It has been 35 years since Konopka and Benzer (1) published their landmark paper in PNAS documenting the discovery of three lines of Drosophila with aberrant circadian rhythms. One strain was governed by a clock that ran faster than wild type, one harbored a slower-than-normal clock, and one was arrhythmic. These mutations equivalently affected two different rhythms, in the timing of adult emergence from the pupal case and the flies' rhythms of locomotion, suggesting that they affected a general circadian clock mechanism rather than the observed behaviors per se. The genetic analysis of these mutants, however, is what made this discovery so powerful: By meiotic recombination mapping, analysis of small chromosome deletions, and complementation, the genetic etiology of the rhythm anomalies in all three lines mapped to the same gene, dubbed period, creating an allelic series with the arrhythmic allele (per0) appearing to be a null allele. The remarkable implication of this discovery, that there are specific genes whose functions specifically subserve a general circadian timekeeping mechanism, has been spectacularly borne out with the discovery of at least a half-dozen other such “clock factors” in forward genetic screens and the ensuing construction of a coherent molecular model for circadian clock function. In a recent issue of PNAS, Peschel et al. (2) showed that, 35 years later, classical forward genetic studies continue to yield important insights into the mechanisms of the circadian clock.
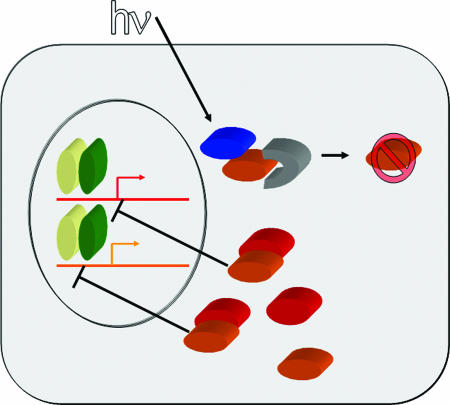
As currently understood, the Drosophila circadian clock mechanism consists primarily of a time-delayed transcription-translation feedback loop (3) (Fig. 1). Two positive transcription factors, encoded by Clock (Clk) and cycle (cyc), drive the transcription of the per locus as well as the clock gene timeless (tim). PER and TIM proteins heterodimerize, accumulate in the cytoplasm, undergo orchestrated translocation to the nucleus, and ultimately repress the CLK–CYC dimer and thus transcription of per and tim. After casein kinase-mediated phosphorylation of PER and TIM, these proteins are degraded via the proteosome, which allows reinitiation of per and tim transcription and renewal of the cycle.
Fig. 1.
A model for circadian entrainment. The core circadian oscillator consists of the genes Clock (yellow), cycle (green), per (red), and tim (orange). Transcription driven by Clock and cycle from E-box elements in per and tim genes led to generation of PER and TIM proteins in the cytoplasm. In dark conditions, these proteins dimerize and translocate to the nucleus where they inhibit their own transcription. After phosphorylation and slimb-mediated degradation of both proteins, the cycle can begin anew. Light (hv) activates the blue light photopigment CRY (blue), causing it to bind to TIM. The bound or modified TIM is then bound by JET (gray), which leads to ubiquitinization of TIM and its degradation, thereby shifting the circadian clock's phase.
The free-running period of most organisms' circadian clocks is close to, but not exactly, 24 hours. A circadian clock is of little value if it cannot be precisely synchronized to local time. The daily light–dark cycle is the primary cue circadian clocks use for synchronization (called entrainment). In vivo, TIM protein undergoes rapid, proteosome-mediated degradation in response to light, linking the circadian time-keeping mechanism to the light–dark cycle. Because the phase of the clock is dependent on the instantaneous levels of TIM and PER proteins, a rapid change in TIM abundance has the potential to shift (and thereby ultimately re-entrain) the clock. TIM itself, however, is not a photopigment. In a previous forward genetic screen, Stanewsky et al. (4) discovered a mutation in the photolyase-like blue light photopigment cryptochrome (cry) that resulted in impaired entrainment of the clock to light–dark cycles, without disrupting the central circadian oscillator itself (4, 5). CRY physically interacts with TIM in a light-dependent manner, both in heterologous yeast expression systems and in vitro (6, 7). However, the mechanism by which photic activation of CRY leads to TIM degradation has been, until recently, unclear.
Like many animals, wild-type Drosophila become behaviorally arrhythmic when kept in constant, 24-hour-per-day light (LL in chronobiological jargon). Disruption of the circadian photoreceptive pathway suppresses this arrhythmicity; the (presumed) loss-of-function cryb mutant fly shows anomalously preserved free-running rhythms when kept in LL (8). Thus, assaying for preserved rhythmicity in LL is a potentially efficient way to screen for mutations in the clock's photic input pathway. Koh et al. (9) recently reported a novel fly strain that, like cryb, abnormally maintains free-running rhythms in LL. The circadian rhythms of these flies could be entrained to light–dark cycles but took longer to re-entrain to a new phase (a test that simulates a trip across time zones) than did wild-type animals. The mutant strain was thus called jetlag (jet). The mutation was mapped by meiotic recombination to a small region containing ≈18 genes on the “left” arm of the second chromosome, near (but clearly separate from) tim. One of the genes in this interval, discovered by the Drosophila genome project and lyrically named CG8873, encodes an F-box protein of the Skp1/Cullin/F-box (SCF) E3 ubiquitin ligase family. A gene encoding another Drosophila F-box protein, named slimb, is required for normal TIM degradation in darkness (10, 11). Thus, the new SCF family member became an immediate candidate within the genetic interval for the jet locus. Sequencing of the open reading frame of CG8873 in the mutant strain, as well as that of seven other strains that failed to complement the LL free-running phenotype, revealed two potential coding mutations. The more common allele, dubbed “c” and found in six of the seven strains, had a phenylalanine to isoleucine mutation at amino acid 209. The other noncomplementing strain (harboring the rare, or “r,” allele) showed a serine to leucine mutation at amino acid 220. jet flies showed reduced phase shifts in response to light, which could be rescued by expressing the normal form of CG8873 in tim-expressing cells (via GAL4-UAS driven expression). In Drosophila Schneider-2R (S2R) cells, TIM does not degrade in response to light. When JET, CRY, and TIM are coexpressed in these cells, TIM degrades in response to light. In cells expressing only CRY and TIM or only JET and TIM, TIM does not degrade after light exposure. In cell culture, JET physically associates with TIM and induces the latter's ubiquitinization (9).
In their work, Peschel et al. (2) describe another strain of Drosophila that exhibits strong rhythmicity in LL, which they named Veela. (One suspects that the authors so dubbed this strain, named for the siren-like creatures whose dancing mesmerizes Harry Potter and his friend Ron but who turn into vengeful, fireball-throwing bird-like creatures when spurned, to summarize the sequential attraction and difficulty in working with these flies.) Initial mapping studies placed the mutation between aristaless (genomic region 21C1) and dumpy (24F4) markers, an interval within the second chromosome that includes tim (region 23F6) but not jet (at 25B4). Genetically, Veela interacts with cryb; the double heterozygote Veela/+;cryb/+ is rhythmic in LL, whereas both single heterozygotes are arrhythmic in this condition. TIM levels in Veela flies are elevated in LL, suggesting a defect in light-mediated degradation. These findings would all be consistent with the discovery of a new tim variant that was resistant to light-mediated degradation. However, unexpectedly, sequencing of the tim locus in Veela flies did not reveal a new mutation but, rather, a previously described naturally occurring variant utilizing a second, upstream translation start site. This allele generates both the originally described TIM and a “long” form containing 23 additional amino acids at the N terminus. The allele is called ls-tim (for long and short) to distinguish it from the originally described s-tim, which produces only shorter TIM (12). ls-tim flies in other genetic backgrounds show the “normal” phenotype of arrhythmicity in LL. Could another gene in this region of chromosome 2 be contributing to the mutant phenotype? Before doing fine-scale mapping to find such a new mutation, the authors examined the status of the jet locus in their novel strain. In genetic mapping tests, all recombinants that were rhythmic in LL carried both ls-tim and the jetc allele. Importantly, two recombinants carried jetc but were arrhythmic in LL. Thus, neither ls-tim nor jetc is sufficient to block light-induced TIM degradation and allow rhythmicity in LL; the Veela phenotype is due to the combination of these two alleles. The authors confirmed this hypothesis by transgenically adding the ls form of tim to homozygous tim-null (tim0) flies carrying Veela. These flies exhibited twice the rate of rhythmicity in LL as did flies carrying Veela/tim01. Were another gene on this interval responsible for the Veela phenotype, one would not have expected enhancement of the phenotype with addition of the ls-tim allele. Finally, the authors looked to see whether jet, like tim, occurs in polymorphic types among strains. In 15 naturally occurring fly strains, no jet variants were found. Thus, the jetc allele seems to be found in laboratory but not wild-type strains.
Classical forward genetic studies continue to yield important insights.
Formally, the authors can conclude that, in Veela flies, jetc interacts with the ls-tim allele of tim to decrease light-mediated TIM degradation. Given the in vitro data of Koh et al. (9), demonstrating biochemical interaction of TIM with JET, it would appear that wild-type forms of jet- and tim-encoded proteins also interact. A coherent model for light-induced phase shifting, therefore, is that light-activated CRY binds TIM. JET consequently recognizes either this complex or a modified TIM, which resulted from its interaction with CRY. JET then facilitates ubiquitinization of TIM and initiates its proteosomal degradation (see Fig. 1).
A slew of interesting and important questions arise from these studies of Veela. Koh et al. (9) reported that placing jetc over a deletion of the gene caused rhythm abnormalities equivalent to jetc/jetc homozygotes, suggesting the c variant may be null or a severe hypomorphic allele. In hindsight, however, this result likely occurred in the genetically sensitized ls-tim background. It is theoretically possible that jetc is a neomorphic or modestly hypomorphic allele that specifically inhibits L+S-TIM degradation. Would a jet allele causing a more severe defect in JET protein show a circadian phenotype in the original s-tim genetic background? Or would such an allele have pleotropic effects masking any circadian phenotype? Does the rhythmicity in LL observed in jetr similarly require the presence of the ls-tim allele? If, as the in vitro experiments suggest, JET does recognize CRY-bound or -modified TIM, how is TIM differentially recognized by two different E3 ligases, SLIMB for degradation occurring in darkness (10, 11) and JET for degradation occurring in the light (2, 9)?
The present work also carries broad lessons about the use of genetics in dissecting behavior in general and the circadian clock in particular. In the “pregenomic” era, analysis of a new mutant strain required meticulous attention to the phenotypic details of increasingly fine mapping crosses. As Peschel et al. (2) demonstrate, such analyses still play a crucial role in the analysis of mutant lines. What at first seemed to be a contradictory anomaly — how can jetc/jetc show a normal light entrainment phenotype? — with careful study demonstrates a genetic interaction of jetc with a subtle allele of tim, providing critical genetic evidence supporting a model for jet and tim interaction that was previously based primarily on in vitro studies.
Footnotes
The author declares no conflict of interest.
See companion article on page 17313 in issue 46 of volume 103.
References
- 1.Konopka RJ, Benzer S. Proc Natl Acad Sci USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peschel N, Veleri S, Stanewsky R. Proc Natl Acad Sci USA. 2006;103:17313–17318. doi: 10.1073/pnas.0606675103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Gelder RN. Sci STKE, 2003 doi: 10.1126/stke.2003.194.cm10. [DOI] [Google Scholar]
- 4.Stanewsky R, Kaneko M, Emery P, Beretta B, Wagersmith K, Kay SA, Rosbash M, Hall JC. Cell. 1998;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- 5.Emery P, So WV, Kaneko M, Hall JC, Rosbash M. Cell. 1998;95:669–679. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- 6.Rosato E, Codd V, Mazzotta G, Piccin A, Zordan M, Costa R, Kyriacou CP. Curr Biol. 2001;11:909–917. doi: 10.1016/s0960-9822(01)00259-7. [DOI] [PubMed] [Google Scholar]
- 7.Ceriani MF, Darlington TK, Staknis D, Mas P, Petti AA, Weitz CJ, Kay SA. Science. 1999;285:553–556. doi: 10.1126/science.285.5427.553. [DOI] [PubMed] [Google Scholar]
- 8.Emery P, Stanewsky R, Hall JC, Rosbash M. Nature. 2000;404:456–457. doi: 10.1038/35006558. [DOI] [PubMed] [Google Scholar]
- 9.Koh K, Zheng X, Sehgal A. Science. 2006;312:1809–1812. doi: 10.1126/science.1124951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko HW, Jiang J, Edery I. Nature. 2002;420:673–678. doi: 10.1038/nature01272. [DOI] [PubMed] [Google Scholar]
- 11.Grima B, Lamouroux A, Chelot E, Papin C, Limbourg-Bouchon B, Rouyer F. Nature. 2002;420:178–182. doi: 10.1038/nature01122. [DOI] [PubMed] [Google Scholar]
- 12.Rosato E, Trevisan A, Sandrelli F, Zordan M, Kyriacou CP, Costa R. Nucleic Acids Res. 1997;25:455–458. doi: 10.1093/nar/25.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]