Abstract
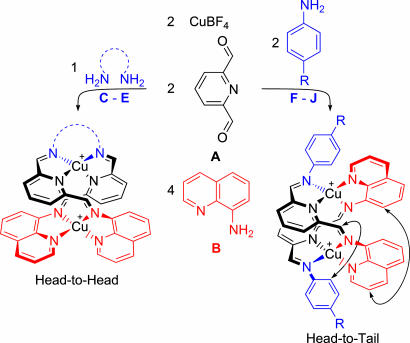
A series of di-copper(I) complexes has been prepared via the reaction of copper(I) tetrafluoroborate, 2,6-diformylpyridine, 8-aminoquinoline, and a series of aliphatic diamines and 4-substituted anilines. To avoid a “valence-frustrated” state, involving a mismatch between the number of ligand donor atoms and the number of metal acceptor sites, the product structures formed selectively: One of the formyl groups of the diformylpyridine reacted specifically with the aminoquinoline, whereas the other formyl group reacted with the diamine or aniline. The observed selectivity was demonstrated to be thermodynamic in nature: When two dicopper complexes that were stable yet “valence-frustrated” were mixed, an imine metathesis reaction was observed to occur spontaneously to generate a “valence-satisfied” structure. In addition to control over the constitution of the ligands, we were able to exercise control over their relative orientations within the complex. Diamines exclusively gave structures in which the ligand exhibited a head-to-head orientation along the copper–copper axis to avoid stretching. Anilines gave predominantly head-to-tail structures, with the proportion of head-to-head isomer decreasing in complexes that incorporate more electron-deficient anilines and disappearing in less polar solvents. We also demonstrated the removal of the metals and the hydrogenation of the imine bonds to generate a molecule containing nonexchanging secondary amines, suggesting potential uses of this technique in the domain of organic synthesis.
Keywords: coordination chemistry, dynamic combinatorial chemistry, synthesis, self-assembly
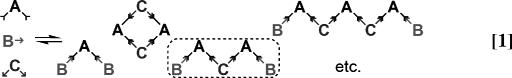
A challenging problem in chemical synthesis is the direction of two different reagents to react at two equivalent sites upon a single substrate molecule. When such a reaction is carried out under thermodynamic control (1), one may expect to generate mixtures of hetero- and homo-coupled products. This problem is compounded when one of the two reagents also possesses two reactive groups. Mixtures of oligomeric products are expected to form, as the difunctional starting materials combine to form polymeric chains and rings of different sizes (Eq. 1).
 |
Various templating (2–9) and self-recognition (10–15) phenomena have been used to shape the thermodynamics of different self-assembly processes, to select a desired product or products from a dynamic library (16–19) of structures. Here we show how this goal may be accomplished through the action of a pair of metal template ions (20), which cooperatively direct each of the two equivalent aldehyde groups of a dialdehyde molecule to react with a different amine, forming two different imine (C N) bonds selectively. The self-assembly of this system was directed by means of the number of acceptor sites present on the CuI template ions and the number of donor sites present on the self-assembled imine ligands. When the number of donor sites cannot readily equal the number of acceptor sites, a “valence-frustrated” or “incommensurate” (21) state results, providing a potential driving force for covalent rearrangement into a “valence-satisfied” structure. In addition to the development of selectivity in the dynamic covalent (1) imine bond formation, means were also discovered to control the orientation of the self-assembled ligands with respect to each other, giving access to either “head-to-head” or “head-to-tail” configurations.
N) bonds selectively. The self-assembly of this system was directed by means of the number of acceptor sites present on the CuI template ions and the number of donor sites present on the self-assembled imine ligands. When the number of donor sites cannot readily equal the number of acceptor sites, a “valence-frustrated” or “incommensurate” (21) state results, providing a potential driving force for covalent rearrangement into a “valence-satisfied” structure. In addition to the development of selectivity in the dynamic covalent (1) imine bond formation, means were also discovered to control the orientation of the self-assembled ligands with respect to each other, giving access to either “head-to-head” or “head-to-tail” configurations.
Results and Discussion
Preparation of Stable Valence-Frustrated Structures.
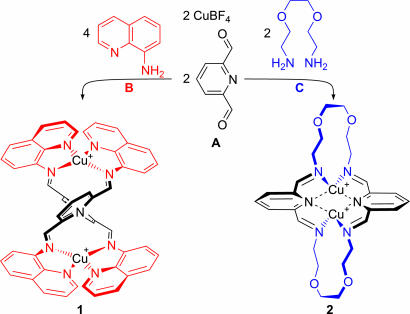
As shown in Scheme 1, the reaction of diformylpyridine A and copper(I) tetrafluoroborate with aminoquinoline B or diamine C in acetonitrile solution generated helicate 1 or macrocycle 2, respectively. Both of these products appear to have D2 symmetry by 1H NMR between 203 and 298 K. It is possible that their true lowest-energy structures possess lower symmetry, and that they undergo fluxional interconversion between isoenergetic structures rapidly on the NMR time scale. This interconversion could lead to a time-averaged D2 symmetry, as has been observed in structures similar to 2 (22).
Scheme 1.
The preparation of valence-frustrated dicopper complexes 1 and 2 from subcomponents A, B, and C and copper(I) tetrafluoroborate.
Despite their thermodynamic stability, complexes 1 and 2 are valence-frustrated: 1 is oversaturated, with five nitrogen donors per copper, and 2 is undersaturated, with only three such donors. In previously described subcomponent self-assembling systems involving copper(I) (23–33), subcomponents were chosen to generate ligands having the optimal number of four imine donors per copper, with the exception of cryptands containing three imine donors per copper(I) (34, 35).
The expression of the template effect (20) in this system is shaped by the possibilities and preferences latent in the ligand subcomponents and the metal ions present in the system. These preferences define the energetic landscape within which self-assembly takes place, working together in parallel to direct the self-assembly process. In the present system, two conclusions may be drawn as to the system's preferences and possibilities based upon our experimental observations.
First, dialdehyde A is well suited to generate multinuclear CuI helical structures such as 1 and 2, and poorly suited to generate mononuclear complexes, despite the entropic driving force favoring complexes of lower nuclearity. A diimine formed by A may readily occupy three coplanar meridional coordination sites in an octahedral complex (36), but its geometry is ill-suited to tridentate chelation within the pseudotetrahedral coordination environment favored by copper(I). Multinuclear helical complexes, in contrast, are well adapted to the preferred geometry of this metal ion (37).
Second, any such dicopper helicate is guaranteed to be valence-frustrated if only one amine is used as a subcomponent. Structures such as 1 and 2 must contain a total of 4n + 2 nitrogen donors, with the 4n deriving from the four subcomponent amines (two diamines in the case of 2) and the 2 coming from the two central pyridines. A mismatch must thus necessarily occur between these 4n + 2 donors and the 4n coordination sites proffered by n copper(I) ions, with two unfulfilled coordination sites present at either ligand or metal.
A structure containing 12 coordination sites, ideal for three copper(I) ions, might be prepared from 4 equivalents each of A and C; no such structure was observed by electrospray ionization mass spectrometry (ESI-MS) or NMR when the reaction was tried using this stoichiometry. Our failure to observe such a structure suggested that the enthalpic penalty for valence-frustration was less than the entropic penalty for creating a large “vernier” (38) structure in which ligand and metal valences are matched. In similar fashion, NMR and ESI-MS spectra suggested that 2 was stable in the presence of additional CuI, showing no tendancy to form an L2Cu33+ structure, nor a circular helicate (3) or other larger assembly to fully satisfy metal and ligand valences.
Selectivity Through Relief of Valence Frustration.
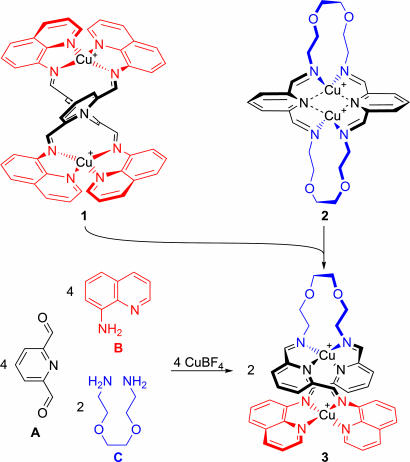
When A, B, and C are present simultaneously, the possibility exists of creating the dinuclear helical structure 3 in which both copper(I) ions and ligands are valence-satisfied: The ligand of 3 possesses 2 (from 2 × A) + 4 (from 2 × B) + 2 (from C) = 8 nitrogen donor atoms, ideally matched to a pair of tetracoordinate copper(I) ions. Structure 3 was the unique product observed when its three precursor subcomponents were mixed with copper(I) (Scheme 2Lower).
Scheme 2.
The preparation of “valence-satisfied” hybrid structure 3 from self-assembly of the free subcomponents (Lower) or from imine metathesis starting with a mixture of 1 and 2 (Upper).
Complex 3 could also be prepared through mixing 1 and 2 together in acetonitrile solution (Scheme 2 Upper). The observation that 1 and 2 were capable of covalent imine metathesis to give 3 indicated that this compound's synthesis proceeded under thermodynamic control (1, 39).
In the absence of copper(I), A, B, and C reacted to form a diverse dynamic library (16–19) of product imines. ESI-MS indicated that this library included a variety of open-chain and macrocyclic products, the ligand of 3 being present only as a minority product. The addition of copper(I) to this mixture thus allowed us to solve the quandary posed by the system of Eq. 1: During the formation of 3, each of A's two aldehyde groups was directed to react with a different amine, selecting one unique structure from among a theoretically limitless collection of cyclic and linear oligomeric structures. The smallest valence-satisfied product was thus selected from among the members of this dynamic library of interconverting products.
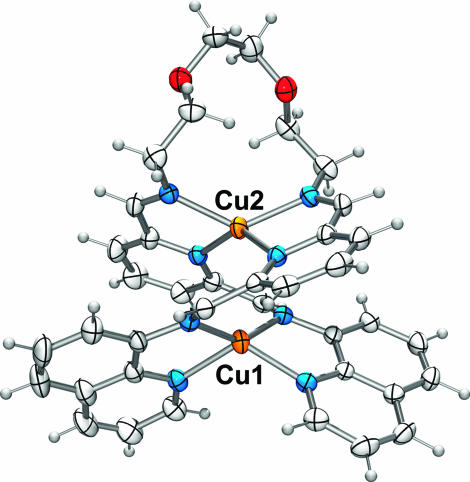
X-ray quality crystals of 3 were grown through vapor counterdiffusion of diisopropyl ether into an acetonitrile solution. An ORTEP diagram of the structure is presented in Fig. 1. The pseudotetrahedral coordination geometry of the copper(I) ions is flattened along the Cu–Cu axis, but no structural details suggest the presence of any great strain (26).
Fig. 1.
ORTEP diagram of 3; the BF4− counterions and acetonitrile of crystallization are not shown. Selected mean bond distances: Cu-Npyridine 2.134 Å; Cu-Namine 2.033 Å; Cu-Nquinoline 2.037 Å; bite angles at: 82.3° (Cu1); 79.7° (Cu2).
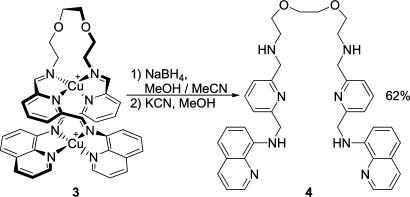
Although the dynamic nature of the imine bonds of 3 played an essential role in its thermodynamic synthesis, it is useful to be able to “turn off” the possibility of dynamic exchange to create a structure that persists even in the absence of the metal templates. This goal was readily attained by treating 3 with sodium borohydride (40) to reduce the imine bonds to secondary amines, followed by potassium cyanide to remove the metal ions (41), as shown in Scheme 3.
Scheme 3.
The hydrogenation and demetalation of 3 to produce 4.
Substrate Generality.
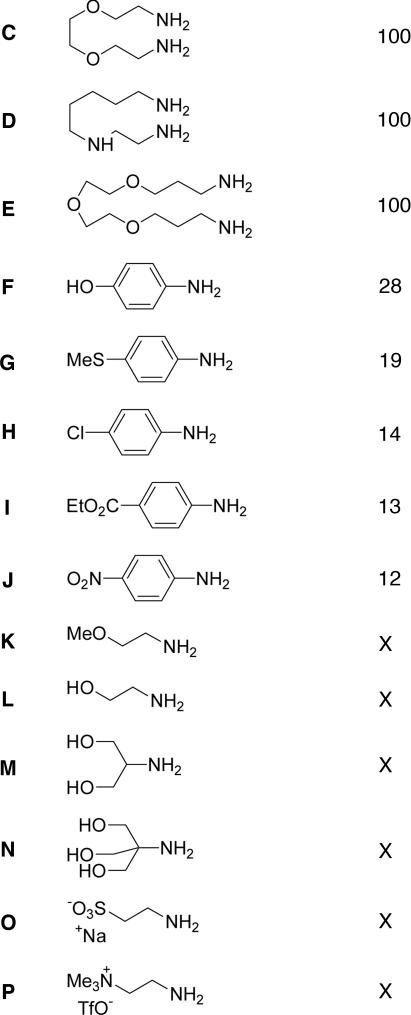
The ability to convert 3 into 4 brings this methodology into the realm of organic synthesis, allowing ready access to a product that could be difficult to prepare using other methods. Thus, we undertook to examine the scope and limitations of this reaction. A variety of different amines were tried in the self-assembly reaction shown in Scheme 4. These amines are listed in Table 1
Scheme 4.
Selective preparation of complexes containing heteroligands from mixtures of subcomponents; double-headed arrows indicate key ROESY (42) signals used to assign the structure of the head-to-tail isomer.
Table 1.
Mono- and di-amines employed in the self-assembly reaction of Scheme 4, and the proportion of head-to-head isomer observed in DMSO solution at equilibrium as determined by 1H integration (remainder is head-to tail)
Both aliphatic diamines C–E and aromatic monoamines F–J gave clean heteroligand formation, in which each of the formyl groups of A reacted with a different amine. ESI-MS indicated the clean formation of dicopper complexes of these heteroligands in all cases. In contrast, the aliphatic monoamines K–P gave ESI-MS and NMR spectra indicating complex mixtures of products. The lack of selectivity in the cases of aliphatic monoamines might be related to the lack of the secondary organizing interactions present in the other cases, such as π-stacking and solvophobic effects (43) in the cases of anilines, and the chelate effect for diamines. Parasitic 1,3-proton shifts from the α-carbon of the amine to the aldehyde carbon might also be occurring in the cases of aliphatic amines; such shifts would be precluded in the cases of anilines and might create considerable strain during the rehybridization of a diamine's α-carbons from sp3 to sp2 (see Fig. 1).
The 1H and NMR spectra of the products obtained with diamines D and E were nearly identical in the aromatic region to the spectrum of 3 obtained from diamine C. The number of NMR signals observed indicated the presence of only a single product isomer for amines C–E. Models suggested that the diamine linker would be stretched into a high-energy configuration in a head-to-tail isomer, in contrast with the strain-free configuration of head-to-head 3 (Fig. 1). Therefore, we concluded that the use of diamines C–E allowed the selection of the head-to-head product isomer as the unique product of the self-assembly process.
In contrast, when 4-substituted anilines were used in place of aliphatic diamines, NMR resonances corresponding to two distinct product isomers were observed in all cases in DMSO solution. Rotating-frame Overhauser effect spectroscopy (ROESY) and correlation spectroscopy (COSY) experiments (42) were used to assign the 1H NMR spectra of the majority product isomer in the case of aniline G. The ROESY experiment showed through-space correlations that were consistent with the assigned structure of the head-to-tail isomer, but not with the head-to-head isomer. The most revealing of the observed ROESY correlations are shown as double-headed arrows in Scheme 4. The 1H NMR spectra of the products derived from the different anilines were all similar in DMSO solution, having the same ordering of aromatic peaks of the same multiplicities, including those peaks corresponding to the minority isomer; the only exceptions were the phenylene resonances of the aniline residue. These observations were consistent with an assignment of the head-to-head isomer as the minority isomer for all of the anilines F–J.
Imine 1H NMR signals separated by 80 Hz at 298 K corresponding to the head-to-head and head-to-tail isomers of the product formed from aniline G coalesced upon heating to 353 K in DMSO, reappearing as a single set of signals above this temperature. This experiment indicated a barrier to isomerization of ≈72 kJ·mol−1 at 353 K (42). This isomerization barrier is similar in magnitude to the racemization barrier (slightly above 67 kJ·mol−1) of structurally similar dicopper double-helicates (27), which suggests that a similar mechanism, possibly involving partial dissociation of one or both ligands, might be operating in both racemization and isomerization.
We attribute the head-to-tail preference in the cases of structures derived from anilines F–J to the favorable dipole–dipole interaction between the two head-to-tail ligand strands, based upon two observations. First, the less polar head-to-tail isomer is favored to a greater extent in less polar solvents, as measured by the Kamlet–Taft π* parameter (44). In the case of aniline G, the head-to-tail isomer is 81% present in DMSO (π* = 1.00), increasing to 88% in nitromethane (π* = 0.85) and becoming the only isomer observed in acetonitrile (π* = 0.75). Secondly, the presence of the head-to-tail isomer was also favored by the presence of an electron-poor aniline subcomponent, which would increase the magnitude of the ligand's dipole. From Table 1, it can be seen that the proportion of head-to-tail isomer increases in going from electron-rich aniline F (72%) to electron-poor aniline J (88%) in DMSO.
The isolated yields of the reactions involving amines D–F and H–J were not measured, because these reactions were only carried out on a milligram scale in NMR tubes (as detailed in supporting information, which is published on the PNAS web site). These reactions appeared to proceed as cleanly as the reactions involving amines C and G, for which the isolated yields of 87% and 74% were obtained. Except in the cases of the very electron-deficient anilines I and J, where small amounts of 1 were found in the reaction mixture, we did not observe any parasitic side reactions during these self-assembly reactions, and no decomposition of any of these products was observed in solution over a time scale of weeks. The only limitations on the yields of these reactions appeared thus to be the difficulty of measuring the four subcomponents on a milligram scale and the difficulty of recovering the products.
It is worth noting that this system is capable of forming a stable imine complex that incorporates the electron-deficient 4-nitroaniline J, although this complex forms less cleanly than in the cases of other anilines. In previous cases involving copper(I) mononuclear complexes and symmetrical dinuclear double-helicates, very little incorporation of 4-nitroaniline was ever observed, which we attributed to its low nucleophilicity (32). The observed stability in the present case may be due to stabilizing dipole–dipole interactions with the opposite ligand, or to delocalization of charge within the π system of the ligand (the formation of a “push-pull” system). Neither of these interactions would be present in the previously described systems (32).
Conclusions
The avoidance of valence frustration thus provides a new means of achieving selectivity in subcomponent self-assembly: ligands of the type (aminoquinoline)–(pyridine)–(amine) may be selectively prepared from mixtures of subcomponents. The resulting asymmetrical di-imines may be demetalated and “fixed” through hydrogenation to provide nonexchanging secondary amines. A wide range of anilines and primary aliphatic diamines took part in this reaction, with different subcomponents allowing the system to express further selectivities. The diamines generated exclusively head-to-head structures, and the anilines gave rise predominantly to head-to-tail structures. For the anilines, further fine control over the head-to-head/head-to-tail ratio could be gained by varying the polarity of the solvent and the electronic nature of the aniline's 4-substituent. Control could thus be exercised over both the ordering and the orientation of the subcomponents.
Two subsequent directions of inquiry are foreseen to branch out from this study. First, the utility of this reaction in organic synthesis will be developed further. Polyamines have a rich set of applications ranging from pharmaceuticals (45) to self-assembled nanostructures (46); generalization of this technique may allow it to be of use within the toolbox of organic synthesis. Second, the asymmetrical head-to-head and head-to-tail motifs shown in Scheme 4 may be of use as subunits in larger assemblies. The use of a rigid dianiline subcomponent, for example, may allow for the preparation of a circular helicate (3).
Materials and Methods
General.
All manipulations were carried out in degassed solvents using reagents of the highest commercially available purity. Cu(NCMe)4BF4 was prepared following literature procedures (47). The 1H NMR spectra of 1, 2, 3, and 4 were assigned with the help of COSY, ROESY, NOESY, HSQC, and HMBC measurements (42). NMR spectra were referenced to the residual 1H or 13C signal of the solvent. The general procedure and characterization data for the products of the self-assembly reactions involving amines C–J (Table 1) are given in supporting information.
Preparative Synthesis of 1.
Into a 50-ml Schlenk flask were added 2,6-diformylpyridine A (30.0 mg, 0.22 mmol), 8-aminoquinoline B (64.0 mg, 0.44 mmol), Cu(NCMe)4BF4 (69.8 mg, 0.22 mmol), and CH3CN (5 ml). The brown solution thus obtained was deoxygenated by three vacuum/argon fill cycles and stirred overnight at room temperature. The solvent was evaporated giving 119 mg (99.7%) of a brown microcrystalline powder, which was pure by NMR spectroscopy. 1H NMR (500 MHz, 298 K, CD3CN): δ = 8.69 (s, 4H, imine), 8.30 (dd, J = 8 Hz, J' = 1.5 Hz, 4H, 4-aminoquinoline), 8.10 (dd, J = 4 Hz, J' = 1 Hz, 4H, 2-aminoquinoline), 7.80 (d, J = 8 Hz, 4H, 5-aminoquinoline), 7.56 (t, J = 7.5 Hz, 2H, 4-pyridinedicarboxaldehyde), 7.43 (dd, J = 8 Hz, J' = 4.5 Hz, 4H, 3-aminoquinoline), 7.32 (m, 8H, 6-aminoquinoline, 3-pyridinedicarboxaldehyde), 7.04 (d, J = 7 Hz, 4H, 7-aminoquinoline). 13C NMR (125.77 MHz, 298 K, CD3CN): δ = 158.08, 150.79, 150.28, 143.14, 141.71, 138.78, 137.92, 130.56, 129.96, 129.55, 128.04, 124.04, 120.74. ESI-MS: m/z = 450.1 ([1]2+), 987.3 ([1 + BF4]+).
Preparative Synthesis of 2.
Into a 50-ml Schlenk flask were added 2,6-diformylpyridine A (18.4 mg, 0.136 mmol), 2-[2-(2-amino-ethoxy)-ethoxy]-ethylamine C (20.2 mg, 0.136 mmol) and CH3CN (5 ml). The mixture was degassed by three vacuum/argon fill cycles. Cu(NCMe)4BF4 (42.8 mg, 0.136 mmol) and CH3CN (5 ml) were then added, giving immediately a red–orange solution that was once again degassed by three vacuum/argon fill cycles. The mixture was heated overnight to 50°C and volatiles were removed under dynamic vacuum. The orange–red glassy product obtained was isolated in 98% yield (53 mg). The product was pure by NMR spectroscopy. 1H NMR (500 MHz, 298 K, CD3CN): δ = 8.35 (s, 4H, imine), 8.19 (t, J = 8.5 Hz, 2H, 4-pyridyl), 8.08 (d, J = 7.5 Hz, 4H, 3,5-pyridyl), 3.2–4 (br m, 24H, aliphatic chain). 13C NMR (125.77 MHz, 298 K, CD3CN): δ = 162.8, 152.3, 140.0, 127.7, 70.9, 69.4, 60.7. ESI-MS: m/z = 311.2 ([2]2+), 557.4 ([2 - Cu]+), 707.3 ([2 + BF4]+).
Preparative Synthesis of 3.
Into a 50-ml Schlenk flask were added A (49.2 mg, 0.36 mmol), B (52.5 mg, 0.36 mmol), C (27.0 mg, 0.18 mmol), and water (5 ml). This mixture was deoxygenated by three vacuum/argon fill cycles. Cu(NCMe)4BF4 (114.6 mg, 0.36 mmol) and water (5 ml) were then added, giving a brown solution immediately, which was once more deoxygenated by three vacuum/argon fill cycles and heated to 50°C overnight. The solution was then cooled to room temperature and filtered under argon through a glass wool plug attached to the end of a steel cannula, and volatiles were removed under dynamic vacuum. The brown microcrystalline powder thus obtained was washed with deoxygenated water (twice, 2 ml) and dried under dynamic vacuum, giving an isolated yield of 146 mg (87%). This product was pure by NMR spectroscopy. 1H NMR (500 MHz, 298 K, CD3CN): δ = 9.30 (s, 2H, quinoline imine), 8.33 (dd, J = 8.5 Hz, J' = 1.5 Hz, 2H, 4-aminoquinoline), 8.17 (dd, J = 4.5 Hz, J' = 1.5 Hz, 2H, 2-aminoquinoline), 7.98 (m, 8H, diamine imine, 5-aminoquinoline, 7-aminoquinoline, 3-pyridinedicarboxaldehyde next to the aminoquinoline), 7.86 (t, J = 8 Hz, 2H, 4-pyridinedicarboxaldehyde), 7.75 (t, J = 8 Hz, 2H, 6-aminoquinoline), 7.36 (dd, J = 8.5 Hz, J' = 4.5 Hz, 2H, 3-aminoquinoline), 7.25 (dd, J = 8 Hz, J' = 1 Hz, 2H, 5-pyridinedicarboxaldehyde, next to the diamine), 3.54 (m, 4H, py-CH=N-CH2-CH2-O-CH2), 3.42 (m, 2H + 2H, py-CH=N-CH2-CH2-O-CH2, py-CH=N-CH2-CH2-O-CH2), 3.19 (m, 2H, py-CH=N-CH2-CH2-O-CH2), 2.99 (m, 2H, py-CH=N-CH2-CH2-O-CH2). 13C NMR (125.77 MHz, 298 K, CD3CN): δ = 160.92, 155.34, 149.97, 149.72, 149.66, 141.38, 140.96, 138.89, 137.88, 130.03, 129.40, 128.94, 128.04, 127.78, 123.12, 70.74, 70.53, 58.55. ESI-MS: m/z = 381.3 ([3]2+), 697.4 ([3 - Cu]+), 847.4 ([3 + BF4]+).
Preparation of 3 Starting From 1 and 2.
Into an NMR tube with a Teflon screw cap were added 1 (0.010 mmol), 2 (0.010 mmol), and CD3CN (1 ml). This brown solution was deoxygenated by three vacuum/argon fill cycles. After 4 days at 50°C, 3 was the only product observed by 1H NMR spectroscopy.
Crystallographic Data for 3.
Crystals were grown through vapor counterdiffusion of diisopropyl ether into an acetonitrile solution under a dinitrogen atmosphere. Cell dimensions and intensities were measured at 200 K on a Stoe IPDS diffractometer with graphite-monochromated MoKα radiation (λ = 0.71073 Å). Data were corrected for Lorentz and polarization effects and for absorption. (C38H34Cu2N8O2) (BF4)2 (CH3CN), Mr = 976.6, monoclinic, P21/n, a = 11.1689 (4), b = 29.7864 (12), c = 12.7313 (6) Å, β = 94.426 (5)°, V = 4222.8 (3) Å3, Z = 4, μ = 1.090 mm−1, dx = 1.536 g·cm−3, S = 1.59 (2), R = ωR = 0.033. Crystallographic data were deposited in the Cambridge Structural Database (CSD reference no. 603273).
Preparative Synthesis of 4.
A solution of NaBH4 (24.7 mg, 0.654 mmol) in MeOH (8 ml) was added to a deoxygenated solution of complex 3 (30.6 mg, 0.033 mmol) in MeCN (5 ml), resulting in gas evolution. The color immediately darkened and a black precipitate appeared. This mixture was stirred at room temperature overnight. The orange solution was then filtered through a plug of cotton wool in a Pasteur pipette directly into a solution of KCN (42.6 mg, 0.654 mmol) in MeOH (2 ml). The resulting yellow solution was stirred at room temperature for 1 h. Solvents were then evaporated, and the product was dissolved in dichloromethane (10 ml). This solution was washed with three portions of water (20 ml each), and the organic solvents were evaporated. The residue was then dissolved in MeOH and the product was precipitated through addition of water. The viscous orange oil was then filtered and dried under vacuum to give 13.2 mg (62.4%) of product. 1H NMR (500 MHz, 298 K, CD2Cl2): δ = 8.74 (dd, J = 4Hz, J' = 1.5 Hz, 2H, 2-aminoquinoline), 8.08 (dd, J = 8.0 Hz, J' = 1.5 Hz, 2H, 4-aminoquinoline), 7.57 (t, J = 8 Hz, 2H, 4-pyridine), 7.39 (dd, J = 8 Hz, J' = 4 Hz, 2H, 3-aminoquinoline), 7.31 (t, J = 8 Hz, 2H, 6-aminoquinoline), 7.22 (d, J = 7.5 Hz, 4H, 3-pyridine + 5-pyridine), 7.05 (d, J = 7 Hz, 2H, 5-aminoquinoline or 7-aminoquinoline), 6.61 (d, J = 7.5 Hz, 2H, 7-aminoquinoline or 5-aminoquinoline), 4.63 (d, J = 5Hz, 4H, CH2-aminoquinoline), 3.60 (m, 8H, CH2-diamine), 3.40 (s, 4H, pyridine-CH2-diamine), 2.84 (t, J = 5.5 Hz, 4H, CH2-diamine). 13C NMR (125.77 MHz, 298 K, CD2Cl2): δ = 159.73, 158.15, 147.04, 144.49, 138.31, 136.93, 135.86, 128.64, 127.65, 121.46, 120.29, 119.25, 114.14, 105.08, 70.65, 70.30, 50.29, 48.94, 48.92. ESI-MS: m/z = 322.6 ([4 + 2H]2+), 643.7 ([4 + H]+).
Preparative Synthesis of the Heterocomplex Incorporating Aniline G.
Into a 50 ml Schlenk flask were added aniline G (30.7 mg, 0.22 mmol), 8-aminoquinoline B (31.8 mg, 0.22 mmol), Cu(NCMe)4BF4 (69.3 mg, 0.22 mmol), diformylpyridine A (0.0298 mg, 0.22 mmol), and CH3CN (5 ml). The brown homogeneous solution thus obtained was deoxygenated by three vacuum/argon fill cycles and stirred overnight at 50°C. The supernatant was removed by cannula-filtration and the precipitate was washed with freshly distilled dichloromethane (twice, 10 ml). The brown microcrystalline powder was dried under vacuum, giving an isolated yield of 86.2 mg (74%). Head-to-tail product isomer: 1H NMR (500 MHz, 298 K, DMSO): δ = 9.13 (s, 2H, quinoline imine), 9.00 (s, 2H, aniline imine), 8.46 (dd, J = 8.5 Hz, J' = 1.5 Hz, 2H, 4-aminoquinoline), 8.41 (dd, J = 4.5 Hz, J' = 1.5 Hz, 2H, 2-aminoquinoline), 8.09 (t, J = 8 Hz, 2H, 4-pyridinedicarboxaldehyde), 8.04 (d, J = 7.5 Hz, 2H, 3-pyridinedicarboxaldehyde next to the aniline), 7.76 (d, J = 8 Hz, 2H, 5-aminoquinoline), 7.67 (dd, J = 8 Hz, J' = 5 Hz, 2H, 3-aminoquinoline), 7.46 (dd, J = 8 Hz, J' = 1 Hz, 2H, 3-pyridinedicarboxaldehyde next to the quinoline), 7.27 (t, J = 8 Hz, 2H, 6-aminoquinoline), 7.18 (d, J = 9 Hz, 4H, aniline next to the -SMe), 7.13 (d, J = 7 Hz, 2H, 7-aminoquinoline), 6.88 (d, J = 9 Hz, 4H, aniline next to the imine), 2.50 (overlapping signal with the DMSO, 6H, -SMe). 13C NMR (125.77 MHz, 298 K, DMSO): δ = 157.20, 156.12, 150.54, 149.63, 148.98, 141.88, 141.39, 140.16, 140.01, 138.61, 137.67, 129.65, 129.60, 128.70, 128.05, 127.05, 126.48, 124.08, 122.60, 117.33, 14.69. Head-to-head product isomer: 1H NMR (500 MHz, 298 K, DMSO): δ = 9.29 (s, 2H, quinoline imine), 8.86 (s, 2H, aniline imine), 8.54 (d, J = 8 Hz, 2H, 4-aminoquinoline), 8.36 (d, J = 4 Hz, 2H, 2-aminoquinoline), 8.17 (d, J = 8 Hz, 2H, 5-aminoquinoline), 8.09 (overlapping signal, 2H, 4-pyridinedicarboxaldehyde), 8.01 (d, J = 7.5 Hz, 2H, 3-pyridinedicarboxaldehyde next to the aminoquinoline), 7.85 (t, J = 8 Hz, 2H, 6-aminoquinoline), 7.74 (d, J = 8 Hz, 2H, 7-aminoquinoline), 7.59 (d, J = 7 Hz, 2H, 3-pyridinedicarboxaldehyde next to the aniline), 7.49 (dd, J = 8 Hz, J' = 4 Hz, 2H, 3-aminoquinoline), 7.19 (overlapping signal, 4H, aniline next to the -SMe), 6.98 (d, J = 8.5 Hz, 4H, aniline next to the imine), 2.50 (overlapping signal with DMSO, 6H, -SMe). ESI-MS: m/z = 445.4 ([M]2+), 891.6 ([M - Cu + 2 MeOH]+), 954.5 ([M + MeOH + MeO−]+), 957.5 ([M + BF4]+).
Supplementary Material
Acknowledgments
We thank P. Perrottet (University of Geneva) for mass spectrometric analyses, A. Pinto (University of Geneva) for ROESY NMR spectra, and Dr. D. Jeannerat for help in interpreting the NMR spectra. This work was supported by the Swiss National Science Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
Data deposition: The atomic coordinates of the x-ray crystal structure of 3 have been deposited in the Cambridge Structural Database, Cambridge Crystallographic Data Centre, Cambridge CB2 1EZ, United Kingdom (CSD reference no. 603273).
References
- 1.Rowan SJ, Cantrill SJ, Cousins GRL, Sanders JKM, Stoddart JF. Angew Chem Int Ed. 2002;41:898–952. doi: 10.1002/1521-3773(20020315)41:6<898::aid-anie898>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.Cacciapaglia R, Di Stefano S, Mandolini L. J Am Chem Soc. 2005;127:13666–13671. doi: 10.1021/ja054362o. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht M. Chem Rev. 2001;101:3457–3497. doi: 10.1021/cr0103672. [DOI] [PubMed] [Google Scholar]
- 4.Otto S, Furlan RLE, Sanders JKM. Science. 2002;297:590–593. doi: 10.1126/science.1072361. [DOI] [PubMed] [Google Scholar]
- 5.Reuter C, Schmieder R, Vögtle F. Pure Appl Chem. 2000;72:2233–2241. [Google Scholar]
- 6.Jiang X, Bollinger JC, Lee D. J Am Chem Soc. 2005;127:15678–15679. doi: 10.1021/ja055225u. [DOI] [PubMed] [Google Scholar]
- 7.Holliday BJ, Mirkin CA. Angew Chem Int Ed. 2001;40:2022–2043. [PubMed] [Google Scholar]
- 8.Stulz E, Scott SM, Bond AD, Teat SJ, Sanders JKM. Chem Eur J. 2003;9:6039–6048. doi: 10.1002/chem.200305265. [DOI] [PubMed] [Google Scholar]
- 9.Elemans J, Rowan AE, Nolte RJM. J Mater Chem. 2003;13:2661–2670. [Google Scholar]
- 10.Saur I, Scopelliti R, Severin K. Chem Eur J. 2006;12:1058–1066. doi: 10.1002/chem.200500621. [DOI] [PubMed] [Google Scholar]
- 11.Wu AX, Isaacs L. J Am Chem Soc. 2003;125:4831–4835. doi: 10.1021/ja028913b. [DOI] [PubMed] [Google Scholar]
- 12.Krämer R, Lehn JM, Marquis-Rigault A. Proc Natl Acad Sci USA. 1993;90:5394–5398. doi: 10.1073/pnas.90.12.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seidel SR, Stang PJ. Acc Chem Res. 2002;35:972–983. doi: 10.1021/ar010142d. [DOI] [PubMed] [Google Scholar]
- 14.Hof F, Craig SL, Nuckolls C, Rebek J. Angew Chem Int Ed. 2002;41:1488–1508. doi: 10.1002/1521-3773(20020503)41:9<1488::aid-anie1488>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 15.Argent SP, Adams H, Riis-Johannessen T, Jeffery JC, Harding LP, Ward MD. J Am Chem Soc. 2006;128:72–73. doi: 10.1021/ja056993o. [DOI] [PubMed] [Google Scholar]
- 16.Lehn JM, Eliseev AV. Science. 2001;291:2331–2332. doi: 10.1126/science.1060066. [DOI] [PubMed] [Google Scholar]
- 17.Rowan SJ, Lukeman PS, Reynolds DJ, Sanders JKM. New J Chem. 1998;22:1015–1018. [Google Scholar]
- 18.Rowan SJ, Hamilton DG, Brady PA, Sanders JKM. J Am Chem Soc. 1997;119:2578–2579. [Google Scholar]
- 19.Albrecht M, Janser I, Runsink J, Raabe G, Weis P, Froehlich R. Angew Chem Int Ed. 2004;43:6662–6666. doi: 10.1002/anie.200453975. [DOI] [PubMed] [Google Scholar]
- 20.Karn JL, Busch DH. Nature. 1966;211:160–162. [Google Scholar]
- 21.Caulder DL, Raymond KN. Dalton. 1999:1185–1200. [Google Scholar]
- 22.Drew MGB, Lavery A, McKee V, Nelson SM. Dalton. 1985:1771–1774. [Google Scholar]
- 23.Brooker S, Hay SJ, Plieger PG. Angew Chem Int Ed. 2000;39:1968–1970. [PubMed] [Google Scholar]
- 24.Childs LJ, Alcock NW, Hannon MJ. Angew Chem Int Ed. 2002;41:4244–4247. doi: 10.1002/1521-3773(20021115)41:22<4244::AID-ANIE4244>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Nitschke JR. Angew Chem Int Ed. 2004;43:3073–3075. doi: 10.1002/anie.200454082. [DOI] [PubMed] [Google Scholar]
- 26.Nitschke JR, Hutin M, Bernardinelli G. Angew Chem Int Ed. 2004;43:6724–6727. doi: 10.1002/anie.200461308. [DOI] [PubMed] [Google Scholar]
- 27.Nitschke JR, Schultz D, Bernardinelli G, Gérard D. J Am Chem Soc. 2004;126:16538–16543. doi: 10.1021/ja046001z. [DOI] [PubMed] [Google Scholar]
- 28.Hutin M, Franz R, Nitschke JR. Chem Eur J. 2006;12:4077–4082. doi: 10.1002/chem.200600079. [DOI] [PubMed] [Google Scholar]
- 29.Schultz D, Nitschke JR. Proc Natl Acad Sci USA. 2005;102:11191–11195. doi: 10.1073/pnas.0502830102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutin M, Nitschke JR. Chem Commun. 2006:1724–1726. doi: 10.1039/b601012a. [DOI] [PubMed] [Google Scholar]
- 31.Hutin M, Schalley CA, Bernardinelli G, Nitschke JR. Chem Eur J. 2006;12:4069–4079. doi: 10.1002/chem.200501591. [DOI] [PubMed] [Google Scholar]
- 32.Schultz D, Nitschke JR. J Am Chem Soc. 2006;128:9887–9892. doi: 10.1021/ja061841u. [DOI] [PubMed] [Google Scholar]
- 33.Schultz D, Nitschke JR. Angew Chem Int Ed. 2006;45:2453–2456. doi: 10.1002/anie.200504447. [DOI] [PubMed] [Google Scholar]
- 34.Drew MGB, Farrell D, Morgan GG, McKee V, Nelson J. Dalton. 2000;9:1513–1519. [Google Scholar]
- 35.Al-Obaidi A, Baranovic G, Coyle J, Coates CG, McGarvey JJ, McKee V, Nelson J. Inorg Chem. 1998;37:3567–3574. doi: 10.1021/ic971274z. [DOI] [PubMed] [Google Scholar]
- 36.Hogg L, Leigh DA, Lusby PJ, Morelli A, Parsons S, Wong JKY. Angew Chem Int Ed. 2004;43:1218–1221. doi: 10.1002/anie.200353186. [DOI] [PubMed] [Google Scholar]
- 37.Lehn JM, Rigault A, Siegel J, Harrowfield J, Chevrier B, Moras D. Proc Natl Acad Sci USA. 1987;84:2565–2569. doi: 10.1073/pnas.84.9.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunter CA, Tomas S. J Am Chem Soc. 2006;128:8975–8979. doi: 10.1021/ja061928f. [DOI] [PubMed] [Google Scholar]
- 39.Lindsey JS. New J Chem. 1991;15:153–180. [Google Scholar]
- 40.Peters AJ, Chichak KS, Cantrill SJ, Stoddart JF. Chem Commun. 2005:3394–3396. doi: 10.1039/b505730b. [DOI] [PubMed] [Google Scholar]
- 41.Sauvage JP, Dietrich-Buchecker C. Molecular Catenanes, Rotaxanes and Knots: A Journey Through the World of Molecular Topology. Germany: Wiley, Weinheim; 1999. [Google Scholar]
- 42.Braun S, Kalinowski H-O, Berger S. 150 and More Basic NMR Experiments. Germany: Wiley, Weinheim; 1998. [Google Scholar]
- 43.Stone MT, Fox JM, Moore JS. Org Lett. 2004;6:3317–3320. doi: 10.1021/ol048770t. [DOI] [PubMed] [Google Scholar]
- 44.Kamlet MJ, Abboud JLM, Abraham MH, Taft RW. J Org Chem. 1983;48:2877–2887. [Google Scholar]
- 45.Thomas T, Thomas TJ. Cell Mol Life Sci. 2001;58:244–258. doi: 10.1007/PL00000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim K. Chem Soc Rev. 2002;31:96–107. doi: 10.1039/a900939f. [DOI] [PubMed] [Google Scholar]
- 47.Ogura T. Transition Met Chem. 1976;1:179–182. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.