Abstract
For staging of bone tumors, TNM and Enneking’s systems are used with some differences. Magnetic resonance imaging is particularly useful for defining the extent of high-grade tumors, including transcortical and intertrabecular infiltration and periosteal extension. The concepts of compartment and curative surgical margins are important for bone tumor staging.
Keywords: Bone tumor staging, MR imaging, TNM system, Enneking (Musculoskeletal Tumor Society) grading
Introduction
Magnetic resonance (MR) imaging is useful for defining bone tumor extent, although its role in differential diagnosis is limited. The roles and limitations of MR imaging in the staging of bone tumors are discussed.
Basic concept of staging
The tumor-node-metastasis (TNM) classification (UICC) and Enneking’s system are two major systems of bone tumor staging . MR imaging is the most useful for evaluation of the tumor extent (T factor), whereas for the N and M factors, computed tomography (CT) is better suited, although MR imaging has some roles.
TNM staging system
The American Joint Commission on Cancer (AJCC)–International Union against Cancer (UICC) system is widely used for the staging of bone tumors . It is applicable to most bone tumors, except malignant lymphoma, multiple myeloma, periosteal and other surface osteosarcomas, and parosteal chondrosarcoma.
The TNM classification consists of T, N and M factors as seen in other malignancies. The T factor represents the tumor extent graded into three stages: T1, tumor 8 cm or less in greatest dimension; T2, tumor more than 8 cm in greatest dimension; and T3, discontinuous tumors in the primary bone site.
The N factor is not so important in bone tumors because lymph node metastases are rare. If lymph node metastasis exists, the prognosis is poor, and it is regarded as distant metastasis. Only axillary and inguinal nodes are considered to be regional nodes. The N factor is divided into two grades: N0, no detectable node metastases; and N1, lymph node metastases.
The M factor includes metastases to the lung (PUL), liver (HEP), brain (BRA), lymph node (LYM), bone marrow (MAR), pleura (PLE), peritoneum (PER), skin (SKI), and others (OTH). Distant metastases most often occur in the lung and liver. The M factor is divided into two grades: M0, absence of metastasis; and M1, presence of metastases.
The G factor, the histological grade, is unique to bone tumors. It is divided into four grades: G1, highly differentiated; G2, moderately differentiated; and G3 and G4, poorly differentiated. G4 is the highest grade, including Ewing’s sarcoma and malignant lymphoma.
Based on these factors, staging is determined as follows: IA, G1 or 2 T1N0M0; IB, G1 or 2 T2N0M0; IIA, G3 or 4 T1N0M0; IIB, G3 or 4 T2N0M0; III, not determined; IVA, G(any) T(any) N1M0; and IVB, G(any) T(any) N(any) M1.
Enneking surgical (Musculoskeletal Tumor Society) grading system
This system, only applicable to mesenchymal tumors, not to hematopoietic tumors, is similar to the AJCC UICC system with some modification .
The T factor is divided into two grades: T1, limited to one compartment; and T2, with transcompartmental extension. The concept of compartment, discussed later, is important and unique for determining the T factor in this system.
The N factor is regarded as metastasis (M factor) in this system.
The G factor includes histological, radiological and clinical features. Three grades, G0–2, include imaging features: G0, benign; G1, low-grade malignant; and G2, high-grade malignant.
These factors are included in the staging as follows: IA, G1T1M0; IB, G1T2M0; IIA, G2T1M0; IIB, G2T2M0; IIIA, G1T1 or 2 M1; IIIB, G2 T1 or 2 M1.
Tumor extent (T factor)
The concept of compartment, intra- vs. transcompartmental, is important for planning tumor resection and reconstruction . An anatomic region surrounded by a natural barrier is called a compartment. Such natural boundaries include the synovial capsule, articular cartilage, bone cortex, periosteum, major fasciae, and tendinous origins. A lesion confined to one of these spaces is called intracompartmental. For example, three compartments, anterior, posterior and medial, exist in the thigh, and four compartments, anterior, deep posterior, posterior and lateral, are present in the calf. The plantar aspect of the foot is divided into three compartments, medial, central and lateral. The regions without natural boundaries, including paraspinal, periclavicular, axillary regions and the dorsum of the hands and feet, are designated as extracompartmental spaces .
MR imaging is suitable for determining tumor extent including muscle, joint and neurovascular bundle involvement (Fig. 1), and is particularly useful in defining the extent of high grade tumors . MR imaging may elucidate growth patterns which cannot be defined by other modalities : (1) transtrabecular infiltration (intramedullary extension through the trabeculae, a sign of high grade infiltrating lesions) (Fig. 2); (2) periosteal extension (extension of the lesion along the periosteum, another growth pattern of high grade lesions) (Fig. 2); (3) transcortical infiltration (infiltrating growth through the cortical bone without localized cortical destruction, a sign of the highest grade tumor) (Fig. 3). Transcortical infiltration is particularly seen in the Ewing/PNET group and lymphomas.
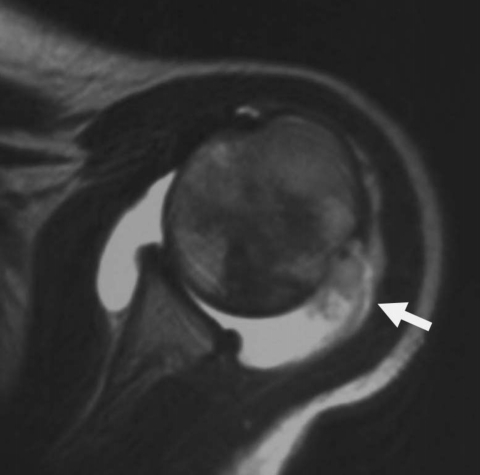
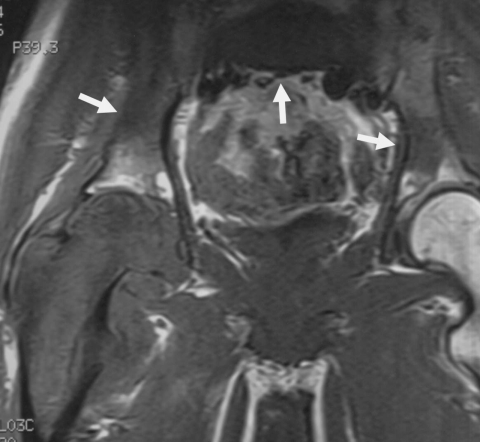
Figure 1.
Intraarticular extension of osteosarcoma (15-year-old girl). Axial T2-weighted image demonstrates a tumor in the proximal humerus infiltrating into the shoulder joint within effusion (arrow).
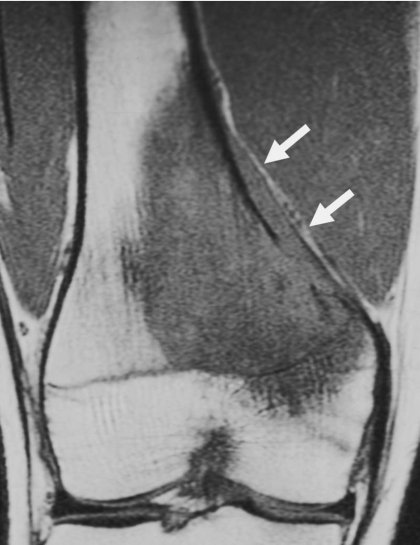
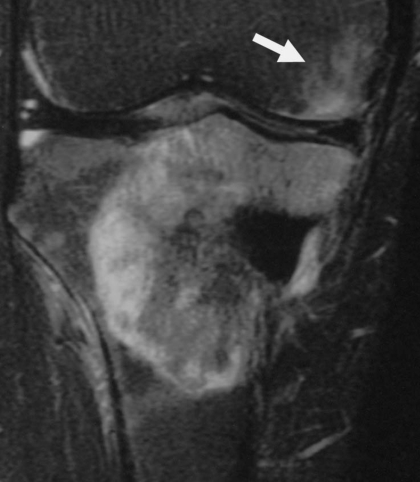
Figure 2.
Intertrabecular infiltration and periosteal extension of osteosarcoma (16-year-old boy). Coronal T1-weighted image shows tumor infiltration among the fine bone trabeculae, and proximal extension along the periosteum (arrow).
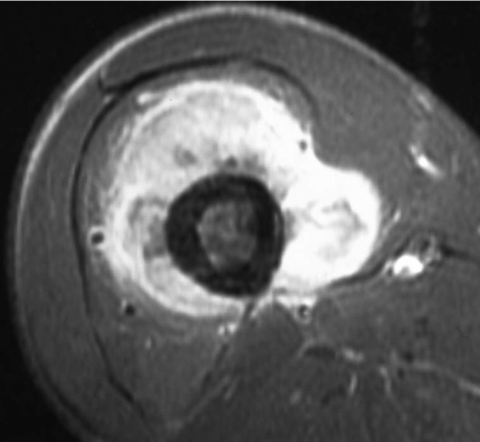
Figure 3.
Transcortical infiltration of osteosarcoma (19-year-old man). Axial T1-weighted image with fat suppression after intravenous injection of Gd-contrast medium. Tumor infiltration across the thick femoral cortex is noted.
Lymph node involvement (N factor)
Malignant mesenchymal tumors rarely metastasize to the lymph nodes. According to the TNM classification, lymph nodes up to the axillary nodes in the upper limbs and up to the inguinal nodes in the lower limbs are considered to be regional nodes. In Enneking’s system (excluding hematopoietic tumors), all lymph node metastases are considered to be distant metastases.
Bone tumors commonly metastasizing to lymph nodes are osteosarcoma , Ewing’s sarcoma and malignant lymphoma (Fig. 4), and soft tissue tumors commonly with lymph node metastases are epithelioid sarcoma, clear cell sarcoma, rhabdomyosarcoma and synovial sarcoma.
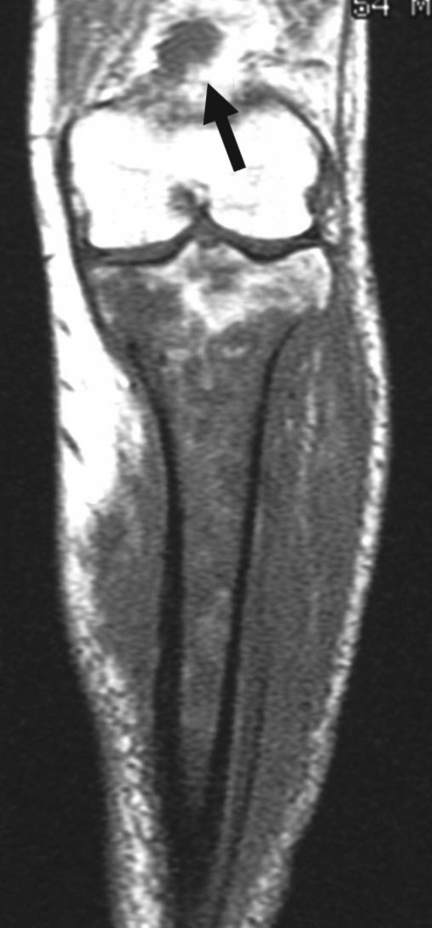
Figure 4.
Popliteal node metastasis of malignant lymphoma (diffuse large B-cell) of the tibia (54-year-old man). Coronal T1-weighted image reveals an enlarged node in the posterior aspect of the distal thigh (arrow), in addition to the primary lesion in the tibia.
CT and MR imaging together are suitable for evaluation, but a CT scan is more suitable to cover wide areas of the body.
Metastases (M factor)
Common sites of bone tumor metastases are the lungs and bones . The lung is the most common site of metastasis and is best evaluated by CT. For liver metastasis, also, CT is the best method of evaluation. Bone scintigraphy is the common method of bone metastasis evaluation. Osteosarcoma and Ewing’s sarcoma are common tumors of metastases to other bones (Fig. 5).
Figure 5.
Multiple skeletal metastases of osteosarcoma (22-year-old man). Coronal T1-weighted image demonstrates a dominant lesion in the right proximal femur with infiltration into the periosteal region. Lesions in the sacrum, right ilium and left acetabulum (arrows) are considered to be metastases. No pulmonary metastases are noted at this time.
Differentiation between multicentric lesions and metastases is difficult. Some authors may consider multiple (more than one) tumors without lung metastases as multicentric tumors, but other authors believe bone metastases with no lung metastases or venous metastases through Batson’s venous plexus to be existent.
Skip metastases are metastases occurring as a separate focus in the same bone as the primary lesion, or occasionally the adjacent bone across the joint (Fig. 6). Enneking reported that it occurred in 20% of osteosarcoma cases, but it is actually not so common . It is seen in high-grade lesions.
Figure 6.
Skip metastasis in the distal femur of osteosarcoma of the proximal tibia (43-year-old man). Coronal T2-weighted image shows a dominant lesion with heterogeneous signal in the proximal tibia. Another lesion, skip metastasis, is seen in the subchondral region of the distal femur (arrow).
Exceptions of grading systems
Malignant lymphoma
Staging of malignant lymphoma involving bone is the same as that of other organs. The Ann-Arbor system is based on the disease extent evaluated by clinical and imaging studies. Determining the disease extent is of prime importance in staging. Evaluation of regional nodes is important for differential diagnosis and staging (Fig. 4).
Multiple myeloma
Multiple myeloma has a different grading system from that of malignant lymphoma. The Durie–Salmon grading system includes a simple plain radiographic scoring system, but it is not so accurate in determining the disease extent. In the current IMWG, only serum albumin and beta-microglobulin are parameters and imaging features are not included. MR imaging is suitable for determining the extent of the bone marrow lesion, and its use for grading may be promising.
Bone-surface sarcomas
The periosteum is an extracompartmental space, and grading of surface tumors is different from other tumors. Bone-surface sarcomas include parosteal osteosarcoma, periosteal osteosarcoma, high-grade surface osteosarcoma, and periosteal chondrosarcoma. Localization is important, and involvement of the medullary canal is a sign of a worse prognosis in parosteal osteosarcoma, but it is still controversial.
MR imaging techniques for staging
Although MR imaging may be superior to CT in defining bone marrow and cortical bone involvement as well as muscular and neurovascular involvement, there are no statistically significant differences between these two modalities . Particularly for defining a reactive zone, which is discussed later, MR imaging is far superior to CT. MR imaging is also sensitive for detecting joint involvement . Gd-chelate contrast has been used to determine tumor extent on MR imaging, particularly in joint involvement.
Whole body MR
Most bone tumors have high signal intensity on short tau inversion recovery (STIR) images. Whole body MRI, as initially described, is a series of STIR images throughout the body, consisting of three or four series of coronal images. This is a way to scan the whole body with MR imaging. It is particularly useful to screen the skeleton and liver with reasonable sensitivity. Its sensitivity on bone involvement appears higher than with bone scintigraphy .
Diffusion-weighted images for staging
This is another way to scan the whole body with MR imaging. This scan is based on the increased signal in the bone tumors on diffusion-weighted imaging. Images are similar to positron emission tomography (PET) scanning with suppression of the body signal . Its clinical value has not been well determined, but the results may be promising.
MR arthrography
Although arthro-tomography has been reported to be useful for staging giant cell tumor of bone , MR arthrography, together with CT arthrography, has rarely been performed. Bone tumors rarely extend across articular cartilage. Intraarticular injection of Gd-chelate may be useful, but intravenous injection usually demonstrates articular involvement (indirect MR arthrography).
MR arteriography
MR arteriography may help evaluate vascular invasion of bone sarcomas. It is useful for the mapping of major arteries, but, for assessing vascular invasion, axial images are important to assess the relationship between the tumor and the blood vessels.
Surgical planning and resection margin
Causes of local recurrence include: (1) inadequate surgical margin; (2) skip metastases; (3) tumor thrombus; and (4) lymph node metastases. To prevent local recurrence, the resection margin is particularly important for curative tumor removal. This concept is based on the fact that microscopic lesions exist at the tumor margin, and for curative resection, some width of peritumoral tissue needs to be excised. Adequate surgical margin and preserving function, muscles and neurovascular bundles, are always dilemmas for limb-salvaging surgery.
Cartilage and connective tissue membranes, including fascia, joint capsule, tendon, tendon sheath, vascular adventitia, and epineurium, are considered to be barriers to tumor infiltration. Such a natural barrier is important when considering a safe margin for curability of tumors. Curative resection is present at more than 3 cm from the reactive margin of the tumor ; the local recurrence rate is 4%. Wide resection is adequately outside the reactive margin; the local recurrence rate is 20%. Marginal resection is resection through the reactive margin; the local recurrence rate is 60%. Intralesional resection is through the tumor itself; the local recurrence rate is 100%.
The reactive zone, an area of abnormal signal on MR imaging, consists of hemorrhage in and around the tumor, muscle degeneration, edema and scar (Fig. 7). It is basically considered to be the region of tumor infiltration. Reactive sclerosis around the tumor is considered to be the reactive zone. Determination of the reactive zone and removal of lesions within the reactive zone are important for curative treatment.
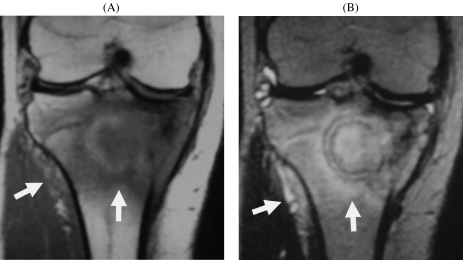
Figure 7.
Wide reactive zone of Ewing’s sarcoma, simulating osteomyelitis (16-year-old boy). (A) Coronal T1-weighted image; (B) coronal T2-weighted image. A round lesion with surrounding sclerosis appears similar to osteomyelitis. The reactive zone is wide in the bone marrow and periosteum laterally (arrows).
Acknowledgements
This work is partly supported by a Grant-in-Aid for Scientific Research, Ministry of Education and Science, Government of Japan (No. 17591289). The author thanks Dr Taro Takahara of Tokai University and Dr Katsuyuki Nakanishi of Osaka-Senin-Hoken Hospital for help in preparing this work.
References
- 1.Wittekind CH, Hutter R, Green FL, et al., eds. 5th ed. UICC; 2005. TNM Atlas. [Google Scholar]
- 2.Musculoskeletal Tumor Society (Enneking WF) Staging of musculoskeletal neoplasms. Skeletal Radiol. 1985;13:183–94. doi: 10.1007/BF00350572. [DOI] [PubMed] [Google Scholar]
- 3.Anderson MW, Temple HT, Dussault RG, Kaplan PA. Compartmental anatomy: relevance to staging and biopsy of musculoskeletal tumor. AJR. 1999;173:1663–71. doi: 10.2214/ajr.173.6.10584817. [DOI] [PubMed] [Google Scholar]
- 4.Bloem JL, Taminiau AH, Eulderink F, et al. Radiologic staging of primary bone sarcoma: MR imaging, scintigraphy, angiography, and CT correlated with pathologic examination. Radiology. 1988;169:805–10. doi: 10.1148/radiology.169.3.3055041. [DOI] [PubMed] [Google Scholar]
- 5.Schima W, Amann G, Stiglbauer R, et al. Preoperative staging of osteosarcoma: efficacy of MR imaging in detecting joint involvement. AJR. 1994;163:1171–5. doi: 10.2214/ajr.163.5.7976895. [DOI] [PubMed] [Google Scholar]
- 6.Swan JS, Grist TM, Sproat IA, et al. Musculoskeletal neoplasms: preoperative evaluation with MR imaging. Radiology. 1995;194:519–24. doi: 10.1148/radiology.194.2.7529935. [DOI] [PubMed] [Google Scholar]
- 7.Panicek DM, Gatsonis C, Rosenthal DI, et al. CT and MR imaging in the local staging of primary malignant musculoskeletal neoplasms: report of the Radiology Diagnostic Oncology Group. Radiology. 1997;202:237–46. doi: 10.1148/radiology.202.1.8988217. [DOI] [PubMed] [Google Scholar]
- 8.Ehara S. Margin analysis of bone tumors: current roles and limitations. J Japan Orthop Assoc. 2006 in press (in Japanese). [Google Scholar]
- 9.Kim SJ, Choi JA, Lee SH, et al. Imaging findings of extrapulmonary metastases of osteosarcoma. Clin Imaging. 2004;28:291–300. doi: 10.1016/S0899-7071(03)00206-7. [DOI] [PubMed] [Google Scholar]
- 10.Enneking WF, Kagan A. “Skip” metastases in osteosarcoma. Cancer. 1975;36:2192–205. doi: 10.1002/cncr.2820360937. [DOI] [PubMed] [Google Scholar]
- 11.Daldrup-Link HE, Franzius C, Link TM, et al. Whole-body MR imaging for detection of bone metastasis in children and young adults: comparison with skeletal scintigraphy and FDG PET. AJR. 2001;177:229–36. doi: 10.2214/ajr.177.1.1770229. [DOI] [PubMed] [Google Scholar]
- 12.Takahara T, Imai Y, Yamashita T, et al. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement with free breathing, STIR and high resolution 3D display. Radiat Med. 2004;22:275–82. [PubMed] [Google Scholar]
- 13.Hudson TM, Schiebler M, Springfield DS, et al. Radiology of giant cell tumor of bone: computed tomography, arthro-tomography, and scintigraphy. Skeletal Radiol. 1984;11:85–95. doi: 10.1007/BF00348795. [DOI] [PubMed] [Google Scholar]
- 14.Kawaguchi N, Ahmed AR, Matsumoto S, et al. The concept curative margin in surgery for bone and soft tissue sarcoma. Clin Orthop. 2004;419:165–72. doi: 10.1097/00003086-200402000-00027. [DOI] [PubMed] [Google Scholar]