Abstract
In testicular germ cell tumour (GCT), imaging plays a central role in assessment of tumour bulk, sites of metastases, monitoring response to therapy, surgical planning and accurate assessment of disease at relapse. The primary modality used for imaging patients with GCT is computed tomography (CT) but plain film radiography, ultrasound, magnetic resonance imaging (MRI) and positron emission tomography (PET) may all have roles to play. This article reviews the role of imaging of testicular germ cell tumours.
Keywords: Testicular tumours, germ cell neoplasia, imaging, CT, MRI, PET
Introduction
Testicular germ cell tumours (GCT) are an increasingly common group of tumours, particularly in young males. The success of current management strategies is such that the majority of patients can expect to be cured. This success hinges on accurate disease assessment and application of chemotherapy and radiotherapy. Serum markers can be useful surrogate markers of disease activity, but they cannot accurately assess disease bulk or locate sites of tumour spread. For these purposes imaging is invaluable and now plays an integral role not only in assessment of tumour bulk and sites of metastases but also in monitoring response to therapy, surgical planning and accurate assessment of disease at relapse. The primary modality used for imaging patients with these tumours is computed tomography (CT) but plain film radiography, ultrasound and magnetic resonance imaging (MRI) also have roles to play. Positron emission tomography (PET) scanning is now being more widely used but its optimal role has yet to be agreed. This article reviews the literature relating to imaging in testicular germ cell tumours. It covers the role of imaging from diagnosis through staging to post-treatment monitoring and surveillance and evidence for the use of imaging.
Diagnosis
The diagnosis of germ cell tumours (GCT) is made by a biopsy or at orchidectomy. Testicular GCT most commonly presents as a painless palpable mass (up to 95% of cases) [1]. In up to 10% of cases, it may present with dull scrotal ache, pain or acute fever [2]. In patients with retroperitoneal metastases or disseminated disease, backache, malaise, lethargy and other systemic features may be the presenting findings [3]. Imaging is largely used to confirm the presence of disease and assess its extent.
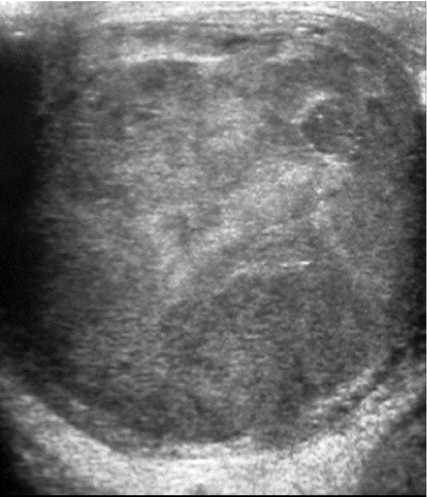
Testicular ultrasound (which should be performed using a 7.5 MHz probe) is used in primary assessment of the testes to confirm diagnosis, to distinguish from other scrotal abnormalities and to screen for abnormalities such as microlithiasis in the contralateral testis [2, 4–6]. Sonographically, testicular tumours are usually well defined and hypoechoic relative to the normal testicle, although some may display a heterogeneous echotexture, calcification or cystic change (Fig. 1). Tumours may display increased vascularity on colour and power Doppler with respect to surrounding normal testicular tissue but this is not specific and may not be demonstrated in small tumours [7]. Ultrasound cannot be used to reliably differentiate between tumour types. For this purpose, MRI may be useful [8, 9].
Figure 1.
Testicular carcinoma. Ultrasound demonstrating heterogeneous echotexture throughout the testicle.
Staging
Once a diagnosis of testicular germ cell tumour has been made, assessment of disease extent must be performed prior to initiating therapy. The European Germ Cell Cancer Consensus Group (EGCCCG) recommend that TNM staging be used [10] (Table 1) and that patients be categorised using the International Germ Cell Cancer Collaborative Group (IGCCCG) classification which stratifies patients into good, intermediate and poor prognostic groups. This latter classification is based on histology, location of primary tumour and metastases and levels of serum markers (Table 2) [11]. A further staging classification, devised at The Royal Marsden Hospital (UK) and widely used in the UK and Europe, is shown in Table 3.
Table 1.
TNM staging classification of testicular tumours [10]
| Primary tumour | |
| The extent of the primary tumour is classified after radical orchidectomy (pT) | |
| pTX | Primary tumour cannot be assessed (if no radical orchidectomy has been performed, TX is used) |
| pT0 | No evidence of primary tumour (e.g. histological scar in testis) |
| pTis | Intratubular germ cell neoplasia |
| pT1 | Tumour limited to testis and epididymis without vascular/lymphatic invasion; tumour may invade into the tunica albuginea but not the tunica vaginalis |
| pT2 | Tumour limited to testis and epididymis with vascular/lymphatic invasion, or tumour extending through tunica albuginea with involvement of tunica vaginalis |
| pT3 | Tumour invades spermatic cord with or without vascular/lymphatic invasion |
| pT4 | Tumour invades scrotum with or without vascular/lymphatic invasion |
| Regional lymph nodes | |
| Clinical involvement | |
| NX | Regional nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis with a lymph node mass ≤2 cm in greatest dimension or multiple lymph nodes none >2 cm in greatest dimension |
| N2 | Metastasis with a lymph node mass >2 cm but <5 cm in greatest dimension, or multiple lymph nodes, any one mass >2 cm but ≤5 cm in greatest dimension |
| N3 | Metastasis with a lymph node mass >5 cm in greatest dimension |
| Pathological involvement | |
| pN0 | No regional lymph node metastases |
| pN1 | Metastasis with a lymph node mass ≤2 cm in greatest dimension and 5 or fewer positive nodes, none >2 cm in greatest dimension |
| pN2 | Metastasis with a lymph node mass >2 cm but ≤5 cm in greatest dimensions; or more than five nodes positive, none >5 cm; or evidence of extranodal extension of tumour |
| pN3 | Metastasis with a lymph node mass >5 cm in greatest dimension |
| Distant metastases | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Non-regional lymph node or pulmonary metastasis |
| M1b | Distant metastasis other than to non-regional lymph nodes and lungs |
Table 2.
International Germ Cell Consensus classification [11]
| Non-seminoma |
| Good prognosis:allof the following |
| —AFP <1000 ng / ml and HCG < 5000 iu / l (1000 ng /ml) and LDH < 1.5 × upper limit of normal (N) and |
| —Non-mediastinal primary |
| —No non-pulmonary visceral metastases (NPVM) |
| Intermediate prognosis:allof the following |
| —AFP 1000–10000 ng /ml, or HCG 5000–50000 iu / l, or LDH 1.5–10 × N and |
| —Non-mediastinal primary site and |
| —No NPVM |
| Poor prognosis:anyof the following |
| —AFP >10000 ng / ml or HCG > 50000 iu / l or LDH >10 × N or |
| —Mediastinal primary site or |
| —NPVM |
| Seminoma |
| Good prognosis |
| —No NPVM |
| —Any primary site |
| —Normal AFP, any HCG, any LDH |
| Intermediate prognosis |
| —NPVM present |
Note: AFP = alphafetoprotein; B-HCG = B-human chorionic gonadotrophin; LDH = lactate dehydrogenase; CNS = central nervous system.
Table 3.
The Royal Marsden Hospital staging classification for testicular germ cell tumours [30]
| Stage | Definitions |
|---|---|
| I | No evidence of metastases |
| IM | Rising serum markers with no other evidence of metastases |
| II | Abdominal node metastases |
| A | < 2 cm in diameter |
| B | 2–5 cm in diameter |
| C | > 5 cm in diameter |
| III | Supradiaphragmatic node metastases |
| M | Mediastinal |
| N | Supraclavicular cervical axillary |
| O | No abdominal node metastases |
| ABC | Node size defined as in Stage II |
| IV | Extralymphatic metastases |
| Lung | |
| L1 | ≤ 3 metastases |
| L2 | > 3 metastases all < 2 cm in diameter |
| L3 | > 3 metastases, one or more > 2 cm in diameter |
| H + | Liver metastases |
| Br + | Brain metastases |
| Bo + | Bone metastases |
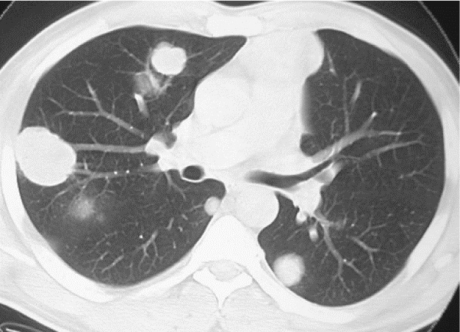
When staging tumours, knowledge of the patterns of spread enables prediction of sites of disease and may improve the accuracy of assessment. Testicular tumours spread via vascular or lymphatic invasion (Figs. 2–5). Vascular spread is most commonly to the lungs. Lung metastases may vary in appearance with respect to the histology of the primary tumour; those from NSGCT appear as multiple small peripheral nodules whereas seminoma metastases tend to be larger masses (Fig. 2). Other sites of metastatic spread include the brain (most common in trophoblastic teratomas), bones and liver (Figs. 3 and 4). Other sites of metastases, though rarely seen and usually only in the setting of advanced disease, include the adrenals, kidneys, spleen, pleura, pericardium and peritoneum.
Figure 2.
Lung metastases. CT image showing multiple lung masses in a patient with metastatic testicular seminoma.
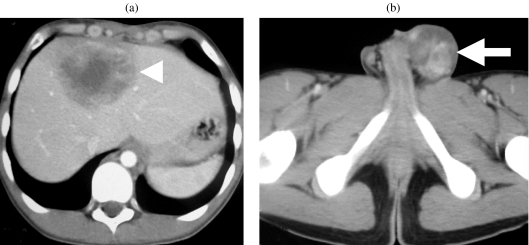
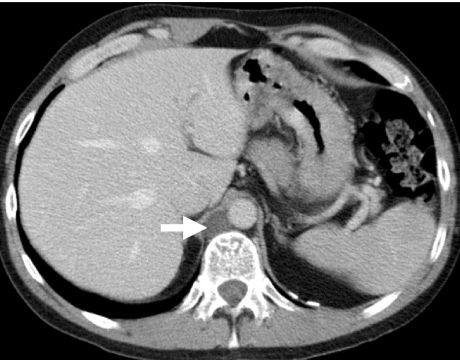
Figure 3.
Metastatic non-seminomatous germ cell tumour. Contrast-enhanced CT shows (a) liver metastasis and (b) the left testicular tumour (arrow).
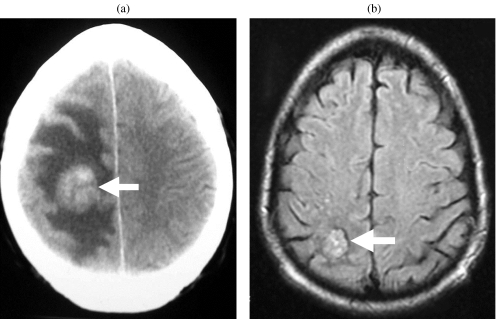
Figure 4.
Brain metastasis. (a) Contrast-enhanced CT showing an enhancing mass (arrow) in the right parietal lobe with surrounding oedema. (b) FLAIR MR image following treatment shows the lesion to be smaller and the surrounding oedema to have resolved.
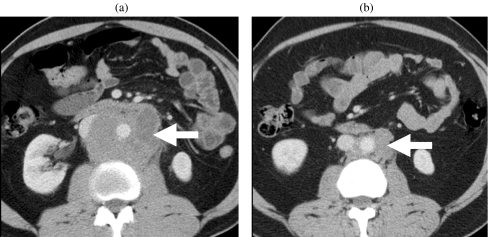
Figure 5.
Retroperitoneal lymphadenopathy. Contrast-enhanced CT (a) before and (b) after chemotherapy shows retroperitoneal lymphadenopathy (arrow) encasing the aorta. The post-chemotherapy scan shows significant reduction in the burden of retroperitoneal lymphadenopathy.
Lymphatic spread reflects the retroperitoneal embryological origin of the testis. Spread occurs via lymphatic channels which pass through the inguinal ring (accompanying the spermatic cord and testicular vessels) to enter retroperitoneal nodes (Fig. 5). Right-sided tumours normally spread to right-sided nodes around the IVC (most commonly lower retroperitoneal, aortocaval or paracaval). Left-sided tumours normally spread to lymph nodes on the left, adjacent to the aorta (most commonly just below the left renal hilum). In the absence of bulky ipsilateral adenopathy, contralateral spread is unusual, and if seen as the only site of metastasis, histological proof of tumour involvement should be sought prior to instigation of therapy [12]. Pelvic adenopathy is also uncommon in the absence of bulky disease elsewhere or history of maldescent or previous scrotal surgery [13]. Echelon nodes, which are sites of nodal disease, identified more frequently at relapse than during primary disease, have been described [14]. On the right the echelon node is sited laterally between the L1 and L3 vertebrae [15]. On the left a similar node has been described as lying on iliopsoas [16].
Nodal disease superior to the level of the renal hila occurs via direct spread. In cases of seminoma spread of disease above the diaphragm may occur via the thoracic duct into the posterior mediastinum. In NSGCT, however, spread is more random involving the anterior mediastinum, aortopulmonary window, hilar, supraclavicular and neck lymph nodes but excluding the posterior mediastinum and subcarinal regions [17].
The primary imaging modality currently used for staging disease is computed tomography (CT). Its overall accuracy approaches 80% [18]. Current EGCCCG guidelines state that patients should receive contrast-enhanced CT of thorax, abdomen and pelvis [19]. The exception to this is in patients with seminoma and no retroperitoneal disease, where chest radiography (in place of thoracic CT) is thought sufficient to assess the thorax for metastases [20].
Computed tomography (CT)
Overall, CT has been shown to be the most sensitive method of assessing metastatic disease in the thorax, abdomen and pelvis, though it is recognised that it may understage disease in up to 25% of cases [21]. It has also been found to be the most sensitive method of assessing supraclavicular, mediastinal and pleural disease [22–28]. With respect to thoracic imaging, use of multidetector CT has not been shown to increase nodule detection when compared to that using single-slice CT for slice thicknesses of 5 mm [29]. However, the ability to produce submillimetre sections will undoubtedly increase the sensitivity of multislice CT for nodule detection.
Lymph node metastases are usually of soft tissue density. Large volume seminomatous disease may, however, have a central low density secondary to necrosis, whereas in large volume NSGCT complex cysts allied with foci of soft tissue may be seen [30]. In large volume disease the diagnosis is rarely in doubt. Assessment of small volume lymphadenopathy, which has significant implications for patient staging and management, is unfortunately the Achilles’ heel of CT. The detection of microscopic deposits of tumour in normal-sized nodes and the distinction between tumoral and inflammatory adenopathy are beyond the scope of CT. In light of these handicaps it comes as no surprise that false negative CT examinations occur, thus limiting its diagnostic accuracy. CT is unable to identify small volume disease in normal-sized nodes in up to 30% of patients with GCT [31–35].
Studies performed to assess the effect of different thresholds of significance for lymph node size have been performed [36, 37]. Essentially, they confirm the logical notion that, by reducing the lymph node size accepted as normal, the likelihood of detecting positive nodes increases, but the specificity of the test decreases. It has been shown that by using 10–15 mm as the upper limit of normal, up to 44% of scans were false negative [38–40]. A further complication of trying to standardise upper limits of normal is that normal nodes in the superior retroperitoneum are smaller than those in the inferior retroperitoneum on CT [41–43]. Standardization of normality as such has not been agreed; thus institutions vary in their practice. An assessment schema is given (Table 4) [30]. For practical purposes a cut-off of 10 mm is used to differentiate between normal and abnormal lymph nodes. Those measuring between 8 and 10 mm are treated as suspicious. These measurements must, however, be taken in the overall context of the patient’s situation such as risk of disease, marker levels, etc. Laterality of the tumour is an additional consideration. Sites suspicious of disease warrant further investigation; this could include tissue sampling, biochemical markers, additional imaging such as PET scanning (see below) and further follow-up imaging.
Table 4.
Lymph node size at various anatomic sites: short axis diameter, upper limits of normal
| Site | Group | Short axis size |
|---|---|---|
| (mm) | ||
| Head and neck | Cervical | 10 (<10 mm with |
| central necrosis) | ||
| Axilla | 10 | |
| Mediastinum | Subcarinal | 12 |
| Paracardiac | 8 | |
| Retrocural | 6 | |
| All other sites | 10 | |
| Abdomen | Gastrohepatic ligament | 8 |
| Porta hepatis | 8 | |
| Portacaval | 10 | |
| Coeliac axis to renal artery | 10 | |
| Renal artery to aortic bifurcation | 12 | |
| Pelvis | Common iliac | 9 |
| External iliac | 10 | |
| Internal iliac | 7 | |
| Obturator | 8 | |
For the detection of disease beyond the chest, abdomen and pelvis, CT of the brain is not undertaken as part of routine staging in all patients but is indicated in those with high-risk factors (e.g. multiple lung metastases, HCG > 10000) and in patients with suspected metastatic disease on clinical grounds [44, 45]. Brain metastases are often haemorrhagic and usually demonstrate enhancement after intravenous contrast administration (Fig. 4) [30].
Ultrasound
Ultrasound is not routinely used in staging of disease. Assessment of retroperitoneal and pelvic nodes has been shown to be not as reliable when compared to CT or MRI [19]. Indeed, it has been reported that up to 17% of small volume disease may be missed. However, ultrasound is useful in the assessment of solid intra-abdominal organs, e.g. the liver, and as a guide for needle placement during biopsy.
Magnetic resonance imaging (MRI)
Use of MRI to date has been limited in part due to its long examination times, high cost and low availability. However, with the advent of new, shorter sequences and techniques and increasing MRI availability, it is likely that there will be an increase in its use. The use of MRI in staging testicular GCT is emerging. When compared to CT, MRI is known to have better soft tissue contrast resolution and be at least as accurate in detection of retroperitoneal lymph nodes [46] though it is not as accurate in detecting lung metastases [47, 48]. MRI is of use in detection and characterisation of central nervous system, musculoskeletal and hepatic metastases. Furthermore it has also been found to be useful in demonstration of IVC tumour invasion, enteric fistulae [8, 9] and demonstration of vascular anatomy in patients prior to retroperitoneal lymph node surgery. MRI may also be used in those patients where intravenous contrast cannot be given and as a problem solving technique for equivocal CT findings [19].
Recently, MR imaging with lymphotrophic nanoparticles (LNMRI) has been shown to be an effective method for evaluating lymph nodes in cancers [49–55]. Lymphatic targeting has been shown to result from slow extravasation of nanoparticles into the interstitial space, from which they are transported to lymph nodes by lymphatics. Within lymph nodes, these nanoparticles are internalized into macrophages, resulting in intracellular trapping and subsequent changes in magnetic properties by MR. A recent study showed that lymphotrophic nanoparticle-enhanced MRI demonstrated higher sensitivity and specificity for detecting nodal metastases when compared with plain MRI alone. Many nodes larger than 10 mm were benign (32%) and were accurately characterized by LNMRI. The two nodes in that study that were falsely positive were larger than 10 mm and had >50% foci of hyalinization. These areas of hyalinization appeared as focal defects on LNMRI, mimicking metastatic nodes. It was also shown that LNMRI showed a high degree of accuracy in detecting metastases in nodes smaller than 10 mm which would otherwise be considered benign on the basis of traditional size criteria [50].
Positron emission tomography (PET)
PET scanning utilises the differentially greater uptake of fluorine-18-labelled fluorodeoxyglucose (18FDG) in malignant cells (owing to their higher metabolic rate) than normal tissue to enable tumour detection. Aside from mature differentiated teratomas (which have a relatively low metabolic rate) most tumours (and their metastases) demonstrate avid 18FDG uptake. With respect to testicular tumours it has been shown that seminomatous lesions have a significantly higher FDG uptake than non-seminomatous germ cell tumours, as expressed by the standard uptake values (SUV) [56, 57].
The use of PET is widely advocated as it has been shown that the sensitivity of PET is greater than CT (but with similar specificity) [58, 59]. In one study the sensitivity and specificity of PET was reported to be 87% and 94%, respectively, compared to 73% and 94% for CT [60]. It has also been stated that the use of PET can alter management in up to 57% of patients [61] although this is not a universally held view [62].
The role of PET in primary staging is minimal if metastatic disease has already been diagnosed. However, as PET images include a greater body area, it may define sites of disease outside the scope of that seen on routine CT scanning. This can have repercussions on management [59]. Identification of sites of metastases have been shown repeatedly to be more accurately performed by PET than CT [63]. However, its poor detection of small volume (sub-centimetre) disease remains a concern.
A possible use of PET is in those patients with raised tumour marker levels but no definite disease on conventional imaging. Hain et al. reviewed cases of patients with raised marker levels (including those with residual mass) [64]. They found that in all but one case PET identified the site of disease. In their study, five false negative PET results were found. Out of these, three cases had no abnormality on any imaging modality. During follow-up of these patients, it was found that PET scans were the first imaging modality to identify the site of recurrence. As a result of this it was suggested that in the presence of raised marker levels and negative imaging (including negative PET), the most appropriate follow-up imaging may be repeat PET. The use of PET to predict relapse in patients with clinical stage I non-seminomatous germ cell tumour has been investigated by the Medical Research Council (MRC) in the UK in the TE22 study. The study showed that PET identified a proportion of patients with disease not detected by CT; however, the relapse rate among PET-negative patients remains high. The study results therefore suggest that 18FDG-PET scanning is not able to identify patients at sufficiently low risk of relapse to replace other treatment options in this setting [65].
Surveillance
In patients with stage I disease surveillance is a common management pathway. Overall, approximately 30% of patients will relapse [66–68]. Vascular or lymphatic invasion is the most powerful predictor of relapse; the absence of yolk sac elements and the presence of undifferentiated cells are also independent prognostic variables [69]. In the prospective TE04 trial, 45% of those that relapsed did not have raised markers at the time of discovery of recurrent disease. Sixty-one percent of relapses occurred in the para-aortic nodes and 10% in mediastinal or supraclavicular nodes. Ninety-five percent of those who did relapse were in the IGCCCG good prognostic group and overall survival free from germ cell tumour was 99% [11]. As relapse is most frequent in the first year after diagnosis (up to 80%) the number of scans is maximal during this time. Surveillance is performed rigorously with clinical follow-up, chest radiography, serum marker analysis and CT. Serial imaging of the thorax and abdomen is routinely performed.
The value of chest CT above chest radiography has been studied. In a series of 168 stage I NSGCT patients on surveillance in whom chest X-ray rather than chest CT was performed [70]. Nineteen percent (42 patients) of these patients relapsed of which 8/42 relapsed with chest disease. Seven out of eight of these latter patients had evidence of disease elsewhere which was identified on abdominal CT. The one patient in this series who had only chest disease at relapse was clearly diagnosed by chest radiography. This led the authors to conclude that chest imaging with CT would not have changed the prognosis of those that relapsed in the chest in their study [69].
The role of pelvic CT has also been called into question. In one series of patients with testicular germ cell tumours pelvic lymphadenopathy was seen in 16 of 167 patients (9.6%). The presence of bulky para-aortic lymphadenopathy was the only significant predictor for pelvic disease and was present in 11 of 16 patients. In the absence of this or other risk factors for pelvic disease (previous scrotal or inguinal surgery, maldescent, tunica vaginalis invasion, retroperitoneal lymph node dissection or bulky abdominal nodes), routine pelvic CT for patients on surveillance for stage I disease may constitute unnecessary radiation [25].
Centres vary in their preference, but most will scan patients between two and six times during the first year. As yet no consensus on optimal management has been derived but it is seen that those centres that scan more frequently do not detect relapse at a significantly earlier stage. Indeed in one study of 46 patients, all relapses detected after the 3 month CT were picked up by clinical suspicion, raised tumour markers or chest X-ray [71]. The question of whether more frequent CT results in earlier diagnosis of relapse and whether this has any outcome on survival still remains to be conclusively answered. Indeed, early results of the MRC TE08 study have not detected any advantage for a five scan schedule over a two scan schedule (NCRI meeting, October 2005).
The potential benefit of repeated scanning must be weighed against the financial and health costs of more frequent scans. A thoracic CT gives a radiation dose equivalent to 400 chest radiographs (8 vs. 0.02 mSv), while for a chest and abdomen CT the dose is increased to approximately 20 mSv (a dose equivalent to 1000 chest radiographs). This results in a 1:1000 lifetime risk of a second cancer/leukaemia in a 25-year-old over the subsequent 40 years. Another approach to reducing radiation exposure is to use alternative technology. The use of ultrasound and magnetic resonance imaging (MRI) has therefore been suggested in surveillance programmes. Ultrasound is not as reliable as CT or MR imaging in the assessment of abdominal para-aortic nodes. Limited data suggest that MR imaging may be used instead of CT for abdominal disease [46].
Positron emission tomography (PET) is an attractive alternative modality to improve surveillance but as discussed above the MRC TE22 protocol suggests the added value of PET scan may be limited [65].
Assessing response to therapy
The EGCCCG guidelines state that radiological restaging must be performed after completion of first-line chemotherapy. However, in patients with slow tumour marker decline or clinical evidence of progression, restaging should be performed earlier.
Chest radiographs are useful screening tools and are often used in the routine follow-up of early-stage patients and those in complete remission following chemotherapy [72]. They enable detection and surveillance of lung parenchymal nodules 1 cm (or greater) in size, pleurally based masses and effusions and mediastinal masses. In a study designed to investigate the predictive capacity of chest radiography, 288 patients were retrospectively studied. Thirty-three cases of relapse were found but none were identified by chest radiography [73]. Despite this, their relatively low radiation burden and cost continue to make them attractive tools for follow-up studies.
CT is the primary imaging modality for assessing response of disease to treatment. Reduction in size of metastases is the primary change found on CT indicating response to therapy even if malignant cells persist within the residual tissue (Fig. 5). In addition to size, CT can help assess residual masses post-chemotherapy by assessing changes in appearance. Cystic and fatty change which is well assessed using CT have been associated with mature differentiated teratoma and may indicate the need for surgical removal (Fig. 6) [74–76].
Figure 6.
Mature teratoma differentiated in retrocrural node (arrow). Contrast-enhanced CT shows low density lesion in the right retrocrural region.
Seminoma is extremely sensitive to chemo- and radiotherapy, such that residual mass post treatment usually only consists of fibrosis and necrosis. Calcification may also be found within post-treatment tissue [77]. The CT findings may be allied to reduction in serum marker levels and reduction in avidity of uptake on PET scanning.
Treated lung metastases may resemble irregular scars in the region of the previous metastases. In some cases they may also be seen to cavitate [78]. Interval CT scanning during and following completion of therapy is important to assess the response [79]. It also allows the selection of patients who may benefit from surgical lymphadenectomy (traditonally those with residual masses greater than 1 cm) [80]. In those patients with large-volume disease the use of CT and MRI has been shown to be useful in planning an operative approach [81].
PET may have a role in the assessment of residual masses after chemotherapy. Cremerius et al. reviewed PET scans in patients with seminoma after treatment [56]. They reported that PET had 90% sensitivity for detecting residual disease. These results indicate that PET may be of value in selecting patients with seminoma for radiotherapy. The SEMPET trial (where PET was used to assess residual disease in patients with seminoma who were on chemotherapy) showed that PET imaging was more accurate than other assessment modalities [82]. In that study, PET was performed in all patients with residual masses ≥1 cm within 4–12 weeks of completion of chemotherapy. The results were compared to histological analysis of tumour viability or CT evidence of progression. They reported that PET correctly identified all cases of residual tumour in lesions >3 cm and in 95% of cases with lesions ≤3 cm. This gave an overall specificity and sensitivity for 100% and 80% respectively for PET, compared with 74% and 70% for CT. A further recent study reported that only 4 of 47 lesions <3 cm (8.5%) were viable, whereas 11 of 27 lesions ≥3 cm (41%) were viable [83]. This led the authors to conclude that using a solely CT-based surgical strategy would have resulted in over-treatment of nearly 60% of the patients. They suggest that, in lesions ≥3 cm, a negative PET scan may justify surveillance of the patient, since no false negative PET scan was registered in lesions ≥3 cm in the present study. In lesions <3 cm, only one of four viable lesions showed FDG uptake. As there were no false positive results in this group either, it was suggested that a positive PET scan, even in small lesions, is highly specific for tumour viability.
In NSGCT patients with residual masses PET is less useful. PET can differentiate between viable disease and fibrosis (Fig. 7) [56, 64, 84]. The sensitivity and specificity of PET in the largest (75 scans in 55 men) series was 88% and 95%, respectively, with high negative predictive value (NPV) and positive predictive value (PPV) of 90% and 96%, compared with a PPV for CT in this circumstance of 56% [64]. However, its use is limited in this setting as differentiated teratoma (MTD) has variable low or no uptake and cannot be distinguished from fibrosis or necrosis. The crucial decision here is whether a marker response requires surgery or not and PET is unable to help these patients.
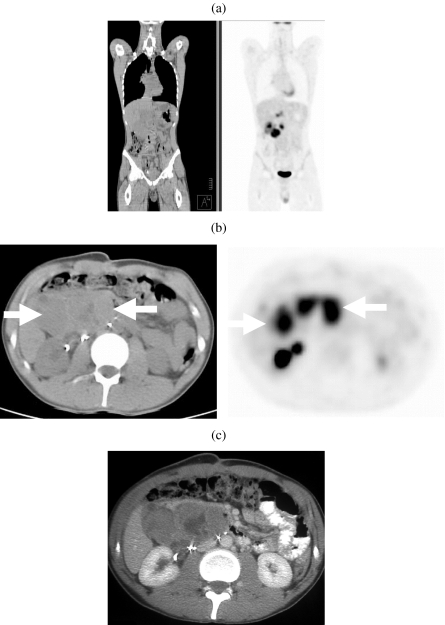
Figure 7.
Non-seminomatous germ cell tumour. (a) Coronal and (b) axial images on 18-FDG enhanced PETCT and (c) iodinated contrast enhanced CT showing a large metabolically active retroperitoneal mass (arrows).
The use of PET to predict response to treatment is uncertain. In contrast to salvage treatment one study found that PET was found more sensitive than CT or serum markers, 100% vs. 59% and 48%, respectively [85]. Though PET was shown not to be as specific as serum tumour markers for this purpose (78% vs. 100%, respectively) it did correctly predict all patients in this study who failed therapy. The overall accuracies of PET, CT and serum markers for prediction of response to high-dose chemotherapy in this study were 91%, 59% and 48%, respectively. There is little information to suggest that PET has a role in first-line treatment when standard assessment tools are successful.
Conclusion
Testicular germ cell tumours have been increasing in incidence and now represent 1% of all male tumours. Tumour presence is normally clinically diagnosed and confirmed by raised levels of serum markers. Imaging can confirm tumour presence but is most useful in assessing the sites of metastases. The most common sites of spread are to retroperitoneal lymph nodes and lung parenchyma. Staging of tumours (which is an integral process in establishing prognosis and therapy) is largely performed using CT. Recent studies have shown that the use of PET in specific scenarios may have significant advantage over CT. MR imaging is used in those patients with neurological disease or where intravenous contrast agent cannot be administered or as an aid to problem solving for equivocal CT.
References
- 1.Coakley FV, Hricak H, Presti JC Jr. Imaging and management of atypical testicular masses. Urol Clin North Am. 1998;25:375–88. doi: 10.1016/s0094-0143(05)70028-8. [DOI] [PubMed] [Google Scholar]
- 2.Guthrie JA, Fowler RC. Ultrasound diagnosis of testicular tumours presenting as epididymal disease. Clin Radiol. 1992;46:397–400. doi: 10.1016/s0009-9260(05)80686-5. [DOI] [PubMed] [Google Scholar]
- 3.Richie JP, Steele GS. Neoplasms of the testis. In: Walsh PC, Retik AB, Vaughan ED, editors. Campbell’s Urology. 8th edn. Philadelphia., PA: Saunders; 2001. pp. 2876–919. [Google Scholar]
- 4.Grantham JG, Charboneau JW, James EM, Kirschling RJ, Kvols LK, Segura JW, et al. Testicular neoplasms: 29 tumors studied by high-resolution US. Radiology. 1985;157:775–80. doi: 10.1148/radiology.157.3.2997838. [DOI] [PubMed] [Google Scholar]
- 5.Senay BA, Stein BS. Testicular neoplasm diagnosed by ultrasound. J Surg Oncol. 1986;32:110–12. doi: 10.1002/jso.2930320214. [DOI] [PubMed] [Google Scholar]
- 6.Rifkin MD, Kurtz AB, Pasto ME, Goldberg BB. Diagnostic capabilities of high-resolution scrotal ultrasonography: prospective evaluation. J Ultrasound Med. 1985;4:13–19. doi: 10.7863/jum.1985.4.1.13. [DOI] [PubMed] [Google Scholar]
- 7.Horstman WG, Melson GL, Middleton WD, Andriole GL. Testicular tumors: findings with color Doppler US. Radiology. 1992;185:733–7. doi: 10.1148/radiology.185.3.1438754. [DOI] [PubMed] [Google Scholar]
- 8.Johnson JO, Mattrey RF, Phillipson J. Differentiation of seminomatous from nonseminomatous testicular tumors with MR imaging. AJR Am J Roentgenol. 1990;154:539–43. doi: 10.2214/ajr.154.3.2106218. [DOI] [PubMed] [Google Scholar]
- 9.Thurnher S, Hricak H, Carroll PR, Pobiel RS, Filly RA. Imaging the testis: comparison between MR imaging and US. Radiology. 1988;167:631–6. doi: 10.1148/radiology.167.3.3283834. [DOI] [PubMed] [Google Scholar]
- 10.Sobin LH, Wittekind CH. 6th edn. New York: Wiley-Liss; 2002. UICC: TNM Classification of Malignant Tumours. [Google Scholar]
- 11.International Germ Cell Cancer Collaborative Group International germ cell consensus classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15:594–603. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]
- 12.Dixon AK, Ellis M, Sikora K. Computed tomography of testicular tumours: distribution of abdominal lymphadenopathy. Clin Radiol. 1986;37:519–23. doi: 10.1016/s0009-9260(86)80001-0. [DOI] [PubMed] [Google Scholar]
- 13.Mason MD, Featherstone T, Olliff J, Horwich A. Inguinal and iliac lymph node involvement in germ cell tumours of the testis: implications for radiological investigation and for therapy. Clin Oncol (R Coll Radiol) 1991;3:147–50. doi: 10.1016/s0936-6555(05)80835-0. [DOI] [PubMed] [Google Scholar]
- 14.Williams MP, Cook JV, Duchesne GM. Psoas nodes: an overlooked site of metastasis from testicular tumours. Clin Radiol. 1989;40:607–9. doi: 10.1016/s0009-9260(89)80319-8. [DOI] [PubMed] [Google Scholar]
- 15.Rouviere H. 1938. Anatomy of the Human Lymphatic System. Ann Arbor, MI: Edwards Brothers. [Google Scholar]
- 16.Macdonald JS, Paxton RM. Lymphography. In: Chisholm GD, editor. Scientific Foundations of Urology. Oxford: Heinemann Medical; 1976. [Google Scholar]
- 17.Wood A, Robson N, Tung K, Mead G. Patterns of supradiaphragmatic metastases in testicular germ cell tumours. Clin Radiol. 1996;51:273–6. doi: 10.1016/s0009-9260(96)80345-x. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez EB, Moul JW, Foley JP, Colon E, McLeod DG. Retroperitoneal imaging with third and fourth generation computed axial tomography in clinical stage I nonseminomatous germ cell tumors. Urology. 1994;44:548–52. doi: 10.1016/s0090-4295(94)80056-1. [DOI] [PubMed] [Google Scholar]
- 19.Schmoll HJ, Souchon R, Krege S, Albers P, Beyer J, Kollmannsberger C, et al. European consensus on diagnosis and treatment of germ cell cancer: a report of the European Germ Cell Cancer Consensus Group (EGCCCG) Ann Oncol. 2004;15:1377–99. doi: 10.1093/annonc/mdh301. [DOI] [PubMed] [Google Scholar]
- 20.White PM, Adamson DJ, Howard GC, Wright AR. Imaging of the thorax in the management of germ cell testicular tumours. Clin Radiol. 1999;54:207–11. doi: 10.1016/s0009-9260(99)91152-2. [DOI] [PubMed] [Google Scholar]
- 21.Richie JP. Detection and treatment of testicular cancer. CA Cancer J Clin. 1993;43:151–75. doi: 10.3322/canjclin.43.3.151. [DOI] [PubMed] [Google Scholar]
- 22.Husband JE, Grimer DP. Staging testicular tumours: the role of CT scanning. J R Soc Med. 1985;78(6):25–31. [PMC free article] [PubMed] [Google Scholar]
- 23.Muhm JR, Brown LR, Crowe JK. Detection of pulmonary nodules by computed tomography. AJR Am J Roentgenol. 1977;128:267–70. doi: 10.2214/ajr.128.2.267. [DOI] [PubMed] [Google Scholar]
- 24.Williams MP, Husband JE, Heron CW. Intrathoracic manifestations of metastatic testicular seminoma: a comparison of chest radiographic and CT findings. AJR Am J Roentgenol. 1987;149:473–5. doi: 10.2214/ajr.149.3.473. [DOI] [PubMed] [Google Scholar]
- 25.White PM, Howard GC, Best JJ, Wright AR. The role of computed tomographic examination of the pelvis in the management of testicular germ cell tumours. Clin Radiol. 1997;52:124–9. doi: 10.1016/s0009-9260(97)80105-5. [DOI] [PubMed] [Google Scholar]
- 26.White PM, Adamson DJ, Howard GC, Wright AR. Imaging of the thorax in the management of germ cell testicular tumours. Clin Radiol. 1999;54:207–11. doi: 10.1016/s0009-9260(99)91152-2. [DOI] [PubMed] [Google Scholar]
-
27.Bassalleck B, Berdoz A, Bradtke C, Broders R, Bunker B, Dennert H, et al. Measurement of spin-transfer observables in
 at 1.637 GeV/c. Phys Rev Lett. 2002;89:212–302. doi: 10.1103/PhysRevLett.89.212302. [DOI] [PubMed] [Google Scholar]
at 1.637 GeV/c. Phys Rev Lett. 2002;89:212–302. doi: 10.1103/PhysRevLett.89.212302. [DOI] [PubMed] [Google Scholar] - 28.Meyer CA, Conces DJ. Imaging of intrathoracic metastases of nonseminomatous germ cell tumors. Chest Surg Clin N Am. 2002;12:717–38. doi: 10.1016/s1052-3359(02)00032-7. [DOI] [PubMed] [Google Scholar]
- 29.Kozuka T, Johkoh T, Hamada S, Naito H, Tomiyama N, Koyama M, et al. Detection of pulmonary metastases with multi-detector row CT scans of 5-mm nominal section thickness: autopsy lung study. Radiology. 2003;226:231–4. doi: 10.1148/radiol.2261010394. [DOI] [PubMed] [Google Scholar]
- 30.Husband JE, Koh DM. Testicular germ cell tumours. In: Husband JE, Reznek RH, editors. Imaging in Oncology. 2nd edn. London: Taylor and Francis; 2004. pp. 401–27. [Google Scholar]
- 31.Freedman LS, Parkinson MC, Jones WG, Oliver RT, Peckham MJ, Read G, et al. Histopathology in the prediction of relapse of patients with stage I testicular teratoma treated by orchidectomy alone. Lancet. 1987;2:294–8. doi: 10.1016/s0140-6736(87)90889-0. [DOI] [PubMed] [Google Scholar]
- 32.Thompson PI, Nixon J, Harvey VJ. Disease relapse in patients with stage I nonseminomatous germ cell tumor of the testis on active surveillance. J Clin Oncol. 1988;6:1597–603. doi: 10.1200/JCO.1988.6.10.1597. [DOI] [PubMed] [Google Scholar]
- 33.Nicolai N, Pizzocaro G. A surveillance study of clinical stage I nonseminomatous germ cell tumors of the testis: 10-year followup. J Urol. 1995;154:1045–9. [PubMed] [Google Scholar]
- 34.Read G, Stenning SP, Cullen MH, Parkinson MC, Horwich A, Kaye SB, et al. Medical Research Council prospective study of surveillance for stage I testicular teratoma. Medical Research Council Testicular Tumors Working Party. J Clin Oncol. 1992;10:1762–8. doi: 10.1200/JCO.1992.10.11.1762. [DOI] [PubMed] [Google Scholar]
- 35.Peckham MJ, Barrett A, Husband JE, Hendry WF. Orchidectomy alone in testicular stage I non-seminomatous germ-cell tumours. Lancet. 1982;2:678–80. doi: 10.1016/s0140-6736(82)90710-3. [DOI] [PubMed] [Google Scholar]
- 36.Stomper PC, Fung CY, Socinski MA, Jochelson MS, Garnick MB, Richie JP. Detection of retroperitoneal metastases in early-stage nonseminomatous testicular cancer: analysis of different CT criteria. AJR Am J Roentgenol. 1987;149:1187–90. doi: 10.2214/ajr.149.6.1187. [DOI] [PubMed] [Google Scholar]
- 37.Lien HH, Stenwig AE, Ous S, Fossa SD. Influence of different criteria for abnormal lymph node size on reliability of computed tomography in patients with non-seminomatous testicular tumor. Acta Radiol Diagn (Stockh) 1986;27:199–203. doi: 10.1177/028418518602700212. [DOI] [PubMed] [Google Scholar]
- 38.Thomas JL, Bernardino ME, Bracken RB. Staging of testicular carcinoma: comparison of CT and lymphangiography. AJR Am J Roentgenol. 1981;137:991–6. doi: 10.2214/ajr.137.5.991. [DOI] [PubMed] [Google Scholar]
- 39.Rowland RG, Weisman D, Williams SD, Einhorn LH, Klatte EC, Donohue JP. Accuracy of preoperative staging in stages A and B nonseminomatous germ cell testis tumors. J Urol. 1982;127:718–20. doi: 10.1016/s0022-5347(17)54015-x. [DOI] [PubMed] [Google Scholar]
- 40.Richie JP, Garnick MB, Finberg H. Computerized tomography: how accurate for abdominal staging of testis tumors? J Urol. 1982;127:715–7. doi: 10.1016/s0022-5347(17)54013-6. [DOI] [PubMed] [Google Scholar]
- 41.Dorfman RE, Alpern MB, Gross BH, Sandler MA. Upper abdominal lymph nodes: criteria for normal size determined with CT. Radiology. 1991;180:319–22. doi: 10.1148/radiology.180.2.2068292. [DOI] [PubMed] [Google Scholar]
- 42.Magnusson A. Size of normal retroperitoneal lymph nodes. Acta Radiol Diagn (Stockh) 1983;24:315–8. doi: 10.1177/028418518302400407. [DOI] [PubMed] [Google Scholar]
- 43.Forsberg L, Dale L, Hoiem L, Magnusson A, Mikulowski P, Olsson AM, et al. Computed tomography in early stages of testicular carcinoma. Size of normal retroperitoneal lymph nodes and lymph nodes in patients with metastases in stage II A. A SWENOTECA study: Swedish-Norwegian Testicular Cancer Project. Acta Radiol Diagn (Stockh) 1986;27:569–74. doi: 10.1177/028418518602700516. [DOI] [PubMed] [Google Scholar]
- 44.Bokemeyer C, Nowak P, Haupt A, Metzner B, Kohne H, Hartmann JT, et al. Treatment of brain metastases in patients with testicular cancer. J Clin Oncol. 1997;15:1449–54. doi: 10.1200/JCO.1997.15.4.1449. [DOI] [PubMed] [Google Scholar]
- 45.Fossa SD, Bokemeyer C, Gerl A, Culine S, Jones WG, Mead GM, et al. Treatment outcome of patients with brain metastases from malignant germ cell tumors. Cancer. 1999;85:988–97. doi: 10.1002/(sici)1097-0142(19990215)85:4<988::aid-cncr29>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 46.Sohaib SA, Huddart R, Dearnaley DP, Horwich A, Husband J. Sensitivity of MRI in the diagnosis of retroperitoneal disease in testicular germ cell tumour. Am J Roentegenol. 2005;184(4):63. [Conference proceedings]. [Google Scholar]
- 47.Ellis JH, Bies JR, Kopecky KK, Klatte EC, Rowland RG, Donohue JP. Comparison of NMR and CT imaging in the evaluation of metastatic retroperitoneal lymphadenopathy from testicular carcinoma. J Comput Assist Tomogr. 1984;8:709–19. doi: 10.1097/00004728-198408000-00023. [DOI] [PubMed] [Google Scholar]
- 48.Lawton AJ, Mead GM. Staging and prognostic factors in testicular cancer. Semin Surg Oncol. 1999;17:223–9. doi: 10.1002/(sici)1098-2388(199912)17:4<223::aid-ssu2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 49.Rockall AG, Sohaib SA, Harisinghani MG, Babar SA, Singh N, Jeyarajah AR, et al. Diagnostic performance of nanoparticle-enhanced magnetic resonance imaging in the diagnosis of lymph node metastases in patients with endometrial and cervical cancer. J Clin Oncol. 2005;23:2813–21. doi: 10.1200/JCO.2005.07.166. [DOI] [PubMed] [Google Scholar]
- 50.Harisinghani MG, Saksena M, Ross RW, Tabatabaei S, Dahl D, McDougal S, et al. A pilot study of lymphotrophic nanoparticle-enhanced magnetic resonance imaging technique in early stage testicular cancer: a new method for noninvasive lymph node evaluation. Urology. 2005;66:1066–71. doi: 10.1016/j.urology.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 51.Harisinghani MG, Saini S, Weissleder R, Hahn PF, Yantiss RK, Tempany C, et al. MR lymphangiography using ultrasmall superparamagnetic iron oxide in patients with primary abdominal and pelvic malignancies: radiographic–pathologic correlation. AJR Am J Roentgenol. 1999;172:1347–51. doi: 10.2214/ajr.172.5.10227514. [DOI] [PubMed] [Google Scholar]
- 52.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348:2491–9. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 53.Anzai Y, Piccoli CW, Outwater EK, Stanford W, Bluemke DA, Nurenberg P, et al. Evaluation of neck and body metastases to nodes with ferumoxtran 10-enhanced MR imaging: phase III safety and efficacy study. Radiology. 2003;228:777–88. doi: 10.1148/radiol.2283020872. [DOI] [PubMed] [Google Scholar]
- 54.Bellin MF, Lebleu L, Meric JB. Evaluation of retroperitoneal and pelvic lymph node metastases with MRI and MR lymphangiography. Abdom Imaging. 2003;28:155–63. doi: 10.1007/s00261-001-0182-9. [DOI] [PubMed] [Google Scholar]
- 55.Koh DM, Brown G, Temple L, Raja A, Toomey P, Bett N, et al. Rectal cancer: mesorectal lymph nodes at MR imaging with USPIO versus histopathologic findings: initial observations. Radiology. 2004;231:91–9. doi: 10.1148/radiol.2311030142. [DOI] [PubMed] [Google Scholar]
- 56.Cremerius U, Effert PJ, Adam G, Sabri O, Zimmy M, Wagenknecht G, et al. FDG PET for detection and therapy control of metastatic germ cell tumor. J Nucl Med. 1998;39:815–22. [PubMed] [Google Scholar]
- 57.Ravi R, Ong J, Oliver RT, Badenoch DF, Fowler CG, Hendry WF. The management of residual masses after chemotherapy in metastatic seminoma. BJU Int. 1999;83:649–53. doi: 10.1046/j.1464-410x.1999.00974.x. [DOI] [PubMed] [Google Scholar]
- 58.Albers P, Bender H, Yilmaz H, Schoeneich G, Biersack HJ, Mueller SC. Positron emission tomography in the clinical staging of patients with Stage I and II testicular germ cell tumors. Urology. 1999;53:808–11. doi: 10.1016/s0090-4295(98)00576-7. [DOI] [PubMed] [Google Scholar]
- 59.Hain SF, O’Doherty MJ, Timothy AR, Leslie MD, Partridge SE, Huddart RA. Fluorodeoxyglucose PET in the initial staging of germ cell tumours. Eur J Nucl Med. 2000;27:590–4. doi: 10.1007/s002590050547. [DOI] [PubMed] [Google Scholar]
- 60.Cremerius U, Wildberger JE, Borchers H, Zimny M, Jakse G, Gunther RW, et al. Does positron emission tomography using 18-fluoro-2-deoxyglucose improve clinical staging of testicular cancer? Results of a study in 50 patients. Urology. 1999;54:900–4. doi: 10.1016/s0090-4295(99)00272-1. [DOI] [PubMed] [Google Scholar]
- 61.Gambhir SS, Czernin J, Schwimmer J, Silverman DH, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med. 2001;42:1S–93S. [PubMed] [Google Scholar]
- 62.Karapetis CS, Strickland AH, Yip D, Steer C, Harper PG. Use of fluorodeoxyglucose positron emission tomography scans in patients with advanced germ cell tumour following chemotherapy: single-centre experience with long-term follow up. Intern Med J. 2003;33:427–35. doi: 10.1046/j.1445-5994.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 63.Hain SF, Maisey MN. Positron emission tomography for urological tumours. BJU Int. 2003;92:159–64. doi: 10.1046/j.1464-410x.2003.04284.x. [DOI] [PubMed] [Google Scholar]
- 64.Hain SF, O’Doherty MJ, Timothy AR, Leslie MD, Harper PG, Huddart RA. Fluorodeoxyglucose positron emission tomography in the evaluation of germ cell tumours at relapse. Br J Cancer. 2000;83:863–9. doi: 10.1054/bjoc.2000.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huddart R, O’Doherty M, Padhani AR, Rustin GJ, Mead GM, Selby P, et al. A prospective study of 18FDG PET in the prediction of relapse in patients with high risk clinical stage 1 non-seminomatous germ cell cancer (MRCstudy TE22) European Journal of Cancer. 2005;3(2):227. [Abstract]. [Google Scholar]
- 66.Jones RH, Vasey PA. Part I: testicular cancer: management of early disease. Lancet Oncol. 2003;4:730–7. doi: 10.1016/s1470-2045(03)01278-6. [DOI] [PubMed] [Google Scholar]
- 67.Freedman LS, Parkinson MC, Jones WG, Oliver RT, Peckham MJ, Read G, et al. Histopathology in the prediction of relapse of patients with stage I testicular teratoma treated by orchidectomy alone. Lancet. 1987;2:294–8. doi: 10.1016/s0140-6736(87)90889-0. [DOI] [PubMed] [Google Scholar]
- 68.Read G, Stenning SP, Cullen MH, Parkinson MC, Horwich A, Kaye SB, et al. Medical Research Council prospective study of surveillance for stage I testicular teratoma. Medical Research Council Testicular Tumors Working Party. J Clin Oncol. 1992;10:1762–8. doi: 10.1200/JCO.1992.10.11.1762. [DOI] [PubMed] [Google Scholar]
- 69.Nathan PD, Rustin GR. The role of CT scanning in the surveillance of testicular tumours. Clin Oncol (R Coll Radiol) 2003;15:121–2. doi: 10.1053/clon.2002.0194. [DOI] [PubMed] [Google Scholar]
- 70.Harvey ML, Geldart TR, Duell R, Mead GM, Tung K. Routine computerised tomographic scans of the thorax in surveillance of stage I testicular non-seminomatous germ-cell cancer: a necessary risk? Ann Oncol. 2002;13:237–42. doi: 10.1093/annonc/mdf032. [DOI] [PubMed] [Google Scholar]
- 71.Crawford SM, Rustin GJ, Begent RH, Newlands ES, Bagshawe KD. Safety of surveillance in the management of stage I anaplastic germ cell tumours of the testis. Br J Urol. 1988;61:250–3. doi: 10.1111/j.1464-410x.1988.tb06389.x. [DOI] [PubMed] [Google Scholar]
- 72.Hellerstedt BA, Pienta KJ. Germ cell tumors: review of selected studies from 2002. Curr Opin Oncol. 2003;15:234–8. doi: 10.1097/00001622-200305000-00010. [DOI] [PubMed] [Google Scholar]
- 73.Gietema JA, Meinardi MT, Sleijfer DT, Hoekstra HJ, van der Graaf WT. Routine chest X-rays have no additional value in the detection of relapse during routine follow-up of patients treated with chemotherapy for disseminated non-seminomatous testicular cancer. Ann Oncol. 2002;13:1616–20. doi: 10.1093/annonc/mdf282. [DOI] [PubMed] [Google Scholar]
- 74.Lentini JF, Love MB, Ritchie WG, Sedlacek TV. Computed tomography in retroconversion of hepatic metastases from immature ovarian teratoma. J Comput Assist Tomogr. 1986;10:1060–2. doi: 10.1097/00004728-198611000-00037. [DOI] [PubMed] [Google Scholar]
- 75.Moskovic E, Jobling T, Fisher C, Wiltshaw E, Parsons C. Retroconversion of immature teratoma of the ovary: CT appearances. Clin Radiol. 1991;43:402–8. doi: 10.1016/s0009-9260(05)80570-7. [DOI] [PubMed] [Google Scholar]
- 76.Connor S, Guest P. Conversion of multiple solid testicular teratoma metastases to fatty and cystic liver masses following chemotherapy: CT evidence of ‘maturation’. Br J Radiol. 1999;72:1114–16. doi: 10.1259/bjr.72.863.10700831. [DOI] [PubMed] [Google Scholar]
- 77.Williams MP, Naik G, Heron CW, Husband JE. Computed tomography of the abdomen in advanced seminoma: response to treatment. Clin Radiol. 1987;38:629–33. doi: 10.1016/s0009-9260(87)80345-8. [DOI] [PubMed] [Google Scholar]
- 78.Charig MJ, Williams MP. Pulmonary lacunae: sequelae of metastases following chemotherapy. Clin Radiol. 1990;42:93–6. doi: 10.1016/s0009-9260(05)82075-6. [DOI] [PubMed] [Google Scholar]
- 79.Husband JE, Barrett A, Peckham MJ. The role of computed tomography in the assessment of tumour volume in patients with malignant testicular teratoma. Br J Radiol. 1981;15:50–3. [Google Scholar]
- 80.Hendry WF, A’Hern RP, Hetherington JW, Peckham MJ, Dearnaley DP, Horwich A. Para-aortic lymphadenectomy after chemotherapy for metastatic non-seminomatous germ cell tumours: prognostic value and therapeutic benefit. Br J Urol. 1993;71:208–13. doi: 10.1111/j.1464-410x.1993.tb15920.x. [DOI] [PubMed] [Google Scholar]
- 81.Sugawara Y, Zasadny KR, Grossman HB, Francis IR, Clarke MF, Wahl RL. Germ cell tumor: differentiation of viable tumor, mature teratoma, and necrotic tissue with FDG PET and kinetic modeling. Radiology. 1999;211:249–56. doi: 10.1148/radiology.211.1.r99ap16249. [DOI] [PubMed] [Google Scholar]
- 82.De Santis M, Becherer A, Bokemeyer C, Stoiber F, Oechsle K, Sellner F, et al. 2-18fluoro-deoxy-D-glucose positron emission tomography is a reliable predictor for viable tumor in postchemotherapy seminoma: an update of the prospective multicentric SEMPET trial. J Clin Oncol. 2004;22:1034–9. doi: 10.1200/JCO.2004.07.188. [DOI] [PubMed] [Google Scholar]
- 83.Becherer A, De Santis M, Karanikas G, Szabo M, Bokemeyer C, Dohmen BM, et al. FDG PET is superior to CT in the prediction of viable tumour in post-chemotherapy seminoma residuals. Eur J Radiol. 2005;54:284–8. doi: 10.1016/j.ejrad.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 84.Stephens AW, Gonin R, Hutchins GD, Einhorn LH. Positron emission tomography evaluation of residual radiographic abnormalities in postchemotherapy germ cell tumor patients. J Clin Oncol. 1996;14:1637–41. doi: 10.1200/JCO.1996.14.5.1637. [DOI] [PubMed] [Google Scholar]
- 85.Bokemeyer C, Kollmannsberger C, Oechsle K, Dohmen BM, Pfannenberg A, Claussen CD, et al. Early prediction of treatment response to high-dose salvage chemotherapy in patients with relapsed germ cell cancer using [(18)F]FDG PET. Br J Cancer. 2002;86:506–11. doi: 10.1038/sj.bjc.6600122. [DOI] [PMC free article] [PubMed] [Google Scholar]