Abstract
Objective: To study the value of high-frequency sonography (HFS) and colour Doppler sonography (CDS) in evaluating the 5 year metastatic potential of primary cutaneous melanomas (CM). Materials and methods: 111 CM were studied before surgical resection and 107 were depicted on HFS. The maximal HFS thickness was measured and compared with the Breslow thickness. A CDS study was performed in each tumour. Results: HFS thickness ranged from 0.26 to 8.0 mm and Breslow thickness from 0.15 to 8.0 mm. HFS and Breslow thickness correlated strongly (r>0.93). Intratumour vessels were depicted in 43 of the 107 CM, of which 40 were thicker than 2 mm. The median follow-up was 61 months and 27 patients developed relapses. In the univariate analyses, neovascularization visualized with CDS, sonographic thickness and the Breslow thickness were significantly linked to relapses (p<0.0001), as were lymph node status and ulceration (p=0.007 and 0.004). Conclusion: Vascularization was observed mainly in thick primary melanoma. A median follow-up of 5 years showed the prognostic value of angiogenesis evaluated by CDS.
Keywords: Melanoma, angiogenesis, Doppler sonography, ultrasonography, histology
Introduction
The Breslow thickness is considered to be the most accurate prognostic factor for the risk of metastasis in malignant melanoma [1]. During the first year, the metastasis rate after excision is less than 1% in tumours with a Breslow thickness of less than 0.76 mm, and it reaches 33% in tumours with a Breslow thickness exceeding 4 mm. Several studies have demonstrated the accuracy of sonography in the preoperative evaluation of melanoma thickness [2–6]. Colour Doppler sonography (CDS) has demonstrated its usefulness for the study of intratumour vascularization and the number and size of vessels visualized with CDS are significantly correlated with pathological parameters evaluated by immunochemical studies using anti-factor VIII antibody [7]. The purpose of the present study was to investigate the accuracy of high-frequency sonography (HFS) in the preoperative evaluation of melanoma thickness and the prognostic significance of vascularization evaluated with CDS in a series of patients with primary cutaneous melanomas after a follow-up period of 5 years.
Materials and methods
Patients
The protocol was approved by our Institutional Review Board and all patients signed an informed consent form.
The patients were included in the study from November 1995 to June 2001. The study population included 111 patients, 49 males and 62 females (mean age 64 years; age range 27–97 years). Four patients had two synchronous primary melanomas. In these four patients, only the thickest melanoma was taken into account for the survival analyses. Four patients with a melanoma not identified by sonography were also excluded from the analyses. Thus the population analysed consisted of 107 patients (107 melanomas).
Surgical margins for re-excision were defined based on the Breslow index: for <1 mm thick lesions, the re-excision margin was 1 cm, between 1.1 and 4 mm, the re-excision margin was 2 cm, and finally the re-excision margin was 3 cm for >4 mm thick lesions. The sentinel node procedure was an option introduced in 1998 for the treatment of primary melanoma thicker than 1.5 mm (or less in the case of pathological regression) [8]. Follow-up clinical examinations of patients were scheduled according to the following scheme over 5 years: (i) Breslow thickness <1.5 mm, visit every 6 months, and (ii) Breslow thickness >1.5 mm, visit every 3 months. Follow-up data were updated in February 2004.
Ultrasonography equipment
The cutaneous melanomas were analysed with HFS and CDS before surgical excision. We used two ultrasound machines:
an AU 5 Idea Sonograph (Esaote-Biomedica, Genoa, Italy) with a high-frequency probe (a 13 MHz linear electronic probe)
a PowerVision 8000 (Toshiba, Japan) with a high-frequency probe (12 MHz linear electronic probe).
Standard sonographic gel was used to separate the probe face from the skin surface and to avoid flattening the melanoma. The focal zone was adapted according to the position of the tumour in the superficial tissue.
Ultrasonography morphological study
A sonographic morphological study was performed for each tumour to analyse the structural sonographic pattern, margin delineation and echogenicity compared with the adjacent cutaneous tissue (hypoechoic or hyperechoic) and to measure tumour thickness on the workstation screen using electronic calipers (accurate to the nearest 0.01 mm). Lesion maximal thickness was determined by measuring from the skin surface to the deepest point of the posterior margin.
Ultrasonography Doppler study
We used CDS with a pulse repetition frequency of 750 Hz, an imaging frequency of 9–16 images per second and a 50 Hz acoustic filter to search for intratumour vessels in 107 visible melanomas. Quantification of the number of vessels per tumour was performed by obtaining multiple cross-sections of the tumours. Tumours with more than one vessel were those in which several pixels corresponding to tumour vessels were not contiguous. Contiguity between vessels was assessed across all sections. A semi-quantitative assessment of the degree of angiogenesis was performed according to the number of vessels visualized. Vascularization was considered present when one vessel was visualized, and abundant when two or more vessels were visualized.
Histological analyses
After surgical resection, tumours were analysed histologically to determine the Breslow thickness. The pathologist was unaware of the thickness of the lesion, as measured by sonography.
Statistical analyses
The study compared: (i) thickness measured both by sonography and according to the Breslow method, (ii) Breslow thickness and vascularization detected by CDS, (iii) tumour progression with sonographic and pathological parameters.
The linear relationship between the Breslow measurement and the sonographic measurement was estimated and Pearson’s correlation coefficient calculated. The association between Breslow thickness and vascularization was tested with the Spearman rank correlation test.
The prognostic value of the Breslow thickness and of vascularization was based on the occurrence of the first relapse and tested by log-rank tests in univariate analyses and by the Cox proportional hazards regression model in a multivariate analysis.
Results
Among the 111 melanomas included, 107 were depicted on sonography. They were hypoechoic with a homogeneous echostructure and well defined lower and lateral margins (Fig. 1). Four tumours were not visible at sonography; all had a Breslow thickness of less than 0.80 mm. As measured by sonography, the thickness of the remaining tumours ranged from 0.26 to 8.0 mm; their Breslow thickness ranged from 0.15 to 8.0 mm. Sonography measurements correlated strongly with the Breslow thickness (Pearson’s correlation r>0.93; p<0.0001). The mean difference between the Breslow thickness and the sonography measurement was equal to 0.006 mm.
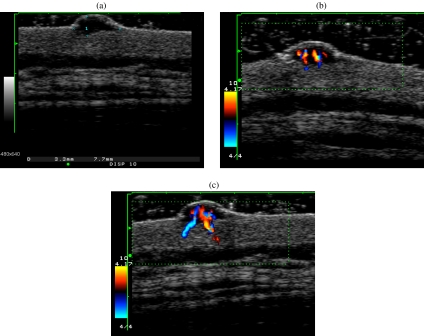
Figure 1.
Melanoma of the lumbar back in a 48-year-old man. (a) The tumour visualized by high-frequency sonography is hypoechoic with a maximal tumour thickness measuring 3.3 mm. (b), (c) The colour Doppler study depicted abundant vascularization in the tumour.
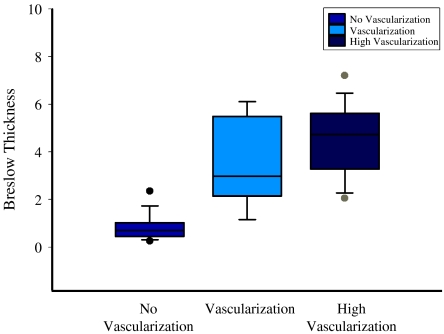
Three categories of melanomas were identified according to vascularization: tumours without visible vessels, vascularized tumours (one visible vessel), tumours with abundant (two or more vessels) vascularization. The relationship between the Breslow thickness and vascularization according to these three categories is shown in Fig. 2.
Figure 2.
Box plot showing the distribution between the Breslow thickness and tumour vascularization according to three categories: tumours without visible vessels, tumours with one visible vessel, tumours with abundant (two or more vessels) vascularization.
On CDS, the 64 melanomas in which vessels were not seen had a mean sonographic thickness of 1 mm (range 0.25–2.5 mm) and a mean Breslow thickness of 0.97 mm (range 0.26–3.2 mm). The 43 melanomas in which vessels were visualized had a mean sonographic thickness of 4.18 mm (range 1.9–8 mm) and a mean Breslow thickness of 4.22 mm (range 0.6–8 mm) (Fig. 1).
In 24 cases with abundant vascularization, the mean Breslow thickness was equal to 4.7 mm (range 2–8 mm) and sonography thickness was 4.4 mm (range 1.9–8 mm). The correlation between vascularization and the Breslow thickness was highly significant (p<0.0001). In the present study population, 22 patients underwent a sentinel node procedure. Only one patient with a non-vascularized primary melanoma had a metastatic sentinel node, but did not present any progression.
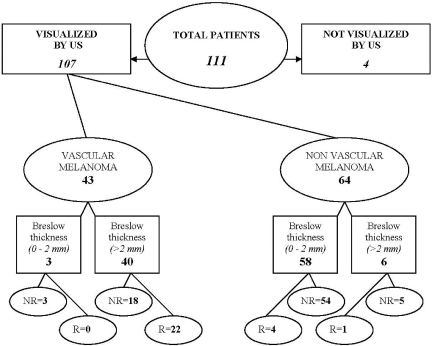
The median follow-up of the 107 patients was 61 months (58.6 ± 2.3 SD, range 1.8–98 months). Twenty-seven patients developed progressive disease (Fig. 3). Of these, 19 patients presented a first relapse in regional lymph nodes (n=10) or visceral metastases (n=9). Only one patient had an isolated local recurrence as a first relapse. The other seven patients had synchronous regional recurrences and distant metastases. Fourteen patients died: 12 of tumour progression and two of cardiovascular problems without tumour relapse.
Figure 3.
5 year follow-up of 107 patients according to the vascularization detected by colour Doppler and the Breslow thickness (US = ultrasonography, R = relapses, NR = no relapses).
In the univariate analyses, vascularization visualized at sonography, sonographic thickness and the Breslow thickness were significantly linked to relapses (p<0.0001). The lymph node status and ulceration were also significantly linked to relapses (p=0.007 and 0.004).
In the multivariate Cox proportional hazards regression analysis, the factor significantly correlated with disease progression was the Breslow thickness (p<0.0001).
However, the Breslow thickness and vascularization were highly correlated. A second analysis was performed without taking into account the Breslow thickness. In this second analysis, vascularization was the only prognostic parameter tested. The probability of disease progression with this second model was not significantly different from that of the original model, suggesting that vascularization and the Breslow thickness confer equivalent prognostic information.
Discussion
This study confirms the value of high-frequency (13 MHz) sonography for the preoperative assessment of primary cutaneous melanomas. Melanomas are visualized and accurately measured with this technique [2–4, 7] and sonographic measurement is highly correlated with the Breslow thickness. These findings are consistent with the results of other studies [3, 9–11], which reported high Pearson correlation coefficients (from 0.88 to 0.95). In the present study, four primary melanomas were not identified by sonography. These melanomas had a Breslow thickness ranging from 0.10 to 0.80 mm. This observation is in accordance with another study, in which some thin melanomas could not be identified by sonography [12].
Tumour growth is dependent on the development of neovessels [13]. Before the growth of tumour blood vessels, cell nutrients are delivered via passive diffusion. Rapid tumour expansion is dependent on the emergence of new capillary blood vessels that vascularize the tumour [14]. Angiogenesis may be an important factor for the development of metastasis from cutaneous malignant melanomas [15, 16]. In the present study, tumour vascularity was investigated in order to determine its prognostic value and to compare this value with that of the Breslow thickness, which is currently considered the most important prognostic factor. Blood flow was studied for the first time in vivo in 71 melanomas by Srivastava et al. using 10 MHz continuous Doppler sonography [17]. They found that 44 melanomas had Doppler flow signals and that this signal was present in all tumours with a Breslow thickness exceeding 0.8 mm except for one tumour. They estimated that the onset of vascularization begins at this thickness. They also found a significant correlation between a higher peak systolic frequency in tumours from patients who developed a relapse than in tumours from patients without relapse. However, this was studied in a small number of patients (n=21) with a follow-up of 2 years. It is not possible to directly visualize blood flow with their technique.
Three studies have shown that CDS can visualize vessels in melanomas or in cutaneous metastases from melanomas [18–20]. We have previously shown that intratumour vessels can be visualized directly with HFS and CDS and that spectra can be recorded with pulsed Doppler sonography. The correlation with three histological parameters (microvessel density, the number of vessels measuring >100 μ m in diameter, and the diameter of the largest vessel) showed that CDS can visualize vessels greater than 100 μm and thus evaluate neoangiogenesis in vivo [21–23].
The present study shows that CDS allows the evaluation of vascularization in melanomas and confirms the relationship between the thickness of the melanoma and the extent of vascularization, in a larger number of patients. Vessels were mainly detected in melanomas that were thicker than 2 mm: 87% of melanomas thicker than 2 mm were vascularized vs. 5% of melanomas thinner than 2 mm. Most tumours (10/13) with at least two vessels were thicker than 4 mm. In their pathological study, Srivastava et al. noted that melanomas with a Breslow thickness between 0.76 and 4.0 mm and with an increased vascular area seen at histology were more likely to metastasize than tumours with a comparable thickness but significantly less neovasculature [24]. Barnhill and Levy, who specifically studied the vascular aspects of melanomas with a very low metastatic potential in theory (<1.0 mm thick), showed that melanomas associated with angiogenesis had a poorer prognosis than those without angiogenesis [25]. Pathological results reported by Straume and Akslen also confirmed that microvessel density (MVD) was an independent prognostic variable in vertical growth phase melanomas [26]. In addition, they showed that the intratumour/tumour base ratio for MVD increased with tumour thickness.
The present study shows that angiogenesis assessed by CDS before surgical excision of primary melanomas is significantly correlated with relapse. Among patients with a primary melanoma thicker than 2 mm, 55% patients with a vascularized melanoma relapsed compared with 17% whose melanoma was not vascularized.
Vessels identified on CDS seem to be indicative of tumours with a high metastatic potential. Abundant vascularity detectable with CDS corresponds to the phase of rapid tumour growth and is correlated with the rate of metastasis.
These results indicate that the follow-up strategy should be particularly attentive to patients with vascularized primary melanomas.
Although sonography is a reliable method for the measurement of tumour thickness, and a useful tool for surgical planning, sonography alone cannot reliably assess differences between benign and malignant tumours [27, 28]. Sonography with colour Doppler appears to be an efficient tool for the differential diagnosis between benign and malignant tumours (size > 2 mm) and for the management of these neoplasms [29].
Treatment of primary cutaneous melanoma is usually a two-step procedure. An excisional biopsy is performed in order to confirm both the diagnosis of malignancy and prognostic features: Breslow thickness, ulceration, regression phenomena. Thereafter, the treatment is planned according to these prognostic features. Surgical treatment consists of re-excision with free margins whose size is determined according to the Breslow thickness and, possibly, regression phenomena. These criteria are also used to propose a sentinel node procedure. Sonography accurately depicts the maximum thickness of the great majority of primary cutaneous melanomas. A one-step treatment procedure is conceivable, provided the malignancy can be assessed following a small biopsy at the edge of the tumour. Benefits in terms of pain attenuation, patient comfort, time saving, and lower costs need to be assessed in a randomized prospective study.
Contrast agents can also be used with sonography to improve tumour vessel detection. Ongoing studies are investigating higher frequencies (40–100 MHz) for better tissue characterization and vessel detection with contrast enhancement [30].
This study confirms that high-frequency sonography is a simple, reliable, noninvasive method for accurate preoperative measurement of the thickness of melanomas. Surgical planning could be adapted according to this measurement. The angiogenesis evaluated with CDS could be used to identify melanomas with a high metastatic potential.
Acknowledgment
The authors are grateful to Lorna Saint Ange for editing.
Footnotes
This study was presented at the ASCO meeting in 2002 (oral communication). This study was presented at the RSNA meeting in 2002 (oral communication).
References
- 1.Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970;172:902–8. doi: 10.1097/00000658-197011000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gassenmaier G, Kiesewetter F, Schell H, Zinner M. Value of high resolution ultrasound in determination of vertical tumor thickness in malignant melanoma of the skin. Hautarzt. 1990;41:360–4. [PubMed] [Google Scholar]
- 3.Hoffmann K, Jung J, el Gammal S, Altmeyer P. Malignant melanoma in 20-MHz B scan sonography. Dermatology. 1992;185:49–55. doi: 10.1159/000247403. [DOI] [PubMed] [Google Scholar]
- 4.Harland CC, Bamber JC, Gusterson BA, Mortimer PS. High frequency, high resolution B-scan ultrasound in the assessment of skin tumours. Br J Dermatol. 1993;128:525–32. doi: 10.1111/j.1365-2133.1993.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 5.Fornage BD, McGavran MH, Duvic M, Waldron CA. Imaging of the skin with 20-MHz US. Radiology. 1993;189:69–76. doi: 10.1148/radiology.189.1.8372222. [DOI] [PubMed] [Google Scholar]
- 6.Lassau N, Spatz A, Avril MF, Tardivon A, Margulis A, Mamelle G, et al. Value of high-frequency US for preoperative assessment of skin tumors. Radiographics. 1997;17:1559–65. doi: 10.1148/radiographics.17.6.9397463. [DOI] [PubMed] [Google Scholar]
- 7.Lassau N, Mercier S, Koscielny S, Avril MF, Margulis A, Mamelle G, et al. Prognostic value of high-frequency sonography and color Doppler sonography for the preoperative assessment of melanomas. Am J Roentgenol. 1999;172:457–61. doi: 10.2214/ajr.172.2.9930803. [DOI] [PubMed] [Google Scholar]
- 8.Gershenwald JE, Thompson W, Mansfield PF, Lee JE, Colome MI, Tseng CH, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999;17:976–83. doi: 10.1200/JCO.1999.17.3.976. [DOI] [PubMed] [Google Scholar]
- 9.Tacke J, Haagen G, Hornstein OP, Huettinger G, Kiesewetter F, Schell H, et al. Clinical relevance of sonometry-derived tumour thickness in malignant melanoma—a statistical analysis. Br J Dermatol. 1995;132:209–14. doi: 10.1111/j.1365-2133.1995.tb05015.x. [DOI] [PubMed] [Google Scholar]
- 10.Solivetti FM, Thorel MF, Di Luca Sidozzi A, Bucher S, Donati P, Panichelli V. Role of high-definition and high frequency ultrasonography in determining tumor thickness in cutaneous malignant melanoma. Radiol Med (Torino) 1998;96:558–61. [PubMed] [Google Scholar]
- 11.Serrone L, Solivetti FM, Thorel MF, Eibenschutz L, Donati P, Catricala C. High frequency ultrasound in the preoperative staging of primary melanoma: a statistical analysis. Melanoma Res. 2002;12:287–90. doi: 10.1097/00008390-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Pellacani G, Seidenari S. Preoperative melanoma thickness determination by 20-MHz sonography and digital videomicroscopy in combination. Arch Dermatol. 2003;139:293–8. doi: 10.1001/archderm.139.3.293. [DOI] [PubMed] [Google Scholar]
- 13.Fallowfield ME, Cook MG. The vascularity of primary cutaneous melanoma. J Pathol. 1991;164:241–4. doi: 10.1002/path.1711640309. [DOI] [PubMed] [Google Scholar]
- 14.Denekamp J. Review article: angiogenesis, neovascular proliferation and vascular pathophysiology as targets for cancer therapy. Br J Radiol. 1993;66:181–96. doi: 10.1259/0007-1285-66-783-181. [DOI] [PubMed] [Google Scholar]
- 15.Folkman J. What is the role of angiogenesis in metastasis from cutaneous melanoma? Eur J Cancer Clin Oncol. 1987;23:361–3. doi: 10.1016/0277-5379(87)90370-1. [DOI] [PubMed] [Google Scholar]
- 16.Barnhill RL, Mihm MC Jr, Ceballos PI. Angiogenesis and regressing cutaneous malignant melanoma. Lancet. 1992;339:991–2. doi: 10.1016/0140-6736(92)91569-t. [DOI] [PubMed] [Google Scholar]
- 17.Srivastava A, Hughes LE, Woodcock JP, Laidler P. Vascularity in cutaneous melanoma detected by Doppler sonography and histology: correlation with tumour behaviour. Br J Cancer. 1989;59:89–91. doi: 10.1038/bjc.1989.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nazarian LN, Alexander AA, Kurtz AB, Capuzzi Jr DM, Rawool NM, Gilbert KR, et al. Superficial melanoma metastases: appearances on gray-scale and color Doppler sonography. Am J Roentgenol. 1998;170:459–63. doi: 10.2214/ajr.170.2.9456964. [DOI] [PubMed] [Google Scholar]
- 19.Alexander AA, Nazarian LN, Capuzzi DM Jr, Rawool NM, Kurtz AB, Mastrangelo MJ. Color Doppler sonographic detection of tumor flow in superficial melanoma metastases: histologic correlation. J Ultrasound Med. 1998;17:123–6. doi: 10.7863/jum.1998.17.2.123. [DOI] [PubMed] [Google Scholar]
- 20.Giovagnorio F, Andreoli C, De Cicco ML. Color Doppler sonography of focal lesions of the skin and subcutaneous tissue. J Ultrasound Med. 1999;18:89–93. doi: 10.7863/jum.1999.18.2.89. [DOI] [PubMed] [Google Scholar]
- 21.Lassau N, Leclere J, Vassal G, Guinebretiere JM, Roche A. Etude de l’angiogénèse tumorale par échographie Doppler couleur, énergie et pulsé sur un modèle animal. J Radiol. 1996;77:851. [Google Scholar]
- 22.Lassau N, Paturel-Asselin C, Guinebretiere JM, Leclere J, Koscielny S, Roche A, et al. New hemodynamic approach to angiogenesis: color and pulsed Doppler ultrasonography. Invest Radiol. 1999;34:194–8. doi: 10.1097/00004424-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Asselin-Paturel C, Lassau N, Guinebretiere JM, Zhang J, Gay F, Bex F, et al. Transfer of the murine interleukin-12 gene in vivo by a Semliki Forest virus vector induces B16 tumor regression through inhibition of tumor blood vessel formation monitored by Doppler ultrasonography. Gene Ther. 1999;6:606–15. doi: 10.1038/sj.gt.3300841. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava A, Laidler P, Davies RP, Horgan K, Hughes LE. The prognostic significance of tumor vascularity in intermediate-thickness (0.76–4.0 mm thick) skin melanoma. A quantitative histologic study. Am J Pathol. 1988;133:419–23. [PMC free article] [PubMed] [Google Scholar]
- 25.Barnhill RL, Levy MA. Regressing thin cutaneous malignant melanomas (< or = 1.0 mm) are associated with angiogenesis. Am J Pathol. 1993;143:99–104. [PMC free article] [PubMed] [Google Scholar]
- 26.Straume O, Akslen LA. Expresson of vascular endothelial growth factor, its receptors (FLT-1, KDR) and TSP-1 related to microvessel density and patient outcome in vertical growth phase melanomas. Am J Pathol. 2001;159:223–35. doi: 10.1016/S0002-9440(10)61688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dummer W, Blaheta HJ, Bastian BC, Schenk T, Brocker EV, Remy W. Preoperative characterization of pigmented skin lesions by epiluminescence microscopy and high-frequency ultrasound. Arch Dermatol. 1995;131:279–85. [PubMed] [Google Scholar]
- 28.Hoffmann K, Happe M, Schuller S, Stucker M, Wiesner M, Gottlober P, et al. Ranking of 20 MHz sonography of malignant melanoma and pigmented lesions in routine diagnosis. Ultraschall Med. 1999;20:104–9. doi: 10.1055/s-1999-14245. [DOI] [PubMed] [Google Scholar]
- 29.Bessoud B, Lassau N, Koscielny S, Longvert C, Avril MF, Duvillard P, et al. High-frequency sonography and color Doppler in the management of pigmented skin lesions. Ultrasound Med Biol. 2003;29:875–9. doi: 10.1016/s0301-5629(03)00035-8. [DOI] [PubMed] [Google Scholar]
- 30.Albrecht T, Urbank A, Mahler M, Bauer A, Dore CJ, Blomley MJ, et al. Prolongation and optimization of Doppler enhancement with a microbubble US contrast agent by using continuous infusion: preliminary experience. Radiology. 1998;207:339–47. doi: 10.1148/radiology.207.2.9577478. [DOI] [PubMed] [Google Scholar]