Abstract
Conventional, single-slice helical computed tomography (SSCT) allowed for scanning the majority of the liver during the critical portal venous phase. This was often referred to as the ‘optimal temporal window’. The introduction of current day multislice CT (MSCT) now allows us to acquire images in a much shorter time and more precisely than ever before. This yields increased conspicuity between low attenuation lesions and the enhanced normal liver parenchyma and optimal imaging for the vast majority of hepatic hypovascular metastases. Most importantly, these scanners, when compared to conventional non-helical scanners, avoid impinging upon the ‘equilibrium’ phase when tumors can become isodense/invisible. MSCT also allows for true multiphase scanning during the arterial and late arterial phases for detection of hypervascular metastases. The MSCT imaging speed has increased significantly over the past years with the introduction of 32- and 64-detector systems and will continue to increase in the future volumetric CT. This provides a number of important gains that are discussed in detail.
Keywords: MSCT, liver metastasis, CT technology
Introduction
The development of multislice computed tomography (MSCT) technology represents a substantial technological advance in computed tomography (CT). The dramatic four-, eight-, and sixteen-fold increase in imaging speed affords several benefits: (1) makes the examination more comfortable; (2) provides a higher quality of examination with improved liver lesion conspicuity; (3) provides scanning with thinner sections; (4) gives increased flexibility in scanning during multiple phases of hepatic enhancement; and (5) offers the ability to perform exquisite three-dimensional (3D) vascular imaging.
Discussion
When conventional single-slice helical CT (SSCT) is used there is continuous data acquisition. This is related to the intrinsic technology of the scanner that consists of a detector made up of an array of rectangular channels. With the evolution to MSCT scanners, manufacturers have developed different arrays which, instead of being long and rectangular, are divided into matrices that generally fall into two different categories: one group has detector elements of equal width along the z-axis (matrix detectors) and the other group has detector elements of unequal width (adaptive array detectors) acquisition of multiple slices.
There are specific clinical advantages in assessing both primary and metastatic liver disease using MSCT. Two clinical applications are directly obvious: first, to increase coverage using the same thickness as with earlier scanners, and, second, to generate much thinner sections with similar anatomical coverage. Hybrid approaches yield a combination thinner sections and greater anatomic coverage.
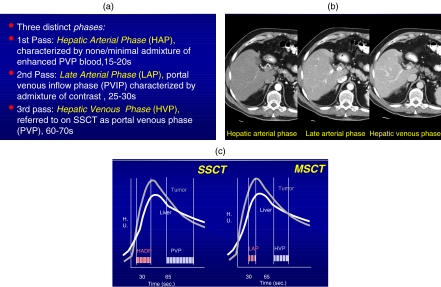
The options and methods of scanning the liver are numerous with no one singular approach being definitive; often different approaches are appropriate when specific disease entities are suspected. In conventional (SSCT), the two major phases of a liver study are the hepatic arterial phase (HAP) and the portal venous phase (PVP) (Fig. 1). Scans can also be performed pre-contrast and in the equilibrium phase (limited value, i.e. cholangiocarcinoma). For most protocols, a scan delay of 60–70 s after initiating contrast injection is appropriate using conventional rates of contrast injection (2–4 ml /s). At faster rates, earlier scanning may be desired. One option to a fixed delay time is to use computer-assisted scanning technology (CAST) and begin scanning at a 50 HU threshold to optimize hitting the peak liver enhancement (SmartPrep, GE, General Electric Medical Systems, Milwaukee, WI) and assure scanning at the optimal PVP (Fig. 2). In some cases, adequate hepatic enhancement has been reported with iodine doses that are 25% lower than conventional CT using such technology. This is especially important in certain economic climates. If a patient’s weight is taken into account, an even more pronounced reduction in contrast of up to 40% can be achieved resulting in significant cost savings.
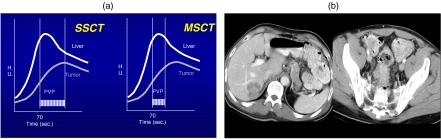
Figure 1.
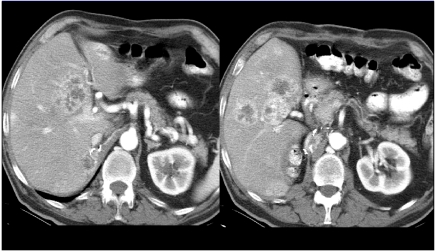
(a) A comparison of single slice CT (SSCT) and multislice CT (MSCT) for hypovascular liver metastases. MSCT allows for a much faster scanning within the optimal portal venous phase (PVP) allowing for images to capture the optimal contrast enhancement. (b) Hypovascular liver metastases in the right lobe of the liver and the associated primary tumor in the sigmoid colon.
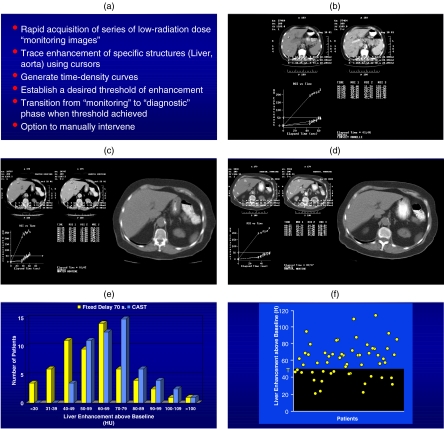
Figure 2.
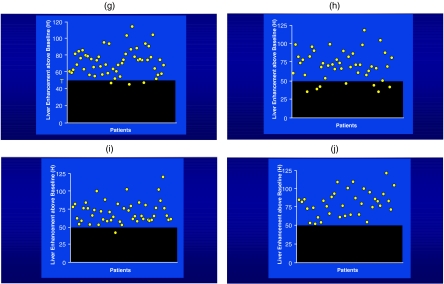
(a) Computer automated scanning technology (CAST) . (b) Graph showing the aortic and liver enhancement curves with a threshold set of 50 HU for the liver. (c) Graphic demonstrating good contrast enhancement with a rapid rise of liver enhancement to the threshold. (d) Same patient as in (c) except following chemotherapy where the rise of the liver enhancement is attenuated due to the poor clinical status of the patient. (e) Patient distribution showing better liver enhancement overall when CAST technology is used. (f) Graphic demonstration showing the number of patients above and below the threshold of 50 HU of enhancement relatively equally distributed. (g) With the use of CAST, this allows many more patients to have a contrast enhancement greater than 40 HU and, therefore, better liver lesion contrast. (h) The use of a fixed delay with a contrast load of 150 ml (300 mg I /ml) of contrast showing approximately two-thirds of patients above the threshold of 50 HU. (i) When 25 ml less contrast is given, an equivalent quality study can be achieved using CAST. (j) When 150 ml of contrast is used, the contrast enhancement of the liver is optimal with all the patients achieving more than 50 HU of enhancements.
Contrast dynamics
The liver, because of its unique dual blood supply, 20% from the hepatic artery and 80% from the portal venous system, remains just as much or more of a challenge for optimizing protocols in the current era of MSCT. The previously termed PVP for SSCT has now been more appropriately named the hepatic venous phase (HVP) on MSCT as this phase captures the opacification of these veins and maximal liver enhancement . This is the only phase necessary to image the vast majority of metastatic disease to the liver. It includes common neoplasms such as lung, colon, lymphoma, and genitourinary tumors . The main impact of MSCT has been on the ability to now examine an organ such as the liver in multiple phases of contrast dynamics with the hope of allowing increased detection of lesions as well as improved lesion characterization. The reason for imaging during an earlier phase of contrast is to improve the detectability of tumors which are hypervascular . This relates to the study of primary neoplasms, i.e. hepatocellular carcinoma (HCC), as well as metastatic disease. The most common hypervascular tumors that benefit from the more comprehensive examination include neuroendocrine tumors, melanoma, sarcoma, carcinoid, renal cell, thyroid, and choriocarcinoma . Although breast carcinoma may be hypervascular, it generally has peripheral rather than dense focal enhancement and can be seen effectively during standard imaging. Optimizing protocols for multiphasic imaging includes arterial phase(s) to the HVP (i.e. dual phase imaging) and/or inclusion of a very early arterial phase for 3D imaging of the vascular system (i.e. triple-phase imaging). In contrast to SSCT, MSCT is able to define three rather than just two distinct phases of contrast enhancement. With SSCT, the two phases are hepatic arterial dominant phase (HADP) and the PVP; with MSCT these phases have been termed the HAP, late arterial phase (LAP) or portal venous inflow phase (PVIP), and a HVP (Figs. 3 and 4). The first two phases were incorporated in the HADP described with SSCT. The ability to rapidly scan with MSCT allows one to separate these and scan in two phases what could only be done in one phase previously. Hypervascular lesions, either primary or metastatic, are usually best seen in the LAP; however, some lesions are seen only in either the HAP or LAP phases (Figs. 5 and 6). Hypervascular lesions have always presented a challenge to the radiologist. Failing to image hypervascular lesions during the HAP results in an insensitive examination similar to failing to image hypovascular lesions in the PVP. The HAP is best identified 10–20 s after the administration of contrast and is characterized by enhancement of the hepatic artery. The LAP is best identified 25–30 s after injection and shows enhancement of the hepatic artery and some enhancement of the portal venous structures. The HVP is marked by opacification of the hepatic veins at the dome of the liver. The speed results in one of the most important challenges in developing optimized protocols for this new, robust technology. Although multiphasic studies could be performed with helical scanners, high-quality, whole organ imaging with multiple phases awaited the introduction of MSCT.
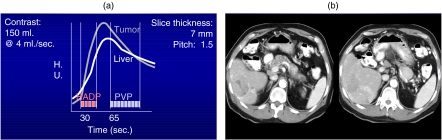
Figure 3.
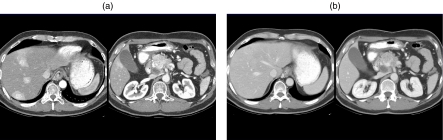
(a) Graph showing the hepatic arterial dominant phase (HADP) and the portal venous phase (PVP) using dual phase scanning. (b) Patient with a hypervascular metastasis from an Islet cell tumor of the pancreas showing the early HADP phase on the left and the later PVP phase on the right.
Figure 4.
(a) Definitions of the various contrast phases using MSCT. (b) Demonstration of contrast dynamics during the HAP, LAP, and HVP stages showing initially hepatic arterial enhancement while the second phase shows a mixture of hepatic artery and portal venous enhancement and the latest phase showing good portal venous enhancement. (c) The most effective terminology for MSCT is the late arterial phase (LAP) and the second phase, the hepatic venous phase (HVP) replacing the previous terminology (HADP and PVP).
Figure 5.
A patient with multiple metastases to the liver from renal cell carcinoma (RCC) demonstrating hypervascular lesions.
Figure 6.
(a) Liver metastases in a patient with Islet cell tumor show the dense hypervascular enhancement of multiple liver metastases. Scan is performing the corticomedullary (CM) phase of the kidneys. (b) Scan later during a nephrogram phase; this shows that the earlier hypervascular metastases have now essentially disappeared.
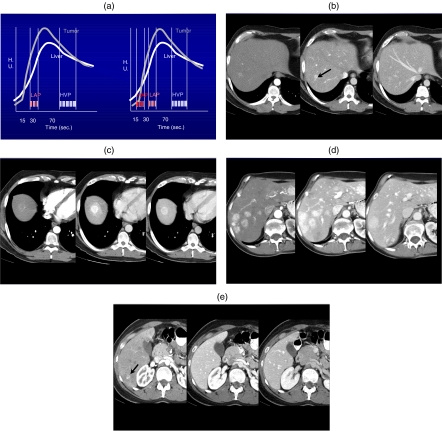
The addition of the HAP component initiated at 15–20 s into the study can increase lesion detection by 8–13% compared to PVP imaging alone (Fig. 7). Thinner collimations (5, 3.75, and 2.5 mm) can detect more lesions but these often remain indeterminate because of their extremely small size. In one series where images of the liver were reconstructed with overlapping collimation, 7% more lesions could be detected. An early arterial phase has the additional value of producing superb 3D imaging of the vascular system depicting hepatic arterial anatomy preoperatively. It is also of value in assessing patients who are candidates for intra-arterial chemotherapy. This flexibility of multislice scanning generally avoids more invasive techniques, i.e. angiographic CT arterial portography (CTAP).
Figure 7.
(a) Triple phase scanning employing MSCT. (b) Tri-phasic scanning in a patient with medullary thyroid carcinoma shows the focal metastases best seen in the late arterial phase (LAP). (c) Metastatic thyroid cancer. The lesion is best seen in the LAP but can be seen more subtly on the HAP and HVP phases. (d) Medullary thyroid carcinoma with multiphasic imaging. The lesions are best seen on both the HAP and LAP phases and much more poorly seen on the delayed HVP phase. (e) Hypervascular metastases from carcinoid. The very small sub-centimeter lesion is only identified on the HAP.
Higher concentration contrast in MSCT
Detection of liver lesions is not only dependent on scanning during the phase that optimally distinguishes normal from abnormal tissue as discussed . Optimized imaging requires using adequate amounts of contrast, i.e. grams of iodine. The grams of iodine have a direct impact on the difference in hepatic attenuation relative to lesion detection which defines the relative conspicuity of lesions (normal hepatic attenuation liver lesion attenuation results in lesion conspicuity). Most recently, with the rapid proliferation of MSCT technology, the concept of using higher concentrations of contrast material has begun to be explored (Fig. 8). The impetus for this has been that the standard contrast concentrations of 300–320 mg I /ml have required volumes of the order of 150 ml to deliver adequate grams of iodine as the ‘drug’ necessary to effectively image the liver. This is in contrast to examining other areas of the body such as the chest where the dose and volume of iodinated contrast can be significantly reduced (i.e. 150–100 ml (helical CT) to 60–75 ml (MSCT)). Imaging of liver lesions requires more precise protocols. Studies of the liver with less than optimal contrast enhancement result in compromised lesion detectability.
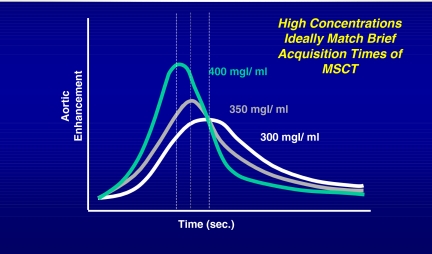
Figure 8.
The graph demonstrates that better contrast enhancement can be achieved when higher concentrations of contrast are being used. In MSCT, the need has shifted from desiring longer contrast enhancement to requiring a greater peak contrast enhancement as scanning can be performed quickly.
Fortunately, to date, prices of contrast material are not directly tied to grams of iodine within the product but are most closely linked with the volume of contrast. Thus, if we can use lower volumes and higher concentrations of contrast, it has the additional benefit of becoming highly cost effective. With SSCT and helical scanning, protocols for body CT require volumes of contrast in the range of 150 ml with 300 and 320 mg I /ml to be able to have optimal enhancement of the liver and also provide adequate enhancement of abdominal and pelvic structures. With MSCT this can be accomplished without requiring these large volumes since scans can be completed rapidly. Thus, it becomes the challenge for radiologists to adopt new protocols to take advantage of this continually evolving technology. Higher concentrations of contrast, 350, 370, and even 400 mg I /ml, have been developed and are being employed clinically. If a target of 37–48 g of iodine is considered to image the liver, then this can be achieved by a number of different permutations of volume and concentration of contrast. Higher concentrations of contrast also allow contrast delivery of the same grams of iodine per second to the target organ at lower rates. For example, the administration of 150 ml of 300 mg I /ml at 5 ml /s delivers an iodine dose of 1.5 g /s, whereas the administration of 100 ml or 370 mg I /ml at only 4 ml /s delivers essentially the same iodine does of 1.48 g /s. The ability to decrease the total volume of contrast will result in overall substantial cost savings in a busy clinical CT service.
Conclusion
The introduction of MSCT has revolutionized the approach to imaging the liver by providing increased flexibility. In order to effectively utilize this technology, protocols designed for imaging hepatic metastatic disease must be carefully constructed.
References
- 1.Berland LL, Lee JY. Comparison of contrast media injection rates and volumes for hepatic dynamic incremented computed tomography. Invest Radiol. 1988;23:918–22. doi: 10.1097/00004424-198812000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Freeny PC. Hepatic CT. State of the art. Radiology. 1988;168:319–23. doi: 10.1148/radiology.168.2.3293101. [DOI] [PubMed] [Google Scholar]
- 3.Hughes KS, Rosenstein RB, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases. A multi-institutional study of long-term survivors. Dis Colon Rectum. 1988;31:1–4. doi: 10.1007/bf02552560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foley WD. Dynamic hepatic CT scanning. AJR Am J Roentgenol. 1989;152:272–4. doi: 10.2214/ajr.152.2.272. [DOI] [PubMed] [Google Scholar]
- 5.Heiken JP, Weyman PJ, Lee JK, et al. Detection of focal hepatic masses: prospective evaluation with CT, delayed CT, CT during arterial portography, and MR imaging. Radiology. 1989;171:47–51. doi: 10.1148/radiology.171.1.2538862. [DOI] [PubMed] [Google Scholar]
- 6.Baron RL. Understanding and optimizing use of contrast material for CT of the liver. AJR Am J Roentgenol. 1994;163:323–31. doi: 10.2214/ajr.163.2.8037023. [DOI] [PubMed] [Google Scholar]
- 7.Baker ME, Pelley R. Hepatic metastases: basic principles and implications for radiologists. Radiology. 1995;197:329–37. doi: 10.1148/radiology.197.2.7480672. [DOI] [PubMed] [Google Scholar]
- 8.Bonaldi VM, Bret PM, Reinhold C, Atri M. Helical CT of the liver: value of an early hepatic arterial phase. Radiology. 1995;197:357–63. doi: 10.1148/radiology.197.2.7480677. [DOI] [PubMed] [Google Scholar]
- 9.Hollett MD, Jeffrey RB Jr, Nino-Murcia M, Jorgensen MJ, Harris DP. Dual-phase helical CT of the liver: value of arterial phase scans in the detection of small (< or = 1.5 cm) malignant hepatic neoplasms. AJR Am J Roentgenol. 1995;164:879–84. doi: 10.2214/ajr.164.4.7726040. [DOI] [PubMed] [Google Scholar]
- 10.Kopka L, Funke M, Fischer U, Vosshenrich R, Oestmann JW, Grabbe E. Parenchymal liver enhancement with bolus-triggered helical CT: preliminary clinical results. Radiology. 1995;195:282–4. doi: 10.1148/radiology.195.1.7892486. [DOI] [PubMed] [Google Scholar]
- 11.Silverman PM, Roberts S, Tefft MC, et al. Helical CT of the liver: clinical application of an automated computer technique, SmartPrep, for obtaining images with optimal contrast enhancement. AJR Am J Roentgenol. 1995;165:73–8. doi: 10.2214/ajr.165.1.7785637. [DOI] [PubMed] [Google Scholar]
- 12.Soyer P, Bluemke DA, Reichle R, et al. Imaging of intrahepatic cholangiocarcinoma: 1. Peripheral cholangiocarcinoma. AJR Am J Roentgenol. 1995;165:1427–31. doi: 10.2214/ajr.165.6.7484579. [DOI] [PubMed] [Google Scholar]
- 13.Soyer P, Bluemke DA, Reichle R, et al. Imaging of intrahepatic cholangiocarcinoma: 2. Hilar cholangiocarcinoma. AJR Am J Roentgenol. 1995;165:1433–6. doi: 10.2214/ajr.165.6.7484580. [DOI] [PubMed] [Google Scholar]
- 14.Oliver 3rd JH, Baron RL. Helical biphasic contrast-enhanced CT of the liver: technique, indications, interpretation, and pitfalls. Radiology. 1996;201:1–14. doi: 10.1148/radiology.201.1.8816509. [DOI] [PubMed] [Google Scholar]
- 15.Silverman PM, Roberts SC, Ducic I, et al. Assessment of a technology that permits individualized scan delays on helical hepatic CT: a technique to improve efficiency in use of contrast material. AJR Am J Roentgenol. 1996;167:79–84. doi: 10.2214/ajr.167.1.8659426. [DOI] [PubMed] [Google Scholar]
- 16.Frederick MG, Paulson EK, Nelson RC. Helical CT for detecting focal liver lesions in patients with breast carcinoma: comparison of noncontrast phase, hepatic arterial phase, and portal venous phase. J Comput Assist Tomogr. 1997;21:229–35. doi: 10.1097/00004728-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Bae KT, Heiken JP, Brink JA. Aortic and hepatic peak enhancement at CT: effect of contrast medium injection rate—pharmacokinetic analysis and experimental porcine model. Radiology. 1998;206:455–64. doi: 10.1148/radiology.206.2.9457200. [DOI] [PubMed] [Google Scholar]
- 18.Berland LL, Smith JK. Multidetector-array CT: once again, technology creates new opportunities. Radiology. 1998;209:327–9. doi: 10.1148/radiology.209.2.9807555. [DOI] [PubMed] [Google Scholar]
- 19.Silverman PM. Philadelphia, PA: Lippincott, Williams & Wilkins; 1998. Helical (Spiral) Computed Tomography: A Practical Approach to Clinical Protocols. [Google Scholar]
- 20.Husband JES, Reznek RH. Oxford: ICIS Medical Media V Ltd; 1998. Imaging in Oncology. [Google Scholar]
- 21.Paulson EK, McDermott VG, Keogan MT, DeLong DM, Frederick MG, Nelson RC. Carcinoid metastases to the liver: role of triple-phase helical CT. Radiology. 1998;206:143–50. doi: 10.1148/radiology.206.1.9423664. [DOI] [PubMed] [Google Scholar]
- 22.Foley WD, Mallisee TA, Hohenwalter MD, Wilson CR, Quiroz FA, Taylor AJ. Multiphase hepatic CT with a multirow detector CT scanner. AJR Am J Roentgenol. 2000;175:679–85. doi: 10.2214/ajr.175.3.1750679. [DOI] [PubMed] [Google Scholar]
- 23.O’Riordan E, Craven CM, Wilson D, Robinson PJ. Dual phase hepatic CT: influence of scanning direction on liver attenuation. AJR Am J Roentgenol. 2000;174:1417–21. doi: 10.2214/ajr.174.5.1741417. [DOI] [PubMed] [Google Scholar]
- 24.2nd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2002. Multislice Computed Tomography (MSCT): A Practical Approach to Clinical Protocols. [Google Scholar]