Abstract
Previous studies have reported low negative predictive values (NPV) for computed tomography (CT)-guided fine needle aspiration of lung nodules in excluding malignancy. Our aim was to determine the NPV of transthoracic core needle biopsy in a tertiary care hospital with a large cancer patient population. The results of 226 consecutive CT-guided transthoracic core needle biopsies were reviewed. Results were classified into one of the following four groups: positive or suspicious for malignancy, benign specific, benign non-specific, and non-diagnostic. The benign specific group included entities such as fungus, hamartoma and schwannoma. In the benign non-specific group, histologic findings such as scar and inflammation were reported. The non-diagnostic group included cases with only normal pulmonary tissue in the specimen and/or insufficient tissue to render any diagnosis. The results were correlated with subsequent proof obtained via surgery or clinical and imaging follow-up. Out of a total of 226 biopsies, 158 were positive or suspicious for malignancy, 8 were benign specific, 32 were benign non-specific and 28 were non-diagnostic. Forty-three benign non-specific or non-diagnostic cases had subsequent proof, and malignancy was subsequently confirmed in 16/43 cases (5/21 non-specific and 11/22 non-diagnostic cases). The NPVs were 76% and 50% for benign non-specific and non-diagnostic biopsies, respectively. The overall NPV and false negative rate were 68% and 9%, respectively. A core biopsy revealing non-specific benign tissue or insufficient tissue for diagnosis is unreliable in excluding malignancy, and patients with these types of biopsy results should have resampling of tissue or close clinical and imaging follow-up.
Keywords: Lung neoplasms, negative predictive value, lung biopsy
Introduction
Computed tomography (CT)-guided biopsy of lung nodules is often performed to obtain a definitive diagnosis. The positive predictive value (PPV) of a CT-guided core biopsy in diagnosing malignancy is assumed to be close to 100%. However, the negative predictive value (NPV) is often the key clinical parameter. In other words, how reliable is a negative CT-guided biopsy for excluding malignancy? Can a nodule with a negative biopsy be safely watched, or should it be resected regardless of the biopsy result? Reported NPVs for fine needle aspiration biopsy (FNAB) of lung nodules range from 59% to 82% [1–4]. There is little data regarding the NPV of core needle biopsies in this setting, and the available results are also somewhat conflicting, ranging from 67% to 92% [4–6].
The aim of this study was to determine the negative predictive value (NPV) of transthoracic core needle biopsy of lung nodules in a tertiary care hospital with a large cancer patient population.
Materials and methods
After obtaining approval of the Institutional Review Board, the thoracic radiology database was searched for all patients who underwent CT-guided core needle biopsy of a thoracic lesion between 26 March 2002 and 26 April 2005. Biopsies were performed using coaxial technique and an automated biopsy gun with a 1 cm or 2 cm throw length (Quick-Core ® Biopsy Needle, Cook Inc. Bloomington, IN). CT imaging was used to confirm placement of the guide needle tip in the lesion. For large lesions that appeared to be centrally necrotic, samples were obtained from the periphery of the lesion. A pathologist was not present during the procedure; the number of needle passes was determined based on the adequacy of the samples obtained, using gross, visual inspection. Specimens were placed in formalin and submitted for histopathological analysis. Occasionally, if the clinical history suggested an infectious etiology, additional fine needle aspiration biopsy was performed and submitted for microbiological analysis, although this was not routine procedure.
Patient records were examined to determine the histological interpretation for each biopsy. Biopsy results were classified into one of the following four groups: (1) positive or suspicious for malignancy; (2) benign specific; (3) benign non-specific; and (4) non-diagnostic. The benign specific group included entities such as fungus, hamartoma and schwannoma. In the benign non-specific group, histologic findings such as scar and inflammation were reported. The non-diagnostic group included cases with only normal pulmonary tissue in the specimen and/or insufficient tissue to render any diagnosis.
Biopsies that were positive for malignancy were considered to be true positive, without further proof. An attempt was made to correlate all other biopsies with subsequent proof obtained via surgery or clinical and imaging follow-up.
True positive, true negative, false positive, and false negative groups were compared with regard to needle gauge, number of needle passes and lesion size using the Wilcoxon rank sum test.
Results
Out of a total of 226 biopsies, 158 were positive (154) or suspicious (4) for malignancy, 8 were benign specific, 32 were benign non-specific, and 28 were non-diagnostic (Figs. 1–3) (Table 1). All 158 positive or suspicious cases were considered to be true positives; of the four suspicious cases, two had clinical and imaging follow-up consistent with malignancy, one had surgical excision that showed malignancy, and one had no follow-up. Of the remaining 68 cases, 50 had proof via either surgery (20 cases) or clinical and imaging follow-up (30 cases). Thus, 208/226 cases were considered to have proof; 174/208 proven cases were malignant and 34/208 were benign.
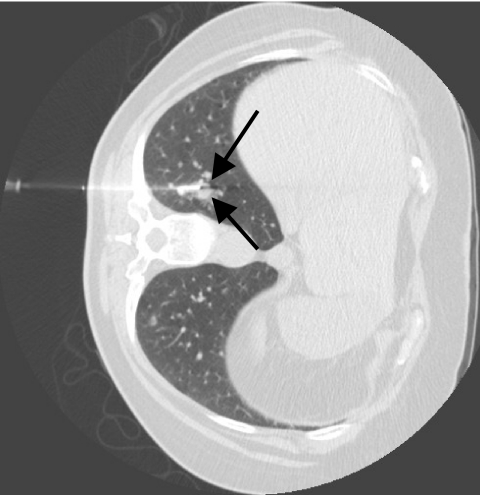
Figure 1.
True positive biopsy: metastatic papillary thyroid carcinoma (arrows).
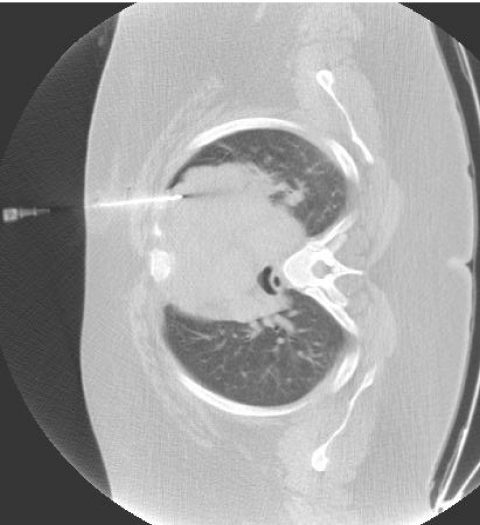
Figure 2.
False negative benign non-specific biopsy; final diagnosis was lymphoma.
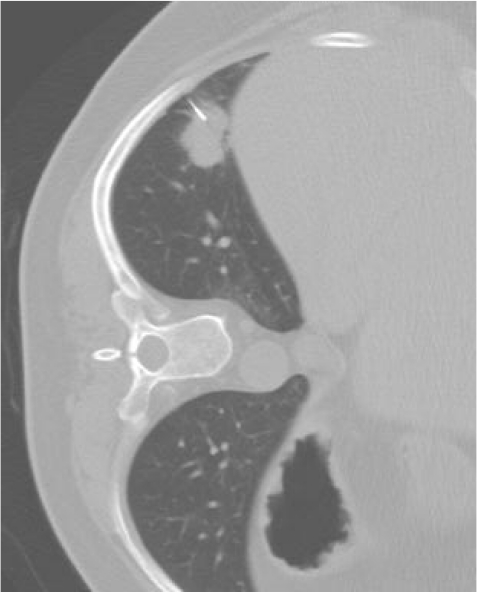
Figure 3.
False negative non-diagnostic biopsy; final diagnosis was non-small cell lung cancer.
Table 1.
Breakdown of biopsy results by group
| Group | No. | No. biopsies | True | True | False | False | Sensitivity | Specificity | Accuracy | NPV | False negative |
|---|---|---|---|---|---|---|---|---|---|---|---|
| biopsies | with proof | positive | negative | positive | negative | (%) | (%) | (%) | (%) | rate (%) | |
| Positive | 158 | 158 a | 158 | 0 | 0 | 0 | — | — | — | — | — |
| Benign specific | 8 | 7 | 0 | 7 | 0 | 0 | — | — | — | 100 | — |
| Benign non-specific | 32 | 21 | 0 | 16 | 0 | 5 | — | — | — | 76 | — |
| Non-diagnostic | 28 | 22 | 0 | 11 | 0 | 11 | — | — | — | 50 | — |
| All combined | 226 | 208 | 158 | 34 | 0 | 16 | 91 | 100 | 92 | 68 | 9 |
aAll positive biopsies were assumed to be true positive.
Positive group
The positive biopsy group (158 cases) included the following types of malignancy: 114 carcinomas, 11 sarcomas, 11 melanomas, 10 lymphomas, 4 malignant (not otherwise specified), 2 germ cell cancers, 2 post-transplant lymphoproliferative diseases (PTLD), and 4 suspicious for malignancy (Table 2). Lesion sizes and number of needle passes used are delineated in Table 3.
Table 2.
Types of malignancy ultimately proven in each biopsy group
| Positive | Benign | Non-diagnostic | |
|---|---|---|---|
| non-specific | |||
| Carcinoma | 114 | 1 | 10 |
| Sarcoma | 11 | — | — |
| Melanoma | 11 | — | — |
| Lymphoma | 10 | 3 | 1 |
| Malignant (not otherwise | |||
| specified) | 4 | — | — |
| Germ cell cancer | 2 | — | — |
| PTLD | 2 | — | — |
| Suspicious for malignancy | 4 | — | — |
| Unknown | — | 1 | — |
| Total | 158 | 5 | 11 |
Table 3.
Breakdown of lesion sizes and number of needle passes in each biopsy group
| Biopsy group | Lesion size (cm) |
No. of needle passes |
|||
|---|---|---|---|---|---|
| Mean | Range | Mean | Range | ||
| Positive | 3.8 | 1–16 | 4.3 | 2–8 | |
| Benign specific | 2.1 | 1–2.6 | 4.0 | 2–5 | |
| Benign non-specific | |||||
| True negative | 3.0 | 1–6.2 | 4.3 | 3–6 | |
| False negative | 3.5 | 0.9–9.3 | 4.6 | 3–6 | |
| Non-diagnostic | |||||
| True negative | 2.4 | 1.1–5.1 | 3.7 | 1–6 | |
| False negative | 2.4 | 1–5.1 | 4.6 | 3–6 | |
Benign specific group
The benign specific group (8 cases) included five infections (4 fungal, 1 bacterial) and 1 case each of hamartoma, schwannoma, and cavernous hemangioma. Clinical and imaging follow-up in 7 of these 8 patients confirmed a non-malignant process; the patient with a cavernous hemangioma did not have definitive follow-up of this finding. Thus 7/7 lesions with proof were true negative. Lesion sizes and number of needle passes used are delineated in Table 3.
Benign non-specific group
Twenty-one of the 32 patients in the benign non-specific group had subsequent proof of the benign or malignant nature of the lesion. Sixteen of 21 lesions with proof were true negative for malignancy, and 5/21 were false negative. Of the 16 true negative cases, 5 were likely infectious in etiology based on clinical and imaging follow-up. The 5 false negative cases included 3 lymphomas and 1 adenocarcinoma. In the fifth patient, the clinical and imaging course was consistent with malignancy, although no tissue was obtained. The NPV of a benign non-specific biopsy was 76% (Table 1). Lesion sizes and number of needle passes used are delineated in Table 3.
Non-diagnostic group
Twenty-two of the 28 patients in the non-diagnostic group had subsequent proof of the benign or malignant nature of the lesion. Eleven of 22 lesions with proof were true negative for malignancy, and 11/22 were false negative. Of the 11 true negative cases, only one was likely infectious in etiology based on clinical and imaging follow-up. The 11 false negative cases included 3 squamous cell carcinomas, 2 adenocarcinomas, and 1 unspecified carcinoma (Table 2). In 5 patients, although no tissue was obtained, the subsequent clinical and imaging course was consistent with malignancy: one was thought to have metastatic colon cancer, one metastatic head and neck cancer, one metastatic lymphoma, and two lung cancers. The NPV of a non-diagnostic biopsy was 50% (Table 1). Lesion sizes and number of needle passes used are delineated in Table 3.
Accuracy
The overall sensitivity, specificity, and accuracy for core needle biopsy were 91%, 100%, and 92%, respectively (Table 1). The overall negative predictive value and false negative rate were 68% and 9%, respectively (Table 1).
Effect of needle gauge
Of the 208 biopsies with proof, 199 were performed using a 20 gauge needle, 8 with an 18 gauge needle, and 1 with a 17 gauge needle. Given the homogeneity of needle sizes throughout the biopsy groups, statistical analysis was not performed.
Effect of number of needle passes
The number of needle passes performed in each lesion ranged from 1 to 8 (mean 4.3) across all groups. There was no significant difference between the true positive group and the group of all false negatives (benign non-specific and non-diagnostic, combined) with regard to number of needle passes. Moreover, there were no significant differences among the benign specific, true negative benign non-specific, and true negative non-diagnostic groups with regard to number of needle passes.
Effect of lesion size
Lesion sizes ranged from 0.9 to 16 cm in diameter (mean 3.5 cm) across all groups. True positive lesions (mean 3.8 cm) were significantly larger than false negatives (benign non-specific and non-diagnostic, combined) (mean 2.7 cm) (p<0.05). However, there was extensive overlap in sizes between the true positive and false negative groups. There were no significant differences among the benign specific, true negative benign non-specific, and true negative non-diagnostic groups with regard to lesion size.
Discussion
The results of our study showed that transthoracic core needle biopsy of lung lesions is often falsely negative in the diagnosis of malignancy, with an overall NPV of 68% and a false negative rate of 9%. Two other studies have found similar figures, with 67–70% NPVs and an 11% false negative rate [4, 5]; prevalence rates of cancer were similar in all studies (76–83%) [4, 5]. A much higher NPV of 92% was reported by Satoh et al. [6]; however, a lower cancer prevalence rate (61%) may have contributed to this discrepancy.
Montaudon et al. [5] looked at factors that might have influenced the accuracy of CT-guided core biopsies in 605 cases. With multivariate analysis, the sole variable significantly associated with a higher rate of false negative diagnosis of malignancy was lesion size equal to or smaller than 10 mm in diameter. Our study also indicated that size was a factor, and true positive lesions were, on average, significantly larger than false negative lesions. However, there was such extensive overlap in size distribution between the groups that use of size would not be useful in any individual case to predict the accuracy of a CT-guided biopsy, and many large cancers were misdiagnosed in our study. In addition, larger benign lesions were not more likely to result in a specific benign diagnosis compared to smaller benign lesions. Montaudon et al. [5] found no correlation between any of the following features and the false negative rate: gender, age, diagnosis of lymphoma, emphysema, lesion location (central vs. peripheral), presence of pleural contact, needle path length, spiculated vs. regular nodule margins, necrosis or cavitation, patient positioning, degree of difficulty of the biopsy, or duration of the procedure.
In our study, the number of needle passes did not correlate with biopsy accuracy, and the number of needle passes in the benign specific group was not larger than the number in the benign non-specific group. We were unable to evaluate the effect of needle gauge, because nearly all patients were biopsied using 20 gauge needles.
Only 21% (7/34) of benign biopsies in our series with proof of benignancy had a specific benign diagnosis. This compares to 41% (43/105) in the report of Montaudon et al. [5]; however, the latter study did not describe the criteria for a specific benign diagnosis, and it is possible that these authors included diagnoses that were considered non-specific in our series. Using our criteria for a specific benign diagnosis, Satoh et al. [6] and Yamagami et al. [4] reported specific benign diagnosis rates of 55% (12/22) and 83% (19/23), respectively. It is unclear why our specific benign diagnosis rate was lower than those found in these studies; these investigations were performed in a different country, and perhaps differing patient populations and manifestations of diseases contributed to this result. If we had routinely sent biopsy specimens for microbiological analysis, it is possible that we would have increased our yield of specific benign diagnoses, thus increasing the value of a negative result; however, this does not appear to be common practice at centers doing percutaneous lung biopsies.
A limitation of our study included lack of proof in 18 biopsies that did not show evidence of malignancy. If these cases were malignant, then the false negative rate would be higher and the negative predictive value would be lower than the rates reported here. On the other hand, if these cases were indeed benign, then the negative predictive value would be higher.
Conclusion
A core biopsy revealing non-specific benign tissue or insufficient tissue for diagnosis is unreliable in excluding malignancy, and all such biopsies need to be viewed with suspicion, regardless of lesion size or number of needle passes used. Patients with these types of biopsy results should have resampling of tissue with biopsy or surgical resection or close clinical and imaging follow-up.
References
- 1.Ohno Y, Hatabu H, Takenaka D, et al. CT-guided transthoracic needle aspiration biopsy of small (< or = 20 mm) solitary pulmonary nodules. AJR Am J Roentgenol. 2003;180:1665–9. doi: 10.2214/ajr.180.6.1801665. [DOI] [PubMed] [Google Scholar]
- 2.Romano M, Griffo S, Gentile M, et al. CT guided percutaneous fine needle biopsy of small lung lesions in outpatients. Safety and efficacy of the procedure compared to inpatients. Radiol Med (Torino) 2004;108:275–82. [PubMed] [Google Scholar]
- 3.Savage C, Walser EM, Schnadig V, Woodside KJ, Ustuner E, Zwischenberger JB. Transthoracic image-guided biopsy of lung nodules: when is benign really benign? J Vasc Interv Radiol. 2004;15:161–4. doi: 10.1097/01.rvi.0000109397.74740.8d. [DOI] [PubMed] [Google Scholar]
- 4.Yamagami T, Iida S, Kato T, et al. Usefulness of new automated cutting needle for tissue-core biopsy of lung nodules under CT fluoroscopic guidance. Chest. 2003;124:147–54. doi: 10.1378/chest.124.1.147. [DOI] [PubMed] [Google Scholar]
- 5.Montaudon M, Latrabe V, Pariente A, Corneloup O, Begueret H, Laurent F. Factors influencing accuracy of CT-guided percutaneous biopsies of pulmonary lesions. Eur Radiol. 2004;14:1234–40. doi: 10.1007/s00330-004-2250-3. [DOI] [PubMed] [Google Scholar]
- 6.Satoh S, Ohdama S, Matsubara O, Okochi Y, Tanaka R, Kimula Y. CT-guided automated cutting needle biopsy by a combined method for accurate specific diagnosis of focal lung lesions. Radiat Med. 2005;23:30–6. [PubMed] [Google Scholar]