Millions of years of evolution leading to a well orchestrated series of events for sperm transport and selection in the female tract and sperm–egg interaction during fertilization can be bypassed by the direct microinjection of spermatozoa into oocytes to alleviate infertility or for the experimental manipulation of model animals (1). Since its introduction in humans (2), many thousands of children have been born by using intracytoplasmic sperm injection (ICSI). ICSI may represent on average approximately one-half of all assisted reproductive techniques used in Europe (3), with considerable differences between countries. Germany and the United Kingdom have many more in vitro fertilizations (IVFs) than ICSIs. France has equal numbers, and Belgium and Spain have each twice as many ICSI cycles than IVF cycles. The widespread use of ICSI relies, in part, on the belief that it is not necessary to pay attention to aspects of sperm (dys)function because they become irrelevant if a spermatozoon is directly microinjected into the oocyte. The article by Morozumi et al. (4) in this issue of PNAS demonstrates that this belief constitutes an oversimplification and that preparation of sperm for ICSI and an understanding of the underlying mechanisms of sperm function during fertilization are important for sperm microinjection. Interestingly, sperm preparation for ICSI does not seem to be of the utmost importance for the initial steps taking place after sperm penetration and leading to the first cell division, but it does have profound effects on subsequent development. The links between early changes after sperm microinjection and mechanisms of embryo and fetal development are not clear; the contribution by Morozumi et al. (4) suggests that efforts to investigate them may be rewarding.
Fertilization is a complex process. Many steps are involved to bring a sperm and an egg together to allow for a dialogue between gametes that is necessary for their mutual “activation” (5). In mammals, the spermatozoon is not ready to interact with the egg upon its transfer to the female tract. The spermatozoon needs to undergo a physiological “switching-on” (known as capacitation) that enables it to interact with the ovum and penetrate its outermost coat, the cumulus oophorus. Capacitation allows the spermatozoon to respond to signals from the ovum and to undergo a process of exocytosis that results in the release or exposure of hydrolytic enzymes present in the acrosome (a modified secretory granule overlying the nucleus). These enzymes allow the sperm to negotiate the barrier surrounding the oocyte (the zona pellucida). Exocytosis of the acrosome results in a massive loss of sperm membranes; the plasma membrane and the underlying outer acrosomal membrane fuse at several points, forming many vesicles that are eventually shed when sperm cells start passing through the zona pellucida. This release of enzymes and membranes renders the remaining sperm plasma membrane able to fuse with the oocyte plasma membrane. Once fusion occurs and the sperm cell is incorporated into the ovum's cytoplasm, the remaining inner acrosomal membrane is disrupted; this event is accompanied by exposure of the sperm cytoplasm and perinuclear material to the oocyte's cytoplasm. The spermatozoon carries in this region an oocyte-activating factor that stimulates Ca2+ release and Ca2+ oscillations important for the initiation of development (6, 7).
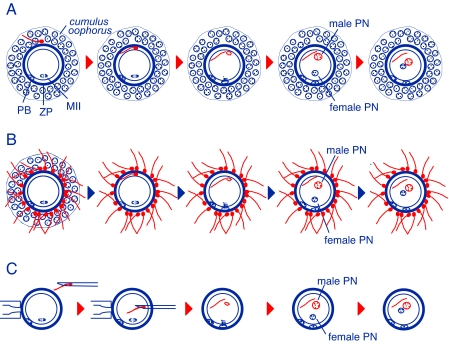
In in vitro fertilization, many sperm cells are needed to ensure fertilization, many more than the number thought to be present at the site of fertilization (Fig. 1), but interaction between sperm and oocyte roughly follows a pattern similar to that seen in vivo. With ICSI, just one sperm is needed for fertilization. However, contrary to the situation in natural fertilization or IVF, the sperm cell is microinjected intact without major preparations (usually a centrifugation wash or “swim-up” to remove secretions from the male reproductive tract). One wonders whether spermatozoa used for ICSI should be subjected to changes that mimic those occurring under natural conditions (such as shedding of membranes and hydrolytic enzymes present in the acrosome) and whether this would affect oocyte activation and embryo development. In addition, there is the question of how to pick the right sperm cell for ICSI (8) when all of the natural barriers of sperm selection have been bypassed. From success stories in the mouse model and humans, it may seem that these issues are not that important after all. Or are they?
Fig. 1.
Fertilization in mammals under natural conditions, in vitro, and by means of sperm microinjection. (A) Fertilization in vivo, for which a single sperm cell interacts with the oocyte and its coats, the cumulus oophorus and zona pellucida (ZP). (B) Fertilization in vitro. Many sperm cells interact with the oocyte. (C) Intracytoplasmic sperm injection. The cumulus-free oocyte is held by a pipette, and the sperm cell is aspirated and microinjected with a capillary micropipette. PB, oocyte's polar body; MII: oocyte's metaphase II; PN: pronucleus.
The success of ICSI has largely eluded domestic animals, such as the bovine, porcine, and equine (9). Detailed studies in the bovine have revealed that, although bull spermatozoa contain a full complement of sperm factors potentially capable of activating oocytes, bovine oocytes are incapable of mounting Ca2+ oscillations after ICSI (9), suggesting that release or activation of the sperm factor is compromised, which leads to a defective Ca2+ stimulus and premature termination of embryo development. In humans, use of ICSI may lead to ≈20% deliveries per cycle; the success rate per embryo is higher because in many instances two or three embryos are transferred with ≈30% multiple pregnancies (3). When embryos produced by ICSI are transferred singly (rather than in groups of two or three embryos) with careful assessment of embryo quality before transfer, the overall pregnancy rate per transfer can reach 35% (10, 11). Could the success of ICSI be improved by using better protocols of sperm preparation? The study by Morozumi et al. (4) suggests that this may be achieved by removal of both sperm membranes and acrosome before microinjection. The current results support and expand earlier investigations revealing that acrosomal contents could be potentially hazardous to embryo development (12). Taken together, these results may be important for human patients as well as for domestic or wild species, for which results have so far been poor and for which it may be important to maximize the use of a limited number of spermatozoa. This work is important because it also highlights the need for adequate experimental studies in model species to identify underlying problems or limitations in techniques and mechanisms before putting them into practice in humans.
The process of acrosomal exocytosis, which results in release of hydrolytic enzymes and shedding of membranes over a large proportion of the sperm head, is triggered by the oocyte-associated signals progesterone and zona pellucida (13). Prominent among the underlying mechanisms of acrosomal exocytosis is the activation of sperm phospholipases, particularly phospholipase A2 (PLA2). PLA2 hydrolyzes membrane phospholipids causing the release of free fatty acids and lysophospholipids. Lysophosphatidylcholine (lysoPC)(=lysolecithin) has been shown to play an important role in the final stages of fusion either by acting as a substrate for the generation of other metabolites or, perhaps more importantly, by destabilizing membranes and acting together with the fusion machinery (14). LysoPC alone can disturb sperm membranes and cause loss of the acrosome under appropriate conditions, and Morozumi et al. (4) have used this to their advantage for the removal of membranes and acrosomal contents in spermatozoa from several species before ICSI. They found that this natural product resulting from the hydrolysis of membrane phospholipids led them to obtain their best results for mouse embryo development after ICSI. Interestingly, treatment of individual spermatozoa for a short period just before microinjection produced much better results than treatment of spermatozoa in suspension (in a mass) and subsequent centrifugation and incubation in culture medium. The latter may cause leaking of sperm-activating factors or release of metabolites needed for oocyte activation. It was found that replacing lysoPC with a detergent (Triton X-100) also was effective, although embryonic and fetal development was lower. The ability of the “natural” lysoPC to disrupt sperm membranes and improve embryonic development after sperm microinjection may represent an attractive approach for the enhancement of ICSI results in a clinical setting. It may also show the way for a breakthrough in the quest for success of ICSI in domestic and wild species.
It is interesting that, although almost all oocytes were activated after ICSI, regardless of whether sperm membranes were removed, there were approximately twice as many mouse embryos developing to term when sperm membranes were eliminated by treatment with lysoPC before microinjection (4). Ca2+ spiking after sperm entry seems to be one major effect of the absence of sperm membranes. The removal of sperm membranes may make the sperm-borne oocyte-activating factor more easily available to the ovum's cytoplasm. The presence or absence of sperm membranes influenced the timing of cleavage to the two-cell stage, with such cleavage occurring much earlier when spermatozoa without membranes were microinjected. However, because all oocytes eventually reached the two-cell stage, the sperm factor must somehow find its way into the ovum's cytoplasm and trigger activation. The timing of this first cleavage division (i.e., time elapsed between sperm injection and cleavage) seems to be an important cue for further development beyond the two-cell stage and, in particular, for postimplantation development. Ca2+ spikes, such as those elicited by the spermatozoon, may represent a “code” for the proper activation and progression of development (15). Support for this idea comes from recent studies showing that Ca2+ oscillatory patterns in fertilized mouse eggs affect embryonic gene expression in blastocysts and postimplantation development to term (16). This finding suggests that the timing of a Ca2+ rise(s) and of Ca2+ spiking and the activation of other events stimulated by sperm entry have to be properly orchestrated. Overall, the work by Morozumi et al. (4) indicates that, when sperm with intact membranes are microinjected, changes underlying oocyte activation may still occur, although at a slower pace, but that there is a disruption in the interaction with other processes required for embryo and fetal development. In the absence of sperm membranes, these changes take place much more rapidly and with far-reaching consequences.
Establishing a link between Ca2+ signaling during oocyte activation at fertilization and development to term (as well as understanding whether the pattern of Ca2+ oscillations is critical for reprogramming somatic nuclei in the cloning of mammals) will be highly informative and potentially of great economic value (15). The results of this paper are a good step in this direction because they help to characterize a good model system for future studies and because they provide initial evidence for such a link.
Footnotes
The author declares no conflict of interest.
See companion article on page 17661.
References
- 1.Yanagimachi R. Reprod Biomed Online. 2005;10:247–288. doi: 10.1016/s1472-6483(10)60947-9. [DOI] [PubMed] [Google Scholar]
- 2.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Lancet. 1992;340:17–18. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- 3.Andersen AN, Erb K. Int J Androl. 2006;29:12–16. doi: 10.1111/j.1365-2605.2005.00577.x. [DOI] [PubMed] [Google Scholar]
- 4.Morozumi K, Shikano T, Miyazaki S, Yanagimachi R. Proc Natl Acad Sci USA. 2006;103:17661–17666. doi: 10.1073/pnas.0608183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanagimachi R. Adv Biophys. 2003;37:49–89. doi: 10.1016/s0065-227x(03)80003-5. [DOI] [PubMed] [Google Scholar]
- 6.Jones KT. Reproduction. 2005;130:813–823. doi: 10.1530/rep.1.00710. [DOI] [PubMed] [Google Scholar]
- 7.Malcuit C, Kurokawa M, Fissore RA. J Cell Physiol. 2006;206:565–573. doi: 10.1002/jcp.20471. [DOI] [PubMed] [Google Scholar]
- 8.Berkovitz A, Eltes F, Yaari S, Katz N, Barr I, Fishman A, Bartoov B. Hum Reprod. 2005;20:185–190. doi: 10.1093/humrep/deh545. [DOI] [PubMed] [Google Scholar]
- 9.Malcuit C, Maserati M, Takahashi Y, Page R, Fissore RA. Reprod Fertil Dev. 2006;18:39–51. doi: 10.1071/rd05131. [DOI] [PubMed] [Google Scholar]
- 10.De Sutter, Van der Elst J, Coetsier T, Dhont M. Reprod Biomed Online. 2003;6:464–469. doi: 10.1016/s1472-6483(10)62169-4. [DOI] [PubMed] [Google Scholar]
- 11.Martikainen H, Orava M, Lakkakorpi J, Tuomivaara L. Hum Reprod. 2004;19:1364–1366. doi: 10.1093/humrep/deh197. [DOI] [PubMed] [Google Scholar]
- 12.Morozumi K, Yanagimachi R. Proc Natl Acad Sci USA. 2005;102:14209–14214. doi: 10.1073/pnas.0507005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roldan ERS, Murase T, Shi QX. Science. 1994;266:1578–1581. doi: 10.1126/science.7985030. [DOI] [PubMed] [Google Scholar]
- 14.Roldan ERS. Front Biosci. 1998;3:D1109–D1119. doi: 10.2741/a348. [DOI] [PubMed] [Google Scholar]
- 15.Ducibella T, Schultz RM, Ozil JP. Sem Cell Dev Biol. 2006;17:324–332. doi: 10.1016/j.semcdb.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Ozil JP, Banrezes B, Tóth S, Pan H, Schultz RM. Dev Biol, 2006 doi: 10.1016/j.ydbio. 2006.08.041. [DOI] [PubMed] [Google Scholar]