Abstract
This paper reviews the potential of ultrasound for assessing the viability and biological behavior of tumors. Unlike color Doppler sonography, modern techniques for contrast-enhanced ultrasound permit the measurement of tissue perfusion irrespective of vessel size or flow velocity. Perfusion can also be assessed quantitatively, using replenishment kinetics or derivates thereof. The perfusion of tumors is a surrogate parameter of their viability and may mirror their response to therapy. Furthermore, the degree of vascularity in a tumor may express its aggressiveness and help to predict its response to treatment. In animal models, a decrease in blood flow has been shown to precede a shrinkage of tumors treated with anti-angiogenic compounds. In liver metastases, arterial and portal blood supply can be assessed separately, and a response to stereotactic radiotherapy was found to go along with a decrease in arterial perfusion. Moreover, a relatively high arterial perfusion of liver metastases may predict a response to chemotherapy. Contrast-enhanced ultrasound may be a potent tool for assessing the effects of anti-angiogenic treatment in patients.
Keywords: Ultrasound, contrast agents, microbubbles, liver neoplasms, metastases
Introduction
Ultrasound contrast agents are gas- or air-containing microbubbles approximately 1–5 μm in diameter which are injected intravenously. They are strong scatterers and enhance the reflected ultrasound signal by about 20–30 dB. In addition they exhibit specific interactions with the transmitted ultrasound pulse which are different from those between sound and tissue, and which can be utilized for contrast-specific imaging. To date, two substances have been approved for non-cardiac human use in Europe. The first, Levovist ® (Schering, Berlin, Germany), consists of air bubbles which are generated by adsorption of galactose particles in an aqueous solution and stabilized by a palmitate layer. The other, SonoVue ® (Bracco-Altana, Konstanz, Germany), consists of sulfur hexafluoride, which forms microbubbles, stabilized by a phospholipid layer, once dissolved in water. Besides their approved use in the liver, where they improve the detection and characterization of focal lesions, contrast ultrasound agents may assist in the assessment of blood flow in tissue, and thereby of tumor viability and perhaps tumor aggressiveness. Most frequently, CT and MRI have been used for this purpose. However, unlike contrast-enhanced ultrasound, they do not solely assess tumor blood flow since the contrast agents used are distributed both intravascularly and in the extracellular extravascular space. Blood flow can be calculated, but only using complex pharmacokinetic modeling.
Interaction between ultrasound and microbubbles
When an ultrasound pulse hits a microbubble it will resonate at a frequency mainly determined by its diameter. It is a lucky coincidence that this resonance frequency is in the range between 2 and 5 MHz and thereby matches the spectrum of most abdominal ultrasound transmitters. Looking closer, however, the reflected signal will not entirely mirror the transmitted pulse, since the oscillations of the microbubbles are asymmetrical. In simple terms, as we can easily see if we take Laplace’s law into consideration, the expansion will be more pronounced than the contraction. When the reflected signal is analyzed in the frequency domain it will therefore, in addition to the fundamental (transmitted) frequency, contain higher-frequency components, i.e. harmonics. Furthermore, at the fundamental frequency, the amplitude of the returning signal is not proportional to the strength of the transmitted pulse. This behavior of microbubbles—concerning both upper and lower harmonics and the fundamental frequency—is referred to as ‘nonlinear backscattering’.
At higher power of the transmitted signal the bubbles will rupture , following a short series of expansion and collapse. As the bubbles perish they emit a final, short-lasting signal, a stimulated acoustical emission (SAE) . This ‘cracking’ sound is very strong, so that each single bubble can be detected. The easiest way to do this is to use the color/power Doppler mode of a commercial ultrasound system, where such sounds will be interpreted as originating from movement, although not with a defined direction and velocity, and encoded by some random color in the image.
Contrast-specific imaging techniques
The first technique used to generate an image where the ultrasound signals are displayed, and the signals from tissue suppressed, was simple filtering (classical harmonic imaging). Put simply, the fundamental frequency (which contained signals from both tissue and contrast medium) is suppressed, leaving only the first harmonics and frequencies above, which originated mainly from contrast medium but only to a lesser degree from tissue . This requires a narrow-band transmit signal. Its disadvantage is that this filtering technique inevitably also suppresses parts of the harmonic signals since the fundamental and harmonic spectra overlap considerably.
Most machines now use a wideband technique, mostly referred to as ‘phase inversion’. Two pulses are emitted, the second being the phase-inverted version of the first, and the reflected echoes are recorded separately, stored in the memory, and added. Whenever the pulse is reflected by tissue, the echo will mirror the transmitted pulse in the time domain. As a result, when the two echoes are added one will be the phase-inverted version of the other and they will cancel each other out. In other words, the signal from tissue is suppressed. Echoes reflected from microbubbles, however, will not cancel each other out, since they do not mirror each other . Likewise, when signals from SAEs are recorded, no cancellation occurs, since the bubble is destroyed before the second pulse arrives.
The techniques for contrast-specific imaging can be divided into high-MI (mechanical index, a measure for the output power) and low-MI techniques. With a high MI (above 0.5), the main phenomenon contributing to the image is SAE, i.e. a strong but short-lasting signal. Lowering the MI—to a range of approximately 0.05 to 0.3—will reduce the amount of bubble destruction. Here, bubble oscillation with generation of harmonics is the main mechanism which contributes to the image. With a low MI the organs as well as the dynamic process of contrast enhancement can be examined in real time. Still, there is some degree of bubble destruction, which can be easily seen in the gradual decay of the signal when the probe is kept constantly over one slice. Lowering the frame rate (e.g. to < 10 Hz) can help to further preserve the signal.
Contrast-enhanced ultrasound has meanwhile been evaluated for numerous organs and has been proven to have a clear role in the detection and differential diagnosis of liver lesions . For this, low-MI imaging is the technique of choice since it allows the liver to be scanned in the usual way.
Quantitative techniques
Most techniques directed at assessing the biological behavior of a malignant tumor aim at quantifying perfusion, vascularity and associated parameters, and so does contrast-enhanced ultrasound. This may serve to better estimate the aggressiveness of a tumor (which is frequently linked to its angiogenic potential ), or to detect treatment effects earlier than tumor shrinkage occurs . Obviously, such assessments are only possible if perfusion etc. can be described quantitatively. In the past, there have been numerous attempts using color Doppler sonography, which, however, describes perfusion only indirectly, if at all, since the flow signal from capillaries is undetectable . Techniques for perfusion measurements using ultrasound contrast media have been developed and used for various organs, e.g. kidney, brain or myocardium . Given the sensitivity of contrast ultrasound, it is not basically inferior to other imaging techniques for quantifying perfusion, although it is challenging technically as well as theoretically. As with any technique, the examiner will also use his or her own judgment regarding the homogeneity of contrast enhancement or the comparison with adjacent normal tissue. In many instances this may be sufficient to serve clinical needs. All further quantification requires that the images be recorded digitally and analyzed in a post-processing session. This is indispensable whenever lesions in different patients are compared, or for longitudinal studies.
Usually, a dynamic series is acquired over a given period of time, while care must be taken to keep the probe in a constant position. The perfusion curves so derived usually show a typical, early rise in signal intensity, a short maximum, and a slow, exponential decay after a bolus injection. Such curves can be characterized by descriptive terms such as the time to maximum, maximal intensity, initial slope, area under the curve, etc. These parameters are well reproducible, but only indirect descriptors for perfusion or blood volume. They are not meaningless, however, considering that, for example, the maximal intensity is a measure for the relative blood volume in the scanned tissue. Therefore, it is legitimate to use such parameters, as has been done for brain or kidney perfusion , for example, or for follow-up of tumors.
For quantitative studies, the examination settings need to be held constant, including the machine settings, injection protocols and acoustic conditions (e.g. insonation window), which may be difficult in some instances. Furthermore, there must be a defined correlation between the ultrasound signal parameters used and the contrast medium concentration in tissue. Therefore, the video signals (gray or color values) need to be antilogged. Some of the on-platform evaluation tools delivered by some manufacturers do already fulfill this requirement.
To quantify absolute perfusion parameters, or parameters which are proportional to the blood flow in the examined region, replenishment kinetics are used . Here, a high-MI pulse is used to destroy all microbubbles contained in the examined slice and follows the increase in signal intensity as fresh microbubbles enter the slice from the adjacent tissue, with the transducer kept strictly in place. Such a protocol consists of defined pulse sequences of an initial high-power pulse and ensuing weaker pulses for eliciting non-linear interactions with the re-entering contrast agent. Put simply, the upslope of the replenishment curve is determined by the mean blood flow velocity inside the vessels in the region. The first model to describe this process was published in 1998 by Wei et al. and served to calculate mean blood flow (ml /s), blood volume (ml), and mean blood velocity (m /s). The theoretical curve was an exponential one, which approximated a maximal plateau in an asymptotic fashion (A=A0[1−exp(−βτ)], with A= ultrasound signal intensity, A0= plateau, β= measure for initial upslope, τ = time after destruction). This function is implemented on some ultrasound platforms and may help to quickly calculate perfusion parameters.
To date, this model has been used to quantify perfusion for myocardium , kidney , brain parenchyma , or tumors . However, it has some shortcomings in that it assumes a constant inflow velocity. New models have been developed which more precisely describe the process of replenishment . These may also help to determine whether the blood velocities inside the vessels in the slice are uniform or not, which may be important to assess functional changes, e.g. in tumors. These models are also suitable for the examination of complex vascular systems such as that found in the liver . However, such analyses are not implemented on US platforms, nor are they available commercially.
All replenishment kinetics rely on the assumption that the concentration of bubbles in the blood is constant, which can only be achieved with a constant infusion of contrast agent. Whenever this is given as an intravenous bolus, the signal in the tumor has to be recorded after a first injection, to obtain a curve which reflects the systemic arrival and degradation of microbubbles. From this curve, time-dependent normalization factors have to be extracted, which are then to be applied to the values measured after a second injection, where the replenishment experiment itself is performed.
Perfusion studies in tumors
With any imaging modality, the arrival of contrast agent indicates that there is viable tissue present, be it healthy tissue or tumor. As a consequence, contrast uptake has been shown to decrease when a tumor responds to chemotherapy . Furthermore, as is known from histological studies, a high vessel density correlates with more aggressive tumor behavior .
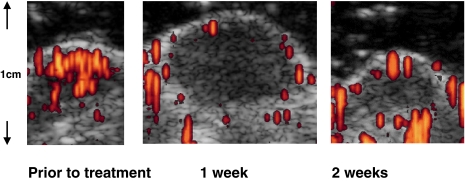
In experimental studies, quantitative contrast ultrasound was used to monitor anti-angiogenic treatment using a VEGF receptor antibody in a murine model. Here, contrast ultrasound showed a marked decline in tumor perfusion induced by the antibody, which preceded a regression in tumor size (Fig. 1) . This matched the results obtained with dynamic contrast-enhanced MRI.
Figure 1.
Subcutaneous heterotransplant of human squamous cell carcinoma cells in nude mice treated with a blocking antibody to vascular endothelial growth factor receptor 2 (DC101), which were repeatedly examined at weekly intervals. At contrast-enhanced intermittent ultrasound the small tumor is highly vascularized at the offset. After 1 week, the tumor has grown, but shows almost no blood flow inside. The final scan, after 2 weeks, shows that the tumor has shrunk and the blood flow is still absent. From , with permission.
Studies in the liver first showed that liver metastases frequently demonstrate a strong and short-lasting enhancement during the arterial phase, which was not visible in CT or MRI studies. The most likely explanation is that US contrast agents exhibit their effect as intravascular compounds, while in CT and MRI the contrast agent needs to fill the interstitial space to exhibit a visible effect. The phase of arterial arrival of contrast agent in liver metastases is probably too short to permit a significant extravasation of contrast medium. In the phase of contrast agent delivery to the liver via the portal vein, however, liver metastases behave as in CT or MRI. Since they lack significant portal blood supply, they appear dark within a bright liver—with the important difference that in contrast-enhanced ultrasound (CE-US) the contrast between liver and lesions is far higher than in CT or MRI, which facilitates the detection. This may be beneficial for finding small lesions, in the range of 1 cm or below . The race between CE-US and CT or MRI is still ongoing. It appears that US is not inferior; however, any premature statement that CE-US will obviate the need for CT or MRI in the workup of cancer patients must be strongly objected to since cancer is a disease of the whole person, not just the liver.
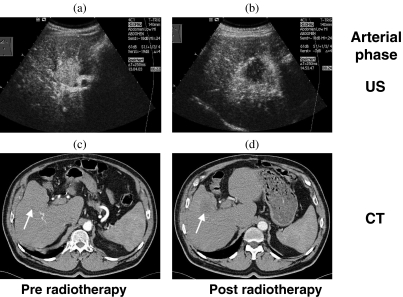
With the ability to assess the arterial phase of perfusion in liver neoplasms, the examiner holds in his or her hands an invaluable tool for judging the tumor’s biological behavior and reactions to treatment which has so far not been available with other methods. In liver metastases treated with stereotactic radiotherapy, for example, we can study how arterial perfusion decreases as a consequence of the sterilization of the tissue (Fig. 2) . In another, as yet unpublished study on liver metastases treated with chemotherapy, it was found that before initiating treatment the arterial contrast enhancement was stronger in later responders than in non-responders. During treatment, it decreased in responders and remained unchanged in non-responders.
Figure 2.
The arterial perfusion of the liver and a metastasis of colon carcinoma before stereotactic radiotherapy (a, c) and 5 months later (b, d), visualized by low-MI sonography after injection of 2.4 ml SonoVue i.v. (a, b) and by contrast-enhanced computed tomography (CT) (b, d). In contrast to CT (130 ml Ultravist 300 i.v.), contrast-enhanced ultrasound (CEUS) clearly shows a high arterial enhancement of the untreated liver metastasis, its reduction after therapy and an arterially hypervascularized perifocal liver reaction after therapy. From , with permission.
In conclusion, contrast-enhanced ultrasound is a promising method to examine tumor blood flow, as a surrogate parameter of tumor vitality and its response to treatment. The mathematical models required to precisely calculate perfusion, however, are highly challenging and require strict protocols, skilled examiners, and computational power. Evaluation tools implemented on newer machines, cumbersome as they still are, raise considerable hope that perfusion assessment may be possible in a clinical setting in the future.
References
- 1.de Jong N, Frinking PJ, Bouakaz A, Goordern M, Schourmans T, Jingping X, et al. Optical imaging of contrast agent microbubbles in an ultrasound field with a 100-MHz camera. Ultrasound Med Biol. 2000;26:487–92. doi: 10.1016/s0301-5629(99)00159-3. [DOI] [PubMed] [Google Scholar]
- 2.Blomley M, Albrecht T, Cosgrove D, Jayaram V, Butler-Barnes J, Eckersley R. Stimulated acoustic emission in liver parenchyma with Levovist. Lancet. 1998;351:568. doi: 10.1016/S0140-6736(05)78554-8. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht T, Blomley MJK, Heckemann RA, Cosgrove DO, Jayaram V, Patel N, et al. Stimulierte akustische Emission mit dem Ultraschallkontrastmittel Levovist: ein klinisch nutzbarer Effekt mit leberspezifischen Eigenschaften. Fortschr Röntgenstr. 2000;172:61–7. doi: 10.1055/s-2000-11101. [DOI] [PubMed] [Google Scholar]
- 4.Burns PN. Harmonic imaging with ultrasound contrast agents. Clin Radiol. 1996;51(1):50–55. [PubMed] [Google Scholar]
- 5.Hope Simpson D, Burns PN. Pulse inversion Doppler: a new method for detecting nonlinear echoes from microbubble contrast agents. IEEE Trans Ultrason Ferroelec Freq Contr. 1999;46:372–82. doi: 10.1109/58.753026. [DOI] [PubMed] [Google Scholar]
- 6.Albrecht T, Hoffmann CW, Schettler S, Overberg A, Ilg M, von Behren PL, et al. B-mode enhancement at phase-inversion US with air-based microbubble contrast agent: initial experience in humans. Radiology. 2000;216:273–8. doi: 10.1148/radiology.216.1.r00jl27273. [DOI] [PubMed] [Google Scholar]
- 7.Albrecht T, Blomley MJ, Burns PN, Wilson S, Harvey CJ, Leen E, et al. Improved detection of hepatic metastases with pulse-inversion US during the liver-specific phase of SHU 508A: multicenter study. Radiology. 2003;227:361–70. doi: 10.1148/radiol.2272011833. [DOI] [PubMed] [Google Scholar]
- 8.Albrecht T, Hoffmann CW, Schmitz SA, Schettler S, Overberg A, Germer CT, et al. Phase-inversion sonography during the liver-specific late phase of contrast enhancement: improved detection of liver metastases. AJR Am J Roentgenol. 2001;176:1191–8. doi: 10.2214/ajr.176.5.1761191. [DOI] [PubMed] [Google Scholar]
- 9.Bryant TH, Blomley MJ, Albrecht T, Sidhu PS, Leen EL, Basilico R, et al. Improved characterization of liver lesions with liver-phase uptake of liver-specific microbubbles: prospective multicenter study. Radiology. 2004;232:799–809. doi: 10.1148/radiol.2323030596. [DOI] [PubMed] [Google Scholar]
- 10.Hohmann J, Skrok J, Puls R, Albrecht T. Charakterisierung fokaler Leberläsionen mit kontrastmittelgestütztem ‘low MI real-time’. Ultraschall und SonoVue Fortschr Röntgenstr. 2003;175:835–43. doi: 10.1055/s-2003-39923. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich CF. Characterisation of focal liver lesions with contrast enhanced ultrasonography. Eur J Radiol. 2004;51((Suppl)):S9–17. doi: 10.1016/j.ejrad.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 12.Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, et al. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232:420–30. doi: 10.1148/radiol.2322031401. [DOI] [PubMed] [Google Scholar]
- 13.Nicolau C, Catala V, Vilana R, Gilabert R, Bianchi L, Sole M, et al. Evaluation of hepatocellular carcinoma using SonoVue, a second generation ultrasound contrast agent: correlation with cellular differentiation. Eur Radiol. 2004;14:1092–9. doi: 10.1007/s00330-004-2298-0. [DOI] [PubMed] [Google Scholar]
- 14.Skjoldbye B, Pedersen MH, Struckmann J, Burcharth F, Larsen T. Improved detection and biopsy of solid liver lesions using pulse-inversion ultrasound scanning and contrast agent infusion. Ultrasound Med Biol. 2002;28:439–44. doi: 10.1016/s0301-5629(02)00484-2. [DOI] [PubMed] [Google Scholar]
- 15.Solbiati L, Ierace T, Tonolini M, Cova L. Guidance and monitoring of radiofrequency liver tumor ablation with contrast-enhanced ultrasound. Eur J Radiol. 2004;51((Suppl)):S19–23. doi: 10.1016/j.ejrad.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 16.Weidner NR, Semple JP, Welch WR. Tumor angiogenesis and metastasis: correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 17.Wasser K, Klein SK, Fink C, Junkermann H, Sinn HP, Zuna I, et al. Evaluation of neoadjuvant chemotherapeutic response of breast cancer using dynamic MRI with high temporal resolution. Eur Radiol. 2003;13:80–87. doi: 10.1007/s00330-002-1654-1. [DOI] [PubMed] [Google Scholar]
- 18.Delorme S, Peschke P, Zuna I, van Kaick G. Sensitivity of color Doppler sonography: an experimental approach. Ultrasound Med Biol. 1999;25:541–7. doi: 10.1016/s0301-5629(98)00186-0. [DOI] [PubMed] [Google Scholar]
- 19.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97:473–83. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- 20.Wei K, Le E, Bin JP, Coggins M, Thorpe J, Kaul S. Quantification of renal blood flow with contrast-enhanced ultrasound. J Am Coll Cardiol. 2001;37:1135–40. doi: 10.1016/s0735-1097(00)01210-9. [DOI] [PubMed] [Google Scholar]
- 21.Seidel G, Claassen L, Meyer K, Vidal-Langwasser M. Evaluation of blood flow in the cerebral microcirculation: analysis of the refill kinetics during ultrasound contrast agent infusion. Ultrasound Med Biol. 2001;27:1059–64. doi: 10.1016/s0301-5629(01)00416-1. [DOI] [PubMed] [Google Scholar]
- 22.Lefevre F, Correas JM, Briancon S, Helenon O, Kessler M, Claudon M. Contrast-enhanced sonography of the renal transplant using triggered pulse-inversion imaging: preliminary results. Ultrasound Med Biol. 2002;28:303–14. doi: 10.1016/s0301-5629(01)00526-9. [DOI] [PubMed] [Google Scholar]
- 23.Krix M, Kiessling F, Vosseler S, Farhan N, Mueller MM, Bohlen P, et al. Sensitive non-invasive monitoring of tumor perfusion during antiangiogenic therapy by intermittent, bolus-contrast power Doppler sonography. Cancer Res. 2003;63:8264–70. [PubMed] [Google Scholar]
- 24.Lucidarme O, Franchi-Abella S, Correas JM, Bridal SL, Kurtisovski E, Berger G. Blood flow quantification with contrast-enhanced US: ‘entrance in the section’ phenomenon: phantom and rabbit study. Radiology. 2003;228:473–9. doi: 10.1148/radiol.2282020699. [DOI] [PubMed] [Google Scholar]
- 25.Krix M, Kiessling F, Farhan N, Schmidt K, Hoffend J, Delorme S. A multivessel model describing replenishment kinetics of ultrasound contrast agent for quantification of tissue perfusion. Ultrasound Med Biol. 2003;29:1421–30. doi: 10.1016/s0301-5629(03)01033-0. [DOI] [PubMed] [Google Scholar]
- 26.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 27.Delorme S, Krix M, Albrecht T. Ultraschallkontrastmittel: Grundlagen und klinische Anwendung. Fortschr Geb Rontgenstr Nuklearmed. 2005;178:155–64. doi: 10.1055/s-2005-858648. [DOI] [PubMed] [Google Scholar]
- 28.Krix M, Plathow C, Essig M, Herfarth K, Debus J, Kauczor HU, et al. Monitoring of liver metastases after stereotactic radiotherapy using low-MI contrast-enhanced ultrasound: initial results. Eur Radiol. 2005;15:677–84. doi: 10.1007/s00330-004-2620-x. [DOI] [PubMed] [Google Scholar]