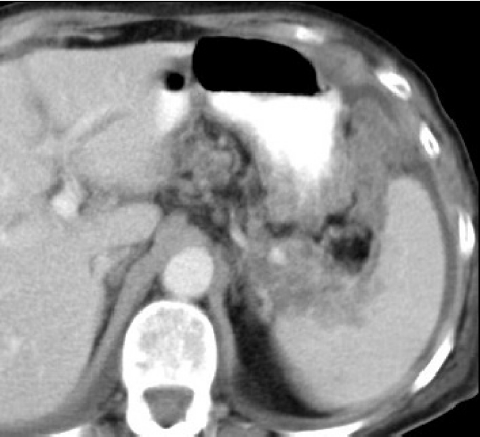
The ready availability of CT makes it the investigation of choice for planning surgery in women believed to have metastatic spread of ovarian cancer. In the presence of bulky metastatic tumour CT predicts when cytoreductive surgery is likely to be incomplete by defining sites of unresectable tumour (Fig. 2). Bulky disease in the supracolic compartment around the spleen and stomach, within suprarenal lymph nodes, and affecting the subdiaphragmatic recesses and parenchyma of the liver is usually beyond the scope of surgery. CT provides the surgeon with the detail required to discuss surgical and other therapeutic options with the patient and her carers. A variety of schemes have been devised to judge the tumour extent at key sites [4].
Figure 2.
CT showing inoperable ovarian cancer in the left supracolic compartment involving the spleen and stomach.
When radical cytoreductive surgery is not considered appropriate for women with bulky disease, with poor performance status or with a history of malignancy which can mimic primary ovarian cancer, CT can help further. Image guided needle core biopsy is an effective, safe and well tolerated alternative to surgery (mini-laparotomy, laparoscopy) in providing a definitive histological diagnosis when cancer surgery is not considered appropriate [5]. There is current interest in neoadjuvant chemotherapy followed by intervention (interval) debulking surgery (IDS) for women with primary ovarian cancer unable to undergo radical surgery at initial diagnosis [6]. With this therapeutic approach primary chemotherapy is administered in the hope that tumour, as monitored by serial CT examination, can be debulked and downstaged to allow subsequent surgery.
A firm histological diagnosis is essential when there is concern that peritoneal carcinomatosis results from disease metastatic to the ovary from other primary sites. With a history of breast cancer image guided biopsy of peritoneal disease is mandatory as in our experience there is a greater likelihood of a new gynaecological primary or primary peritoneal carcinoma (PPC), which is treated in the same way, than of recurrent breast cancer [7]. Simple cytological assessment of ascitic fluid infrequently results in distinction of primary ovarian cancer from disease metastatic from other sites [5]. Fluid can be spun down to form a pellet from which a cell block can be processed but this lacks the cellular architecture of a core biopsy specimen.
Peritoneal core biopsy using CT (or US) guidance is a valuable and useful alternative to laparoscopy or exploratory surgery in several circumstances (Table 1).
Table 1.
Indications for image guided biopsy with peritoneal carcinomatosis
| (1) In women believed to have ovarian cancer but with poor performance status or with advanced disease believed beyond the scope of primary cytoreductive surgery |
| (2) In women with a history of cancer whose metastases may mimic ovarian cancer (e.g. breast, GI tract, melanoma) |
| (3) When there is diagnostic uncertainty, e.g. unusual imaging patterns of disease such as peritoneal carcinomatosis with bilateral solid ovarian masses or non-enlarged ovaries or with an unusual tumour marker profile |
Thus in women with undiagnosed peritoneal carcinomatosis both PPC and secondary ovarian cancer require consideration. Peritoneal biopsy should precede an exhaustive (and potentially hazardous and unpleasant) series of investigations of potential primary sites such as upper and lower bowel endoscopy. Needle core biopsy findings can focus the search for the primary tumour when appropriate. In the majority of women undergoing image guided needle core biopsy standard haematoxylin and eosin (H&E) staining is diagnostic, and this can be compared with historical material in women with prior malignancy. In women with poorly differentiated tumours further special immunohistochemical stains may be required which identify specific tumour markers and other cellular proteins such as cytokeratins. Management of such women is best discussed in a multidisciplinary setting.
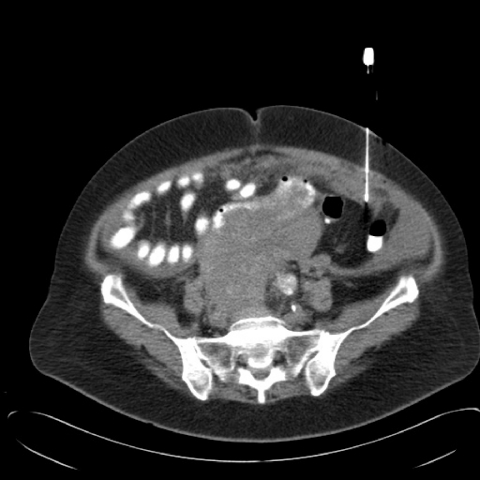
Technique for image guided peritoneal biopsy (Fig. 3)
Figure 3.
CT guided core biopsy of infracolic omental cake.
Criteria for image guided biopsy are: (a) the presence of omental, peritoneal or pelvic mass identified on a staging CT study allowing core biopsy; (b) no bleeding diathesis with platelet count >10×109/L and INR (international normalised ratio) < 1.4; and (c) a decision made after multidisciplinary review that obtaining a definitive diagnosis by non-surgical means was required to plan further treatment.
The image guided biopsy is performed as a separate procedure after multidisciplinary review of the diagnostic studies. With CT guidance the study is performed with only oral contrast preparation beginning with a limited number of localising sections planned from the prior staging study. An 18G cutting needle using a spring loaded device is used (Temno, Bauer Medical International SA, Dominican Republic). The number of needle passes made is judged by the supervising radiologist to provide the equivalent of two full needle cores of solid material for the pathologist.
For US guidance a decision is made from review of the staging CT that the omental cake or other peritoneal mass is likely to be visible with US. It requires some experience to confidently identify omental cake as a structure distinct from bowel loops. No patient preparation is required. With both CT and US guidance the biopsy procedure may be combined with placement of an ascitic drain. The biopsy procedure typically lasts 10–20 min. Aftercare includes bed rest for 6 h with measurement of blood pressure and pulse half hourly for 2 h and then hourly for 4 h after which the patient may eat and drink and become ambulant. The biopsy can be performed as a day case procedure. US guidance is our preferred technique for bulky disease with CT reserved for more inaccessible sites or with small volume infiltrates.
Needle core biopsies are formalin fixed, embedded in paraffin wax and sections cut at 3–4 micrometer thickness. These are stained with haematoxylin and eosin (H&E). There can be significant overlap between the histological patterns of primary ovarian cancer and metastatic disease, especially with mucinous carcinoma [8]. Further immunohistochemical analysis of the biopsy material is performed as deemed necessary for confirmation of diagnosis using the labelled streptavidin-biotin peroxidase system. Most commonly used are monoclonal antibodies to CEA-M, cytokeratin 7 (CK7), CK20 and CA-125. In selected cases additional monoclonal antibodies are used at the discretion of the reporting pathologist in women with previous breast cancer for oestrogen receptor and progesterone receptor and to gross cystic disease protein-15 which is a marker commonly positive in but not specific for primary breast cancer [9]. Isotypes reacting with these antibodies are lineage specific and this characteristic is retained during malignant transformation and progression.
The distinction of metastasis from colorectal cancer from ovarian endometrioid adenocarcinoma remains problematical. As well as recognised histological features characteristic of these tumours immunohistochemistry can be useful [8]. Antibodies to CK7 and CA-125 react with ovarian epithelia but rarely with colonic; antibodies to CK20 and CEA-M react conversely [10–12]. Only strong and widespread positive staining is accepted.
Finally the technique is simple, safe and effective. In an audit of 149 biopsy procedures performed with US (69) and CT (80) guidance there were no significant complications. With a variety of operators including supervised trainee radiologists there was a site-specific primary tumour diagnosis in 93% of cases. In the remainder the pathologist was able to diagnose adenocarcinoma but no primary site with only three patients requiring a surgical biopsy. US and CT guidance had similar results [13].
Conclusion
When used in the context of multidisciplinary team discussion image guided peritoneal core biopsy is a valuable tool in initial diagnosis and management of ovarian cancer, especially prior to neoadjuvant chemotherapy and in patients where there are considerations of cancer metastatic to the ovary from other sites. When integrated into the investigative pathway of women with suspected ovarian cancer it offers improvements in case selection for ovarian cancer surgery and chemotherapy and more effective service delivery.
References
- 1.Kurtz AB, Tsimikas JV, Tempany CM, Hamper UM, Arger PH, Bree RL, et al. Diagnosis and staging of ovarian cancer: comparative values of Doppler and conventional US, CT, and MR imaging correlated with surgery and histopathologic analysis: report of the Radiology Diagnostic Oncology Group. Radiology. 1999;212:19–27. doi: 10.1148/radiology.212.1.r99jl3619. [DOI] [PubMed] [Google Scholar]
- 2.Tempany CM, Zou KH, Silverman SG, Brown DL, Kurtz AB, McNeil BJ. Staging of advanced ovarian cancer: comparison of imaging modalities: report from the Radiological Diagnostic Oncology Group. Radiology. 2000;215:761–7. doi: 10.1148/radiology.215.3.r00jn25761. [DOI] [PubMed] [Google Scholar]
- 3.Brown DL, Zou KH, Tempany CM, Frates MC, Silverman SG, McNeil BJ, et al. Primary versus secondary ovarian malignancy: imaging findings of adnexal masses in the Radiology Diagnostic Oncology Group Study. Radiology. 2001;219:213–18. doi: 10.1148/radiology.219.1.r01ap28213. [DOI] [PubMed] [Google Scholar]
- 4.Meyer JI, Kennedy AW, Friedman R, Ayoub A, Zepp RC. Ovarian carcinoma: value of CT in predicting success of debulking surgery. Am J Roentgenol. 1995;165:875–8. doi: 10.2214/ajr.165.4.7676985. [DOI] [PubMed] [Google Scholar]
- 5.Spencer JA, Swift SE, Wilkinson N, Boon AP, Lane G, Perren TJ. Peritoneal carcinomatosis: image-guided peritoneal core biopsy for tumor type and patient care. Radiology. 2001;221:173–7. doi: 10.1148/radiol.2203010070. [DOI] [PubMed] [Google Scholar]
- 6.van der Burg ME, van Lent M, Buyse M, Kobierska A, Colombo N, Favalli G, et al. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. N Engl J Med. 1995;332:629–34. doi: 10.1056/NEJM199503093321002. [DOI] [PubMed] [Google Scholar]
- 7.Hewitt MJ, Hall GD, Wilkinson N, Perren TJ, Lane G, Spencer JA. Image-guided biopsy in women with breast cancer presenting with peritoneal carcinomatosis. Int J Gynecol Cancer. 2006;16(1):108–10. doi: 10.1111/j.1525-1438.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- 8.McCluggage WG, Wilkinson N. Metastatic neoplasms involving the ovary: a review with an emphasis on morphological and immunohistochemical features. Histopathology. 2005;47:231–47. doi: 10.1111/j.1365-2559.2005.02194.x. [DOI] [PubMed] [Google Scholar]
- 9.Monteagudo C, Merino MJ, LaPorte N, Neumann RD. Value of gross cystic fluid protein-15 in distinguishing metastatic breast carcinomas among poorly differentiated neoplasms involving the ovary. Hum Pathol. 1991;22:368–72. doi: 10.1016/0046-8177(91)90084-3. [DOI] [PubMed] [Google Scholar]
- 10.Ramaekers F, van Niekerk C, Poels L, Schaafsma E, Huijsmans A, Robben H, et al. Use of monoclonal antibodies to keratin 7 in the differential diagnosis of adenocarcinomas. Am J Pathol. 1990;136:641–55. [PMC free article] [PubMed] [Google Scholar]
- 11.Berezowski K, Stastny JF, Kornstein MJ. Cytokeratins 7 and 20 and carcinoembryonic antigen in ovarian and colonic carcinoma. Mod Pathol. 1996;9:426–9. [PubMed] [Google Scholar]
- 12.Loy TS, Calaluce RD, Keeney GL. Cytokeratin immunostaining in differentiating primary ovarian cancer from metastatic colonic adenocarcinoma. Mod Pathol. 1996;9:1040–4. [PubMed] [Google Scholar]
- 13.Anderson KE, Hewitt MJ, Wilkinson N, Swift SE, Weston MJ, Spencer JA. Image-guided peritoneal core biopsy (IGB) in peritoneal carcinomatosis (PC): experience in 149 patients. Cancer Imaging. 2005;5:S40. [Google Scholar]