Abstract
The incidence of hepatocellular carcinoma has been rising in the USA in the past two decades. Hepatocellular carcinoma primarily affects older people and reaches its highest prevalence among those aged between 50 and 70 years. Chronic infection by the hepatitis B virus is the most common cause of this disease. Since hepatocellular carcinoma is an indolent tumor, it has a low life expectancy. In patients with suspected hepatocellular carcinoma, CT, MRI, and ultrasound techniques are useful for formulating the diagnosis based on vascularity and specific enhancement features. In this paper we will discuss the multimodal approach for diagnosis and surveillance of hepatocellular carcinoma. We will also furnish the latest staging and treatment, epidemiology, clinical presentation, pathology and laboratory findings in hepatocellular carcinoma.
Keywords: Hepatocellular carcinoma (HCC), staging, radiology and treatment
Introduction
Hepatocellular carcinoma (HCC) ranks fifth in cancer frequency in the world with an estimated 0.5 to 1 million new cases occurring per year. By the year 2010 [1], HCC will have exceeded lung cancer as the foremost cause of cancer mortality. The peak age of incidence is 50–70 years, with a male predominance of 4:1. The incidence of HCC in the United States has increased approximately 70% [2] during the past two decades, from 1.4 per million in 1976–1980 to 2.4 per million in 1991–1995. We will review the epidemiology, clinical presentation, staging, pathology, laboratory findings, radiology, and treatment of hepatocellular carcinoma.
Epidemiology
Worldwide, HCC is the third most common cause of cancer-related death. Approximately 80% [3] of all cases are found in Asia. The incidence of HCC has been low in the United States, but the rates are increasing with the disease occurring among the younger population. This increase in HCC can be explained by the increased rate of hepatitis C virus infection and an improved clinical management of patients with cirrhosis. Other factors related to increase of HCC incidence in the United States include hepatitis B virus infection, alcoholic liver disease, tyrosinemia, and hemochromatosis. Additional risk factors include excessive androgens, α1-antitrypsin deficiency, as well as exposure to aflatoxins, thorotrast, oral contraceptives, and vinyl chloride [2, 4].
Clinical presentation
The growth of HCC is usually silent in nature and it may go undiagnosed for as long as 3 years from the time of development. Common symptoms include abdominal pain, fatigue, and weight loss. Loss of hepatic synthetic function often leads to hepatic encephalopathy, jaundice, and ascites. High portal pressures result in new or difficult-to-control ascites and variceal bleeding. Hepatomegaly is often noted on physical examination; with hard and irregular palpable borders, although sometimes the liver may appear to be small and shrunken. Paraneoplastic manifestations include hypercalcemia, hypoglycemia, feminization, polycythemia, and watery diarrhea. Right upper quadrant pain can result from either hepatic capsular inflammation or complications related to the tumor, such as intratumoral hemorrhage or necrosis. An enlarging mass compressing the adjacent extrahepatic biliary structure can lead to painless obstructive jaundice or cholangitis. Tumor invasion into the main portal vein can result in splenomegaly and ascites. Sometimes, when a large subcapsular tumor sustains blunt trauma or outgrows its blood supply, it may rupture into the peritoneal cavity. These patients present with sudden severe onset of abdominal pain, peritonism, and hypotension. Peritoneal lavage or laparotomy can assist in confirming the diagnosis.
Staging
Evaluation and treatment of patients with hepatocellular carcinoma is dependent on accurate staging. Major vascular and microvascular invasion act as independent predictors of death. Severe fibrosis and cirrhosis is a negative predictor of survival following resection of HCC [5]. There are a few classification systems: CLIP (Cancer of the Liver Italian Program), BCLC (Barcelona Clinic Liver Cancer), and JIS (Japan Integrated Staging). Tables 1 and 2 present the latest tumor staging by the AJCC (American Joint Committee on Cancer).
Table 1.
TNM staging system devised by the American Joint Committee on Cancer
| Stage I | T1 N0 M0 |
| Stage II | T2 N0 M0 |
| Stage IIIA | T3 N0 M0 |
| Stage IIIB | T4 N0 M0 or any T1 N1 M0 |
| Stage IIIC | Any T N0 M1 |
| Stage IV | Any T—any NM1 |
Table 2.
TNM staging system devised by the American Joint Committee on Cancer
| T1 | Solitary without vascular invasion |
| T2 | Solitary with vascular invasion |
| Multiple <5 cm | |
| T3 | Multiple <5 cm |
| Invades major branch of portal or hepatic vein(s) | |
| T4 | Invades adjacent organs other than gallbladder |
| Perforates visceral peritoneum | |
| N1 | Regional lymph nodes |
| M | Distant metastasis |
Metastasis from hepatocellular carcinoma
The most frequent site of metastasis from HCC is the lung, followed by bone (vertebral body and ribs), lymph nodes, and adrenal glands. Metastases are seldom seen in the peritoneum, brain, skin, or muscles [6]. Most frequently encountered nodal metastasis is within the celiac axis. The other sites of nodal metastasis, in the order of decreasing frequency, occur along the portahepatis, para-aortic, portocaval, mediastinal and cardiophrenic lymphatic chains.
Pathology
The development of hepatocellular carcinoma from premalignant lesions is reported to occur in stages. The regenerative nodules evolve into dysplastic nodules (low and high grade). These may subsequently develop into early HCCs, and if left untreated, become advanced carcinomas.
The gross pathology of HCC is a direct reflection of the imaging findings. HCC may appear as a single mass or as multifocal nodules of variable sizes, and sometimes can be diffusely infiltrative. Macroscopically, small HCCs up to and around 2 cm in diameter are divided into two types: a distinctly nodular type and an indistinctly nodular type [7]. The classic HCC or the distinctly nodular type is seen as a clear nodule with a fibrous capsule and/or fibrous septa. On the other hand, tumors of the indistinctly nodular type show only a vaguely nodular appearance with indistinct margins [7]. The tumor is usually paler than normal liver parenchyma. Early HCCs have a unique blood supply, and receive blood from the portal vein in addition to the hepatic artery [7]. In well-differentiated HCCs containing less-differentiated cancerous tissues a ‘nodule-in-nodule’ appearance is observed. This appearance is not only regarded as a morphological marker but also the radiological marker [7] in the dedifferentiation process of well-differentiated HCC.
Microscopically, tumors range from well differentiated to highly anaplastic. The most common histologic pattern is the trabecular pattern. The scirrhous type is the least common pattern [7].
Laboratory findings
Approximately half of hepatocellular carcinomas have alpha-fetoprotein (AFP) as one of the earliest recognized oncofetal markers [8]. AFP is produced in large amounts by the fetal liver, but its expression reduces sharply at birth. Elevated AFP is reported in yolk sac tumors, cirrhosis, massive liver necrosis, chronic hepatitis, pregnancy, fetal distress, and fetal neural tube defects [8]. AFP can be used as an unfavorable prognostic marker in patients with HCC [8]. β-catenin [7] positivity is common among the AFP-negative liver tumors. Other tumor markers with less sensitivity include des-gamma-carboxyprothrombin, α-L- and isoenzymes of γ-glutamyl transferase [8].
Radiology
Ultrasound
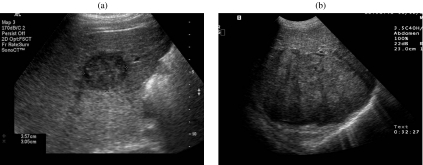
Ultrasound (US) has been utilized as a screening imaging modality for HCC in patients with a history of chronic liver disease (hepatitis or alcohol abuse). US is relatively inexpensive and is widely available in developing countries, where the incidence of HCC is high due to the underlying prevalence of hepatitis and liver infection, including parasitic infections. The most common US appearance of small well-differentiated HCC (<3 cm) is a well-circumscribed hypoechoic mass. The US appearance in larger masses is variable and depends on the presence of fat, calcium, and necrosis. The presence of dense cellular elements, necrosis, or sinusoidal dilatation gives a hypoechoic appearance (Fig. 1(a)) whereas existence of hemorrhage, fat, or fibrosis presents as a heterogeneous hyperechoic mass (Fig. 1(b)). The surrounding capsule, if present, is generally hypoechoic.
Figure 1.
(a) A 64-year-old man with hepatocellular carcinoma and chronic transfusion-related hepatitis C. Transverse sonogram shows a small, 3 cm, hypoechoic mass in the right lobe of the liver. (b) A 51-year-old man with a history of hemochromatosis and hepatocellular carcinoma. Transverse sonogram shows a heterogeneous large mass in the right lobe of the liver.
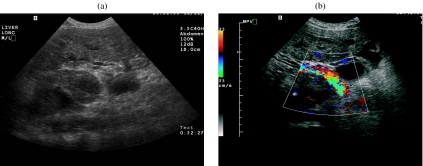
In staging, US can evaluate tumor size and number, and with the help of color Doppler, vascular invasion into the hepatic and the portal veins can be assessed (Fig. 2(a, b)). Ultrasound can provide guidance for percutaneous biopsy and in delivering therapy for a suspected liver mass. Color Doppler US can also illustrate hypervascularity and arteriovenous shunting.
Figure 2.
A 51-year-old man with a history of hemochromatosis and hepatocellular carcinoma. (a) Transverse sonogram shows portal vein thrombus. (b) Transverse color Doppler sonogram of the right upper quadrant shows heterogeneous flow within the tumor thrombus.
Computed tomography
Computed tomography (CT) is the most commonly used imaging modality in diagnosing HCC in the United States. On unenhanced CT, HCC appears hypodense, except in diffuse hepatic steatosis where it may appear denser relative to the liver parenchyma. Increased attenuation within the HCC may be due to hemorrhage or calcifications. Fatty metamorphosis of HCC will appear as areas of low attenuation.
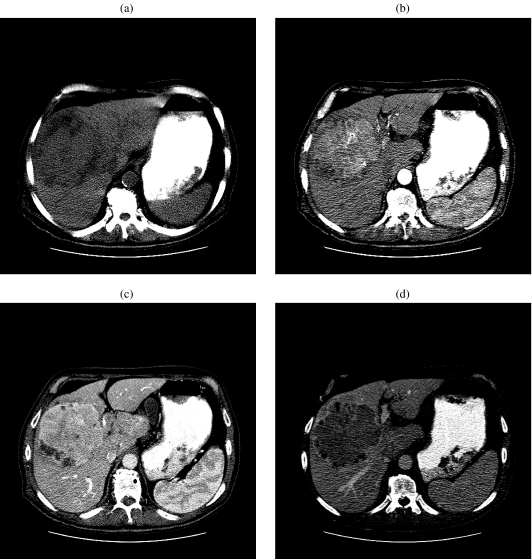
CT evaluation of the liver in a patient with a clinical suspicion of HCC should be performed at three stages of contrast enhancement: the early arterial at 13–25 s, late arterial at 30–40 s, and portal venous phase at 45–60 s. Most hypervascular HCCs are usually seen during the late hepatic arterial phase of contrast enhancement. Areas of internal necrosis or fat remain hypodense. As a consequence of rapid washout, the tumor will become hypodense compared with the liver parenchyma in the portal phase of contrast enhancement (Fig. 3(a–d)). Although most lesions are hyperdense in the early arterial phase of contrast enhancement, some may be isodense or hypodense [9] compared with the liver. A heterogeneous pattern of enhancement has been termed the ‘mosaic’ attenuation pattern and can often be caused by internal necrosis. The rate of injection also plays a key role in the sensitivity of liver lesion detection; a rate of 4–8 mL /s is recommended. Patient-related (cardiovascular status) or lesion-related (tumor vascularity or permeability) variables and definite hemodynamic changes occurring in the circulation of the cirrhotic liver, change the timing of intravenous contrast material delivered to the liver. To compensate for these patient-related factors bolus tracking technique [10] SmartPrep (GE Medical Systems Milwaukee, WI) technique [10] may be required. Some authors have suggested that the use of the double arterial phase [10](early arterial phase 25 s compared to late arterial 40 s phase) shows no significant difference compared with single late arterial phase imaging alone for detecting hypervascular hepatocellular carcinoma if fixed scanning delay is used with bolus tracking techniques.
Figure 3.
A 70-year-old man with hepatocellular carcinoma. (a) Unenhanced CT of the liver shows a heterogeneous mass in segment VIII. (b) Contrast-enhanced CT of the liver during the early arterial phase shows an enhancing mass in segment VIII. (c) Contrast-enhanced CT of the liver during the late arterial phase shows a progressively enhancing mass in segment VIII. (d) Contrast-enhanced CT in the delayed phase of contrast enhancement shows an increase in contrast between low-attenuation hepatocellular carcinoma and liver parenchyma.
A capsule is present in up to 10% of HCCs; it is often hypodense on the hepatic arterial phase, and may show enhancement on the delayed images. The optimal time for acquiring delayed images is 180 s [11] for evaluation of suspected HCC. The addition of delayed phase CT to biphasic CT allows detection of a significantly higher number of HCC nodules [11].
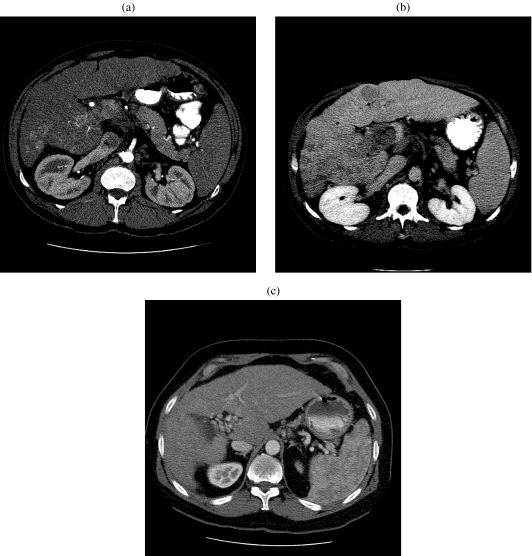
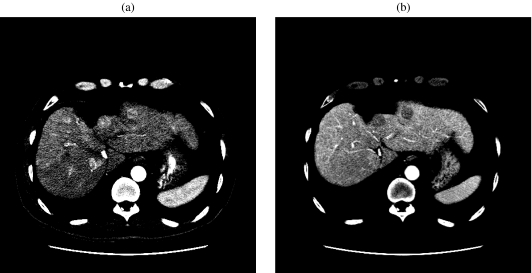
CT is highly accurate in the staging of HCC as the number of lesions, segmental anatomy, regional adenopathy, vascular tumor invasion, and metastases can be easily detected. Distinction between bland thrombus and tumor thrombus is not always feasible, but early enhancement within the thrombus is indicative of tumor (Fig. 4(a, b)). Occlusion of the portal vein can lead to cavernous transformation (Fig. 4(c)). CT also plays a major role in post-treatment evaluation, surveillance, guidance for biopsy, and assessing regeneration of liver parenchyma (Fig. 5(a, b)). The added speed and flexibility of multidetector CT (MDCT) allows high-quality, thin-section imaging and permits three-dimensional reconstruction for preoperative vascular mapping.
Figure 4.
(a) A 51-year-old man with a history of cirrhosis, hemochromatosis, and hepatocellular carcinoma. Arterial phase contrast-enhanced CT of the abdomen at the level of the main portal vein shows heterogeneous enhancement of the portal vein and nodularity of the hepatic contour. (b) In the same patient, late-phase contrast-enhanced CT of the abdomen at the same level as (a) shows washout of enhancement in the portal vein. There is a hypodense lesion within segment IV consistent with hepatocellular carcinoma. (c) A 54-year-old man with hepatocellular carcinoma and non-A/non-B hepatitis. Late-phase contrast-enhanced CT of the abdomen shows cavernous transformation of the portal vein.
Figure 5.
A 65-year-old man with history of partial left hepatic lobectomy. (a) Early arterial contrast-enhanced CT of the abdomen shows enhancing recurrent tumor adjacent to the surgical margin. (b) Delayed contrast-enhanced CT of the abdomen shows contrast between the low-attenuation recurrent tumor and the hepatic parenchyma adjacent to the surgical margin.
Magnetic resonance imaging
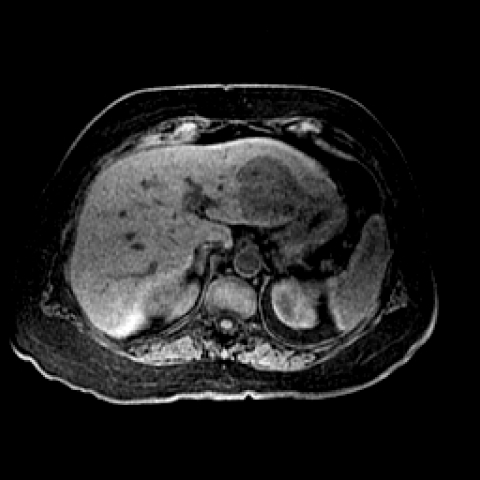
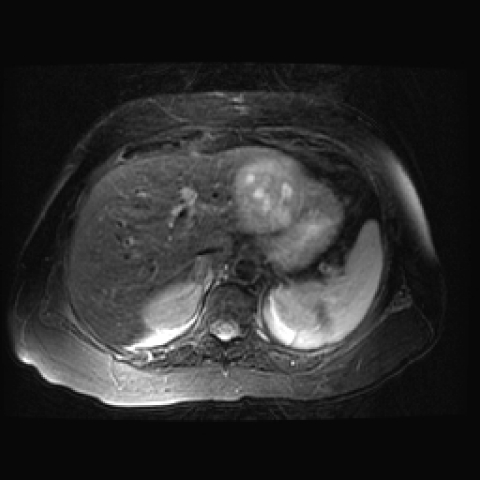
Magnetic resonance imaging (MRI) of HCC should include T1-weighted images, T2-weighted images with fat suppression, and dynamic contrast-enhanced 3D gradient-echo sequences of the liver. The T1 technique is usually a breath-hold gradient-echo sequence with an in-phase TE of 4.1 ms at 1.5-T field-strength magnet. The T2-weighted sequences are obtained at a TE range (60–80 ms). For T2-weighted sequences, a fast spin-echo sequence with fat suppression is performed. On T1-weighted sequences, hepatocellular carcinoma is usually hypointense–isointense to the liver (Fig. 6). Areas of increased intensity may be due to fat, protein, or copper in the tumor. On T2-weighted sequences the tumor is usually hyperintense to the liver (Fig. 7).
Figure 6.
An 85-year-old woman with hepatocellular carcinoma. Axial three-dimensional spoiled gradient-echo unenhanced MR image (TR/TE, 5/2) shows a hypointense mass in segment II/III of the left lobe of the liver.
Figure 7.
An 85-year-old woman with hepatocellular carcinoma. Axial fast spin-echo T2-weighted MR image (381/75; echo-train length, 12) shows hyperintense hepatocellular carcinoma in segment II/III of the left lobe of the liver.
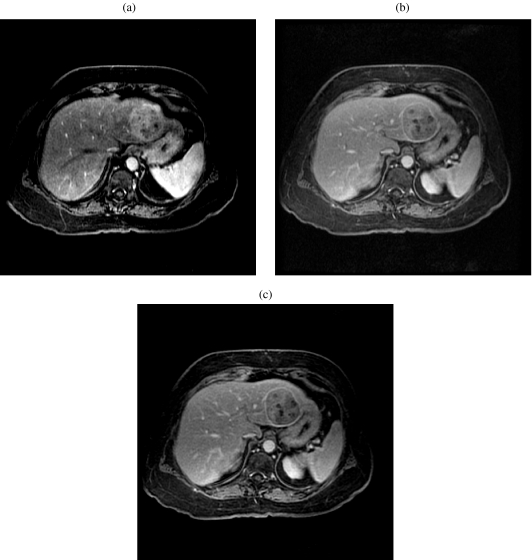
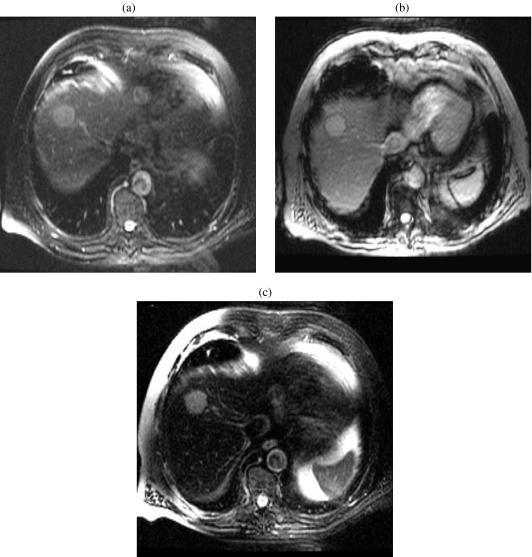
The crucial sequences in the detection of hepatocellular carcinoma are the dynamic gadolinium-enhanced [12] images. Sometimes the arterial phase of enhancement may be the only phase in which a tumor can be seen. Dynamic enhancement illustrates hyperintensity on the hepatic arterial phase because of the hepatic artery supply (Fig. 8(a, b)). The fibrous capsule shows low signal intensity on T1-weighted, T2-weighted images and enhancement on delayed contrast-enhanced images (Fig. 8(c). The tumor may invade the portal vein, hepatic veins, or the biliary system, and this can be detected by MRI.
Figure 8.
An 85-year-old woman with hepatocellular carcinoma. (a) Axial three-dimensional spoiled gradient-echo in the early arterial phase MR image (TR/TE, 5/2) shows a hyperintense mass in segment II/III of the left lobe of the liver. (b) Axial three-dimensional spoiled gradient-echo in the early arterial phase MR image (TR/TE, 5/2) shows a progressive enhancement of the hepatic parenchyma and the mass in segment II/III of the left lobe of the liver. (c) Axial three-dimensional spoiled gradient-echo in the early arterial phase MR image (TR/TE, 5/2) shows washout of contrast from the mass in segment II/III of the left lobe of the liver. Note the hyperintense capsule.
There are two classes of MRI contrast agent available commercially to image the liver: liver-specific and liver-nonspecific contrast agents. The liver-specific agents are divided into two groups: hepatocyte-selective and reticuloendothelial-specific contrast agents. Reticuloendothelial-specific agents are ferumoxides and ferucarbotran; hepatocyte-specific agents are gadobenate dimeglumine and gadoxetic acid [12]. The nonspecific contrast agents are Gd-chelates, such as Gd-DTPA-BMA (Omniscan Nycomed; Amersham Oslo, Norway) and Gd-DTPA (Magnevist Schering AG (Berlin, Germany); Berlex Laboratories (Montville, NJ)) which are routinely used at our institution.
Reticuloendothelial-specific agents affect the susceptibility changes in iron-containing molecules. A precontrast T2 * sequence should be obtained to compare the T2 * sequence acquired following administration of superparamagnetic iron oxide (SPIO). The high T2 * relaxivity of SPIO particles leads to marked reduction in signal intensity of normal liver parenchyma due to consumption by Kupffer cells, and the HCC void of Kupffer cells remains high in signal, thus making the tumor more conspicuous (Fig. 9). The potential pitfall of using this contrast agent in clinical image interpretation is difficulty in reliably differentiating small cysts, hemangiomas, and metastases from well-differentiated HCCs, as these lesions do not have Kupffer cells, do not consume ferumoxides and remain high in signal intensity on T2 * sequences [13]. Feridex (ferumoxides; Berlex Laboratories, Montville, NJ), an SPIO, is used in our institution. Double-contrast MR imaging, i.e. using gadolinium in conjunction with superparamagnetic iron oxide, is considered significantly more accurate than only SPIO-enhanced MR imaging in identifying hepatocellular carcinoma [12].
Figure 9.
An 86-year-old man with melanoma. (a) Axial fast spin-echo T2 axial 2D fast spin echo (TR/TE 4000/85) weighted MR image of the liver shows a hyperintense mass in segment VIII, consistent with a well-differentiated hepatocellular carcinoma. (b) Axial 2D gradient echo T2 * (TR/TE 180/15) weighted MR image of the liver shows a hyperintense mass in segment VIII. (c) Axial 2D gradient echo T2 * (TR/TE 180/15) post Feridex gradient echo T2 weighted MR image of the liver shows a hyperintense mass in segment VIII, due to lack of Feridex uptake. Note the normal liver parenchyma is hypointense. In this case the clinical history is misleading as to the correct diagnosis.
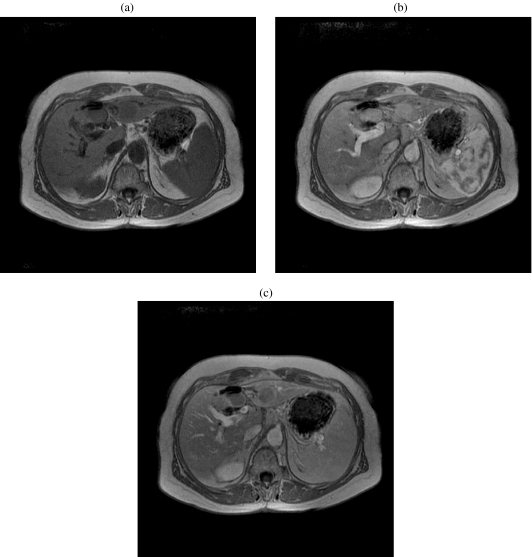
Similar to computed tomography, magnetic resonance imaging (MRI) plays a role in staging, in the detection and number of lesions, their size, and vascular and biliary involvement. MR imaging also plays a critical role in postoperative treatment surveillance as recurrent tumor will demonstrate early enhancement and washout on the delayed phase (Fig. 10). MRI can be used as a modality to guide the treatment of HCCs not visualized by other modalities.
Figure 10.
A 60-year-old woman with hepatocellular carcinoma and underlying Crohn’s disease. (a) Axial three-dimensional precontrast gradient-echo MR image (TR/TE, 220/4) shows a hypointense recurrent tumor in segment IV of the liver, at the site of wedge resection. Note another HCC in segment III. (b) Axial three-dimensional gradient-echo early arterial phase MR image (TR/TE, 220/4) shows a hyperintense recurrent tumor in segment IV of the liver, at the site of wedge resection. (c) Axial three-dimensional gradient-echo portal venous phase MR image (TR/TE, 220/4) shows a hypointense mass relative to the hepatic parenchyma segment IV of the liver, at the site of wedge resection. Note delayed capsular enhancement.
Treatment
Treatment depends on the size of the tumor, on its location and morphology, and the presence of metastatic disease. If HCC is not treated, the 5-year survival is <5%. Surgical treatment, especially partial hepatectomy, remains the treatment of choice for small lesions, while liver transplant [14] remains the treatment of choice in patients with cirrhosis. Unresectable tumors can be treated with radiofrequency ablation (RFA), chemoembolization and selective internal radiation therapy. Chemotherapeutic agents are not shown to have a significant survival benefit [15].
Localized tumors in a noncirrhotic liver may be treated successfully with surgical resection. In the setting of cirrhosis, for the nonsurgical patient localized therapy such as radiofrequency ablation, percutaneous ethanol injection (PEI), chemoembolization, or yttrium-90 microsphere infusion may be options, depending on the liver reserve and resources available. In the setting of advanced cirrhosis, treatment of the tumor may exacerbate liver decompensation, resulting in shortened survival. Liver transplantation has been employed, but it is plagued with many barriers. Patients may have limited therapy options if they have poor performance status, are not surgical candidates, have a tumor that extends into the main portal vein, or have metastases to distant lymph nodes or organs. Emerging therapies exploiting molecular targets are being explored with some promise. Table 3 is a summary of mean survival for various treatments of HCC. This table is merely a review of the literature and should not be judged for efficacy of treatment. Direct comparison of these treatment modalities is limited because of difference in patient population, lesion size, study design and number of patients. Nevertheless, surgery seems to be the best treatment option in patients with HCC (Table 3). The reported 1–2-year survival rate of patients after orthotopic liver transplantation is as high as 85%–90% [14]. However, the results are from a highly selected population. For hepatic resection, the 5-year survival rate has been reported as being as high as 35.8% [16].
Table 3.
Survival rates reported in literature for various treatment modalities for hepatocellular carcinoma [16–22]
| Treatment | Survival rate |
||
|---|---|---|---|
| 12 months | 24 months | 36–60 months | |
| PEI [19] | 81.6–60.3 | ||
| RFA [18] | 73.9 | 52.1 | 71.0–48.0 |
| TACE [20] | 97.0 | 89.0 | 40.7 |
| TACE + PEI [21] | 61.5 | 38.7 | |
| TACE + RFA [22] | 89.7 | 67.1 | |
| Wedge resection [16] | 88.1 | 60.1 | 35.8 |
| Transplant [14] | 90 | 85 | |
Key: RFA = radiofrequency ablation; PEI = percutaneous ethanol injection; TACE = transcatheter arterial chemoembolization.
Portal vein embolization (PVE) is commonly utilized at our institution in the preoperative management of patients selected for major hepatic resection. PVE redirects portal blood flow to the potential liver remnant. It induces hypertrophy of the healthy portion of the liver and improves the functional reserve of the future liver remnant (FLR) prior to surgery. Agents used for PVE include fibrin glue, ethiodized oil, gelatin sponge and thrombin, coils, micro-particles (e.g., PVA particles or tris-acryl gelatin microspheres), and absolute alcohol.
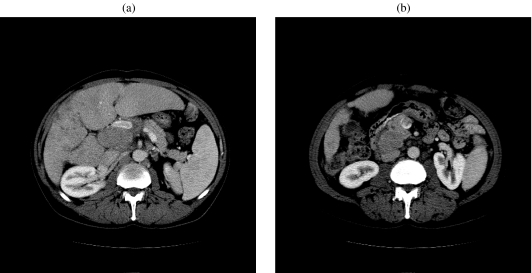
Subcapsular hematoma, hemoperitoneum, hemobilia, pseudoaneurysm, arteriovenous fistula, arterioportal shunts, portal vein/superior mesenteric vein thrombosis (Fig. 11(a, b)), transient liver failure, pneumothorax, and sepsis are some of the complications resulting from portal vein embolization.
Figure 11.
A 55-year-old man with hepatocellular carcinoma, in the setting of hepatitis C, following PVE. (a) Axial CT of the abdomen shows portal vein thrombus following portal vein embolization. (b) Axial CT of the abdomen shows superior mesenteric vein thrombus following portal vein embolization.
Overt clinical portal hypertension is an absolute contraindication to PVE. In cases of tumor invasion of the portal vein, PVE is not appropriate because portal flow is already diverted [17]. PVE can reduce postoperative mortality and morbidity and enables potential therapeutic hepatectomy for patients previously not assumed candidates for resection based on anticipated marginal FLRs.
Percutaneous radiofrequency ablation is considered the best treatment option for patients with underlying cirrhosis. Other indications for RFA include a single nodular-type HCC smaller than 5 cm or as many as three HCC lesions, each smaller than 3 cm, when surgical resection or liver transplantation is not suitable. Radiofrequency ablation (RFA) has emerged as a powerful method for percutaneous treatment of early-stage HCC and currently is being used to treat patients with more advanced cancers [18].
Percutaneous ethanol injection (PEI) [19] for tumor ablation has been reported to be an effective form of direct ablation for HCC in smaller lesions. The 5-year survival rate has been reported as 60.3% [19] with a recurrence rate of 10%. Percutaneous ethanol injection is contraindicated in the presence of gross ascites, bleeding, or obstructive jaundice.
Transcatheter arterial chemoembolization (TACE) [18] uses a combination of agents to compromise the flow of the hepatic artery. The agents include gelatin sponge particles (Gelfoam), iodized oil (Lipiodol (Guerbet, Aulnay-sous-Bois, France)), PVA and hydrophile microspheres. The selective nature of hepatic artery infusion of chemotherapy minimizes adverse effects while maximizing drug delivery to the tumor. Agents used include 5-fluorouracil, PEG-IFN-alpha, floxuridine, doxorubicin, mitoxantrone, epirubicin, and cisplatin. TACE is an effective therapeutic option for cirrhotic patients with unresectable HCC.
Systemic chemotherapy [15] has been used as a palliative with single and multiple agents including 5-fluorouracil, doxorubicin, interferon, cisplatin, octreotide, and tamoxifen. Biologic and chemotherapeutic agents are typically administered to control symptoms in patients with overwhelming disease.
Summary
Hepatocellular carcinoma is one of the most common malignancies worldwide. Imaging plays a crucial role in the detection, diagnosis, staging, treatment, and surveillance of these patients. Reports to clinicians should include all pertinent diagnostic information for staging, including lesion size, number, location, presence of adenopathy, ascites, cirrhosis, vascular involvement, biliary tree involvement, and metastases. Irrespective of sophisticated imaging techniques and the large number of therapeutic options, the 5-year survival rate in patients with HCC remains dismal. Close surveillance with imaging now offers the opportunity to diagnose recurrences early and to direct the most effective therapy.
References
- 1.Clark HP, Carson WF, Kavanagh PV, Ho CP, Shen P, Zagoria RJ. Staging and current treatment of hepatocellular carcinoma. Radiographics. 2005;25(1):S3–S23. doi: 10.1148/rg.25si055507. [DOI] [PubMed] [Google Scholar]
- 2.Merle P. Epidemiology, natural history and pathogenesis of hepatocellular carcinoma. Cancer Radiother. 2005;9:452–7. doi: 10.1016/j.canrad.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Lai EC, Lau WY. The continuing challenge of hepatic cancer in Asia. Surgeon. 2005;3:210–15. doi: 10.1016/s1479-666x(05)80043-5. [DOI] [PubMed] [Google Scholar]
- 4.Oral contraceptives and liver cancer: results of the Multicentre International Liver Tumor Study (MILTS) Contraception. 1997;56:275–84. [PubMed] [Google Scholar]
- 5.Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–92. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 6.Katyal S, Oliver JH 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698–703. doi: 10.1148/radiology.216.3.r00se24698. [DOI] [PubMed] [Google Scholar]
- 7.Kojiro M. Histopathology of liver cancers. Best Pract Res Clin Gastroenterol. 2005;19:39–62. doi: 10.1016/j.bpg.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Gorog D, Regoly-Merei J, Paku S, Kopper L, Nagy P. Alpha-fetoprotein expression is a potential prognostic marker in hepatocellular carcinoma. World J Gastroenterol. 2005;11:5015–18. doi: 10.3748/wjg.v11.i32.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angelelli G, Ianora AA, Scardapane A, Pedote P, Memeo M, Rotondo A. Role of computerized tomography in the staging of gastrointestinal neoplasms. Semin Surg Oncol. 2001;20:109–21. doi: 10.1002/ssu.1024. [DOI] [PubMed] [Google Scholar]
- 10.Ichikawa T, Kitamura T, Nakajima H, Sou H, Tsukamoto T, Ikenaga S, et al. Hypervascular hepatocellular carcinoma: can double arterial phase imaging with multidetector CT improve tumor depiction in the cirrhotic liver? AJR Am J Roentgenol. 2002;179:751–8. doi: 10.2214/ajr.179.3.1790751. [DOI] [PubMed] [Google Scholar]
- 11.Iannaccone R, Laghi A, Catalano C, Rossi P, Mangiapane F, Murakami T, et al. Hepatocellular carcinoma: role of unenhanced and delayed phase multi-detector row helical CT in patients with cirrhosis. Radiology. 2005;234:460–7. doi: 10.1148/radiol.2342031202. [DOI] [PubMed] [Google Scholar]
- 12.Huppertz A, Haraida S, Kraus A, Zech CJ, Scheidler J, Breuer J, et al. Enhancement of focal liver lesions at gadoxetic acid-enhanced MR imaging: correlation with histopathologic findings and spiral CT—initial observations. Radiology. 2005;234:468–78. doi: 10.1148/radiol.2342040278. [DOI] [PubMed] [Google Scholar]
- 13.Nasu K, Kuroki Y, Nawano S, Kuroki S, Tsukamoto T, Yamamoto S, et al. Hepatic metastases: diffusion-weighted sensitivity-encoding versus SPIO-enhanced MR imaging. Radiology. 2006;239:122–30. doi: 10.1148/radiol.2383041384. [DOI] [PubMed] [Google Scholar]
- 14.Merli M, Nicolini G, Gentili F, Novelli G, Iappelli M, Casciaro G, et al. Predictive factors of outcome after liver transplantation in patients with cirrhosis and hepatocellular carcinoma. Transplant Proc. 2005;37:2535–40. doi: 10.1016/j.transproceed.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 15.Kim SJ, Seo HY, Choi JG, Sul HR, Sung HJ, Park KH, et al. Phase II study with a combination of epirubicin, cisplatin, UFT, and leucovorin in advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2006;57:436–42. doi: 10.1007/s00280-005-0067-7. [DOI] [PubMed] [Google Scholar]
- 16.Chan KM, Lee WC, Hung CF, Yu MC, Jan YY, Chen MF. Aggressive multimodality treatment for intra-hepatic recurrence of hepatocellular carcinoma following hepatic resection. Chang Gung Med J. 2005;28:543–50. [PubMed] [Google Scholar]
- 17.Madoff DC, Abdalla EK, Vauthey JN. Portal vein embolization in preparation for major hepatic resection: evolution of a new standard of care. J Vasc Interv Radiol. 2005;16:779–90. doi: 10.1097/01.RVI.0000159543.28222.73. [DOI] [PubMed] [Google Scholar]
- 18.Lencioni R, Della Pina C, Bartolozzi C. Percutaneous image-guided radiofrequency ablation in the therapeutic management of hepatocellular carcinoma. Abdom Imaging. 2005;30:401–8. doi: 10.1007/s00261-004-0254-8. [DOI] [PubMed] [Google Scholar]
- 19.Ebara M, Okabe S, Kita K, Sugiura N, Fukuda H, Yoshikawa M, et al. Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J Hepatol. 2005;43(3):458–64. doi: 10.1016/j.jhep.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 20.Xiao EH, Hu GD, Li JQ, Huang JF. Transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi. 2005;27:478–82. [PubMed] [Google Scholar]
- 21.Becker G, Soezgen T, Olschewski M, Laubenberger J, Blum HE, Allgaier HP. Combined TACE and PEI for palliative treatment of unresectable hepatocellular carcinoma. World J Gastroenterol. 2005;11:6104–9. doi: 10.3748/wjg.v11.i39.6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veltri A, Moretto P, Doriguzzi A, Pagano E, Carrara G, Gandini G. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC) Eur Radiol. 2006;16:661–9. doi: 10.1007/s00330-005-0029-9. [DOI] [PubMed] [Google Scholar]