Abstract
Morphology as demonstrated by CT is the basis for radiotherapy planning. Intensity-modulated and adaptive radiotherapy techniques would greatly benefit from additional functional information allowing for definition of the biological target volume. MRI techniques include several which can characterize and quantify different tissue properties and their tumour-related changes. Results of perfusion MRI represent microvascular density and permeability; MR spectroscopy depicts particular metabolites; diffusion weighted imaging shows tissue at risk and tumour cellularity; while dynamic 3D acquisition (4D MRI) shows organ motion and the mobility of tumours within them.
Keywords: Magnetic resonance imaging (MRI), functional imaging, MR perfusion, MR spectroscopy, radiotherapy planning, biological target volume
Introduction
Radiotherapy planning relies on visualization of the morphology of tumours and the surrounding normal tissue. The basic workhorse for radiotherapy planning is CT because of its well-known ability to demonstrate structure with high spatial resolution and important information about the radiodensity of the tissues. Recent and current developments in radiotherapy such as intensity-modulated and adaptive techniques would greatly benefit from image-based ‘functional’ information of tumour heterogeneity beyond structure. Magnetic resonance imaging (MRI) provides numerous techniques for image-based surrogates of different facets of function. The following pairs of topics and techniques will be covered in this review: angiogenesis—perfusion MRI; metabolism—MR spectroscopy; tissue at risk and tumour cellularity—diffusion weighted imaging; and motion—4D MRI.
Angiogenesis—perfusion MRI
Contrast-enhanced dynamic MRI provides information about tissue perfusion. Such techniques have been applied to many different tumours, such as cancers of the breast, prostate, cervix, rectum and liver, as well as gliomas, pulmonary nodules and multiple myeloma. From signal intensity time curves descriptive parameters such as lag time, amplitude, slope and area under the curve (wash-out) can be calculated. They are determined by perfusion and flow and related to microvascular density and permeability. The results can be improved and perfusion can be quantified by the introduction of pharmacokinetic compartmental models. The models provide results for amplitude and exchange rate and finally lead to quantification for regional blood volume, regional blood flow and mean transit time. They can take the different properties of the contrast agents and the tissue of interest into consideration . Depending on indication T1- or T2 *-weighted sequences will be preferred. Some assumptions have to be made to make the handling of these models feasible. However, in case of different tissue properties the results might not be correct. It has been demonstrated that the amplitude of the signal correlates with the microvascular density of the tumour whereas the exchange rate points towards an increased permeability of the tumour vasculature (Fig. 1) . These parameters are also helpful to assess the angiogenic potential of tumours and are well-suited to follow-up particular therapies with anti-angiogenic compounds such as thalidomide for the treatment of multiple myeloma . Perfusion MRI is also capable of predicting outcome in patients with cerebral metastases who have already undergone stereotactic radiotherapy at 6 week follow-up . Perfusion can also be assessed by non-contrast techniques such as arterial spin labelling (Fig. 2).
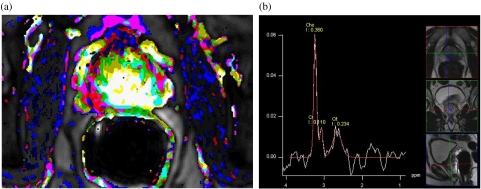
Figure 1.
Prostate cancer. (a) Parameter image of perfusion and permeability with the cancer showing increased values given as light colours representing gross tumour extension at pathology. (b) MR spectroscopy shows elevated choline and decreased citrate and creatine indicating the adenocarcinoma seen as a hypointense area in the T2-weighted planning sequences. This area also corresponds to the area of increased perfusion and permeability (a).
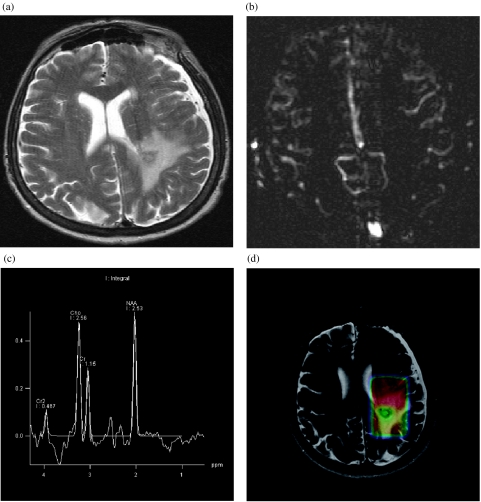
Figure 2.
Anaplastic glioma. (a) T2-weighted turbo-spin-echo image shows the tumour in the left parietal lobe. (b) Perfusion map obtained by arterial spin labelling, a non-contrast technique, shows tumour blood flow not elevated compared to grey matter. (c) Single voxel spectroscopy from tumour tissue shows an increase of Cho indicating increased cellularity and cell membrane synthesis. (d) Colour-coded map of the ratio of N-acetyl-aspartate (NAA)/creatine superimposed on the T2-weighted TSE image shows reduced NAA signal suggesting loss of normal neural elements.
Metabolism—MR spectroscopy
MR spectroscopy (MRS) can provide metabolic information about tumour cells and the surrounding tissue. Shifts in the distribution of certain metabolites provide important hints towards both differential diagnosis and the tumour biological behaviour (Fig. 2). Clinical indications are mainly suspicious intracranial masses and prostate cancer. In the brain, MRS is an important adjunct in the diagnosis of gliomas, e.g. separating them from metastases. After radiotherapy, it is very helpful in differentiating recurrence from radionecrosis , where the appearance of lipid peaks is related to necrosis. In the prostate the cancerous region typically does not contain citrate which is a metabolic product of the normal prostate gland. The loss of citrate or an increase in the choline/citrate ratio is an important indicator for prostate cancer (Fig. 1) . Since citrate is also present in benign hyperplastic nodules these two entities can be separated.
Tissue at risk and tumour cellularity—diffusion weighted imaging
Diffusion weighted imaging allows for localization and visualization of axonal tracts using 3D fibre reconstruction called tractography . The displacement of important neural structures, e.g. the pyramidal tract, by a large mass can be exactly localized , and taken into account when the planned target volume is defined (Fig. 3). In a study involving 20 patients exact knowledge of the course of the axonal tracts led to a change of radiotherapy planning in 71%. In this way, the volume receiving less than 15 Gy could be significantly reduced, while the volume encompassed by the 80% isodose was unchanged .
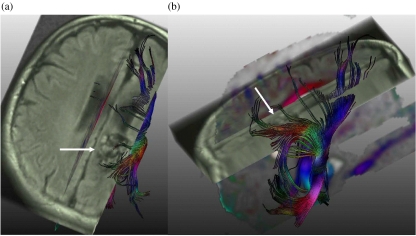
Figure 3.
Intracerebral arteriovenous malformation (AVM). (a) Tractography calculated from diffusion weighted imaging and registered on an axial FLAIR image shows the AVM with flow voids (arrow) and fibres of the corona radiata, including the cortico-spinal tract, which abuts on the AVM. (b) Sagittal view of the complete result of the tractography which superimposes the AVM (arrow).
As an increase in tumour cellularity due to rapid growth leads to a decrease in apparent diffusion constant (ADC) 2D depiction of fibre integrity, e.g. fractional anisotropy, at high b-values can be used to measure tissue vitality. In primary brain tumours, the delineation of the target volume is impeded due to incomplete depiction of the tumour border in conventional MRI. It is known that these tumours grow along the axonal projections, in part displacing, in part infiltrating these structures. Thus, measures from diffusion weighted imaging, such as fractional anisotropy, can be used to assess fibre integrity and have been postulated as sensitive markers for overall tumour extent ; however, research has so far been hampered by limitations in data analysis. Current research is evaluating the role of DTI in predicting tumour extent and shows promising initial results for improved tumour front delineation in patients with primary brain tumours .
Motion—4D MRI
Dynamic three-dimensional (4D) MRI is an attractive means of imaging organ motion and the mobility of tumours over time. This information is especially important for respiratory motion and lung tumours when high precision radiotherapy is used (Fig. 4). Pilot studies of lung tumour mobility used 2D sequences with a temporal resolution of approximately 300 ms. The mobility of lung nodules in X-, Y- and Z-directions was significantly affected by tumour size and location . Obviously, mobility was greatest for small nodules in the caudal lung regions and cranio-caudal direction during deep breathing . However, even during shallow breathing tumour mobility could be substantial. Benefiting from new MR developments such as parallel imaging and view sharing the temporal resolution of a three-dimensional data set could be reduced to 1 s. This makes the 3D registration of lung nodules during continuous breathing feasible . Theoretical calculations show that the integration of such information into radiotherapy planning will result in a substantial increase of the dose that can be delivered to the planned target volume. Parallel assessment of chest wall motion detected by external MR markers and internal tumour motion followed by MRI represents one way of triggering radiotherapy .
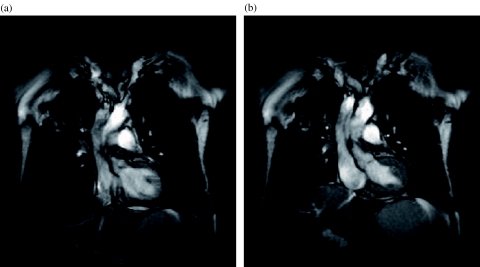
Figure 4.
Lung cancer. (a) Inspiratory image from a dynamic trueFISP sequence obtained with a temporal resolution of three images per second shows the tumour in the right upper lobe with a small clip artifact within the tumour. (b) Expiratory image from the same series shows the displacement of the tumour and also indicates the lack of chest wall infiltration.
Conclusion
Besides mere volumetric visualization of morphology and structure, MRI is also capable of providing ‘functional’ information which can be used to define individual biological target volumes.
References
- 1.Furman-Haran E, Degani H. Parametric analysis of breast MRI. J Comput Assist Tomogr. 2002;26:376–86. doi: 10.1097/00004728-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Kiessling F, Huber PE, Grobholz R, Heilmann M, Meding J, Lichy MP, et al. Dynamic magnetic resonance tomography and proton magnetic resonance spectroscopy of prostate cancers in rats treated by radiotherapy. Invest Radiol. 2004;39:34–44. doi: 10.1097/01.rli.0000095472.37056.0b. [DOI] [PubMed] [Google Scholar]
- 3.Kiessling F, Farhan N, Lichy MP, Vosseler S, Heilmann M, Krix M, et al. Dynamic contrast-enhanced magnetic resonance imaging rapidly indicates vessel regression in human squamous cell carcinomas grown in nude mice caused by VEGF receptor 2 blockade with DC101. Neoplasia. 2004;6:213–23. doi: 10.1593/neo.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wasser K, Moehler T, Neben K, Nosas S, Heiss J, Goldschmidt H, et al. Dynamische MRT des Knochenmarks zum Monitoring des Multiplen Myeloms unter Thalidomid-Monotherapie oder Kombination mit CED-Chemotherapie. Rofo. 2004;176:1285–95. doi: 10.1055/s-2004-813414. [DOI] [PubMed] [Google Scholar]
- 5.Weber MA, Thilmann C, Lichy MP, Gunther M, Delorme S, Zuna I, et al. Assessment of irradiated brain metastases by means of arterial spin-labeling and dynamic susceptibility-weighted contrast-enhanced perfusion MRI: initial results. Invest Radiol. 2004;39:277–87. doi: 10.1097/01.rli.0000119195.50515.04. [DOI] [PubMed] [Google Scholar]
- 6.Lichy MP, Plathow C, Schulz-Ertner D, Kauczor HU, Schlemmer HP. Follow-up gliomas after radiotherapy: (1)H MR spectroscopic imaging for increasing diagnostic accuracy. Neuroradiology. 2005;47:826–34. doi: 10.1007/s00234-005-1434-0. [DOI] [PubMed] [Google Scholar]
- 7.van Dorsten FA, van der Graaf M, Engelbrecht MR, van Leenders GJ, Verhofstad A, Rijpkema M, et al. Combined quantitative dynamic contrast-enhanced MR imaging and (1)H MR spectroscopic imaging of human prostate cancer. J Magn Reson Imaging. 2004;20:279–87. doi: 10.1002/jmri.20113. [DOI] [PubMed] [Google Scholar]
- 8.Stieltjes B, Kaufmann WE, van Zijl PC, Fredericksen K, Pearlson GD, Solaiyappan M, et al. Diffusion tensor imaging and axonal tracking in the human brainstem. Neuroimage. 2001;14: 723–35. doi: 10.1006/nimg.2001.0861. [DOI] [PubMed] [Google Scholar]
- 9.Mori S, Kaufmann WE, Davatzikos C, Stieltjes B, Amodei L, Fredericksen K, et al. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med. 2002;47:215–23. doi: 10.1002/mrm.10074. [DOI] [PubMed] [Google Scholar]
- 10.Aoyama H, Kamada K, Shirato H, Takeuchi F, Kuriki S, Iwasaki Y, et al. Integration of functional brain information into stereotactic irradiation treatment planning using magnetoencephalography and magnetic resonance axonography. Int J Radiat Oncol Biol Phys. 2004;58:1177–83. doi: 10.1016/j.ijrobp.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 11.Provenzale JM, McGraw P, Mhatre P, Guo AC, Delong D. Peritumoral brain regions in gliomas and meningiomas: investigation with isotropic diffusion-weighted MR imaging and diffusion-tensor MR imaging. Radiology. 2004;232:451–60. doi: 10.1148/radiol.2322030959. [DOI] [PubMed] [Google Scholar]
- 12. Stieltjes B, Schlüter M, Didinger B, Weber M-A, Hahn HK, Parzer P, Diffusion tensor imaging in primary brain tumors: reproducible quantitative analysis of corpus callosum infiltration and contralateral involvement using a probabilistic mixture model. Neuroimage (in press). Available online at: doi:10.1016/j.neuroimage.2005.12.052
- 13.Plathow C, Ley S, Fink C, Puderbach M, Hosch W, Schmahl A, et al. Analysis of intrathoracic tumor mobility during whole breathing cycle by dynamic MRI. Int J Radiat Oncol Biol Phys. 2004;59:952–9. doi: 10.1016/j.ijrobp.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 14.Plathow C, Fink C, Ley S, Puderbach M, Eichinger M, Zuna I, et al. Measurement of tumor diameter-dependent mobility of lung tumors by dynamic MRI. Radiother Oncol. 2004;73:349–54. doi: 10.1016/j.radonc.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Giraud P, De Rycke Y, Dubray B. Conformal radiotherapy (CRT) planning for lung cancer: analysis of intrathoracic organ motion during extreme phases of breathing. Int J Radiat Oncol Biol Phys. 2001;51:1081–92. doi: 10.1016/s0360-3016(01)01766-7. [DOI] [PubMed] [Google Scholar]
- 16. Plathow C, Schoebinger M, Fink C, Hof H, Debus J, Meinzer H, Quantification of lung tumor volume and rotation during respiration using 3D dynamic parallel MRI with view sharing—preliminary results. Radiology (in press)
- 17.Plathow C, Zimmermann H, Fink C, Umathum R, Schöbinger M, Huber P, et al. Influence of different breathing manoeuvres on internal and external organ motion: use of fiducial markers in dynamic MRI. Int J Radiat Oncol Biol Phys. 2005;62:238–45. doi: 10.1016/j.ijrobp.2005.01.042. [DOI] [PubMed] [Google Scholar]