Abstract
Direct injection of a single spermatozoon into an oocyte (ICSI) can produce apparently normal offspring. Although the production of normal offspring by ICSI has been successful in mice and humans, it has been less successful in many other species. The reason for this is not clear, but could be, in part, due to inconsistent activation of oocytes because of delayed disintegration of sperm plasma membrane within oocytes and incorporation of the acrosome containing a spectrum of hydrolyzing enzymes. In the mouse, the removal of sperm plasma membrane and acrosome was not a prerequisite to produce offspring by ICSI, but it resulted in earlier onset of oocyte activation and better embryonic development. The best result was obtained when spermatozoa were demembranated individually immediately before ICSI by using lysolecithin, a hydrolysis product of membrane phospholipids.
Keywords: mouse, human, Ca2+ oscillations, fertilization, lysolecithin
Direct injection of a single spermatozoon into an oocyte, commonly called intracytoplasmic sperm injection (ICSI), can produce apparently normal offspring even though it bypasses a number of biological processes necessary for normal fertilization. As long as the sperm nucleus has intact genetic integrity, ICSI can produce healthy offspring regardless of concentrations, morphology, and motility of spermatozoa (1, 2). Only one genomically normal spermatozoon is needed to fertilize one oocyte. A salient difference between natural and ICSI fertilization is that, in the latter, the sperm plasma membrane as well as acrosome (which contains a spectrum of powerful hydrolyzing enzymes) are introduced into an oocyte. For species with small acrosomes, injection of the acrosome into an oocyte apparently does not produce serious problems, but for species like the hamster, with very large acrosomes, injection inevitably results in death of the oocyte (3). We had demonstrated that the contents of the acrosome are potentially harmful to oocytes (4). A notable difference between normal and ICSI fertilization is that repetitive transient increases in intracellular Ca2+ concentration of the oocyte (Ca2+ oscillations), the pivotal signal for oocyte activation (5–8), begins much more slowly in ICSI oocytes than in normally fertilized oocytes. In the mouse, for instance, Ca2+ oscillations begin 1–3 min after plasma membrane fusion between a fertilizing spermatozoon and an oocyte (9), whereas oscillation begins 15–30 min after ICSI (10, 11). In human oocytes, Ca2+ oscillations start 4–12 h after ICSI, when a plasma membrane-intact spermatozoon is injected after immobilization by touching the terminal part of the tail (12). Ca2+ oscillations begin faster (14 ± 6 min) when a spermatozoon is immobilized by applying several piezo pulses to the proximal one-third of the sperm tail before injection (13). Kasai et al. (14) reported that oocyte activation, assessed by the completion of meiosis, occurred earlier when spermatozoa were freed from the plasma membrane before ICSI. Increased fertilization rates after ICSI using plasma membrane-removed porcine spermatozoa were reported (15, 16). Here, we report that removal of both the plasma membrane and acrosome from mouse spermatozoa before ICSI not only accelerates the onset of oocyte activation but also results in improved embryonic development.
Results
Comparison of the “Stability” of Sperm Plasma Membranes in Various Species.
Table 1 shows minimum concentrations of Triton X-100 and lysolecithin (LL) that immobilize (kill) 100% of spermatozoa of various species within 10 sec. Although Triton X-100 failed to demonstrate clear differences in the stability of sperm plasma membranes among several different species, LL clearly showed that human spermatozoa have the most stable membranes (at least most resistant to LL) of all spermatozoa tested.
Table 1.
Minimum concentrations of Triton X-100 and LL to immobilize 100% of spermatozoa within 10 sec (at 24–25°C)
| Species | Triton X-100 (% v/v) | LL (% wt/vol) |
|---|---|---|
| Human | 0.01 | 0.1 |
| Bovine | 0.02 | 0.03 |
| Mouse | 0.02 | 0.02 |
| Porcine | 0.015 | 0.015 |
| Rat | 0.015 | 0.01 |
| Hamster | 0.015 | 0.003 |
Timing of Oocyte Activation After ICSI by Using Spermatozoa With or Without Intact Plasma Membranes.
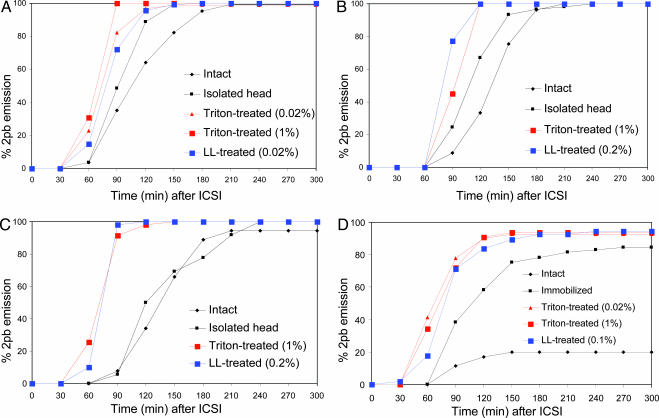
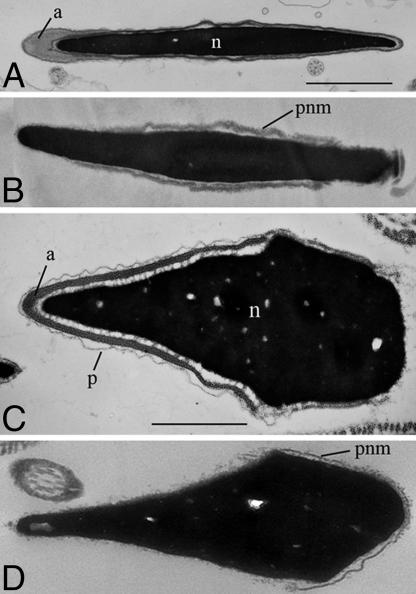
The extrusion of the second polar body was considered as a visible indication of completion of oocyte activation. Fig. 1A shows percentages of mouse oocytes activated after ICSI using mouse spermatozoa. When the entire body of an intact, live spermatozoon was injected into each oocyte, few oocytes were activated at 60 min after ICSI. It was only after 210 min when 100% of oocytes were activated. When the sperm head was isolated from the tail by a piezo pulse and injected, oocytes were activated slightly earlier. The earliest oocyte activation took place when spermatozoa were treated with Triton X-100 or LL before injection of isolated sperm heads. Similar results were obtained after injection of bovine and porcine spermatozoa (Fig. 1 B and C). Acceleration of oocyte activation by plasma membrane removal from spermatozoa was most dramatic for human spermatozoa (Fig. 1D). Membrane-intact human spermatozoa often remained motile within the oocyte's cytoplasm for 1 h after ICSI. Membrane-intact spermatozoa activated only 20% of mouse oocytes even at 300 min after ICSI. More oocytes were activated when spermatozoa were immobilized before injection. The earliest oocyte activation occurred after injection of Triton X-100- or LL-treated spermatozoa. Fig. 2 shows electron micrographs of mouse and human spermatozoa before and after LL treatment. LL removed both the plasma membrane and acrosome from spermatozoa (Fig. 2 B and D). Note that the perinuclear materials (theca) remain on the sperm nucleus.
Fig. 1.
Comparison of the timing of oocyte activation assessed by the emission of the second polar body (2pb). Mouse (A), human (D), porcine (C), and bull (B) spermatozoa were injected individually into a mouse oocyte, and the proportion of oocytes emitting 2pb was determined at 30-min intervals. LL- and Triton X-100-treated spermatozoa activated oocytes much earlier than intact spermatozoa. Immobilized spermatozoa were intermediate. Each point is based on an average of 80 oocytes examined.
Fig. 2.
Electron micrographs of the heads of mouse and human spermatozoa. Mouse spermatozoa before (A) and after (B) LL treatment. Human spermatozoa before (C) and after (D) LL treatment. a, acrosome; n, nucleus; p, plasma membrane; pnm, perinuclear material. Note that perinuclear material (theca) remains on sperm nucleus after removal of the plasma membrane and acrosome (B and D). (Scale bars, 1 μm.)
Onset and Pattern of Intracellular Ca2+ Oscillations After ICSI Using Plasma Membrane-Intact and Membrane-Disrupted Spermatozoa.
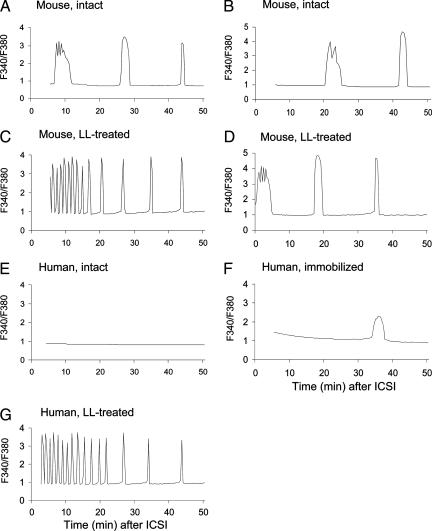
We examined intracellular Ca2+ oscillations in mouse oocytes after injection of (i) motile mouse spermatozoa without any treatments, (ii) mouse spermatozoa treated individually with 0.2% LL, (iii) motile human spermatozoa without any treatments, (iv) human spermatozoa immobilized by applying a few piezo pulses to the midpiece, and (v) human spermatozoa treated individually with 0.2% LL. Time 0 in Fig. 3 means the moment of ICSI. Recording started usually within several minutes after ICSI but, sometimes, almost immediately after ICSI.
Fig. 3.
Ca2+ oscillations in mouse oocytes after ICSI using a spermatozoon indicated.
When an intact, motile mouse spermatozoon was injected into an oocyte, the spermatozoon stopped its tail movement mostly within 10 min. The first Ca2+ transient after normal fertilization (5) or ICSI (11) is characterized by the longer duration than later Ca2+ transients associated with superimposed smaller Ca2+ oscillations. The first Ca2+ response depicted in Fig. 3 A and B was thought to be the first Ca2+ transient, judging from its pattern. Thus, Ca2+ oscillations began 7–20 min after injection of membrane-intact mouse spermatozoa. Ca2+ transients occurred at the interval of ≈20 min. When LL-treated mouse spermatozoa were injected, high-frequency Ca2+ oscillations (8–9 spikes per 10 min) were recorded in some oocytes (4 of 13 oocytes) from the beginning of intracellular calcium ion concentration measurement (Fig. 3C). Probably, Ca2+ oscillations began immediately after ICSI. The high-frequency Ca2+ oscillations continued for ≈20 min before the interspike interval was prolonged for up to 10 min. In 9 of 13 oocytes, the usual first Ca2+ transient occurred immediately after ICSI, followed by succeeding Ca2+ spikes at the interspike interval of 10–15 min (Fig. 3D).
When intact, live human spermatozoa were injected into mouse oocytes, none of five oocytes showed Ca2+ response during recording for 50–300 min (Fig. 3E). When piezo-immobilized human spermatozoa were injected into seven mouse oocytes, one of seven oocytes showed a single Ca2+ transient within 50 min of recording (Fig. 3F). Three oocytes showed one or a few Ca2+ oscillations, at 0.5- to 2-h intervals, during 4 h of recording. The other three oocytes showed typical Ca2+ oscillations from the beginning of recording. In other words, the Ca2+ response was erratic in the oocytes injected with piezo-immobilized human spermatozoa. In contrast, high-frequency Ca2+ oscillations occurred in all four oocytes when they were each injected with a single LL-treated human spermatozoon (Fig. 3G). The amount of LL injected into oocytes was negligible because spermatozoa were thoroughly rinsed before injection. LL, even if it was intentionally injected into oocytes (1 pl of 0.2% solution = ≈1.0 pg into each oocyte), did not induce any Ca2+ increase (data not shown).
Development of Mouse Oocytes Injected with Plasma Membrane-Intact or Plasma Membrane-Disrupted Spermatozoa.
When a single mouse spermatozoon with intact plasma membrane was injected into an oocyte, ≈80% of ICSI-surviving oocytes developed into blastocysts in vitro. The same proportion of oocytes developed into blastocysts after injection of isolated sperm heads with or without prior treatment of spermatozoa with Triton X-100 or LL (data not shown). However, the method of handling spermatozoa before ICSI affected postimplantation development of embryos (Table 2). When the overall efficiency of embryonic development was assessed by the proportion of term fetuses developed from two-cell embryos, the lowest value (40%) was obtained after injection of plasma membrane-intact spermatozoa (experiment A). Developmental efficiency increased (58%) when sperm heads and tails were separated by piezo-pulses, and the heads were individually injected into oocytes (experiment B). The highest efficiency (71%) was recorded when spermatozoa were treated individually with 0.02% LL immediately before injection, and their heads were injected individually into oocytes (experiment C). The efficiency was reduced (46%) when spermatozoa were treated with LL as a mass, and LL-treated sperm heads were left in the medium for some time before ICSI (experiment D). This was true for sperm heads treated with Triton X-100 (compare experiments E and F).
Table 2.
Term development of mouse embryos developed from the oocytes fertilized by injection of intact, immobilized, lysolecithin-treated and Triton X-100-treated mouse spermatozoa
| Exp. | Sperm injected | Total no. of zygotes cultured (no. of reps.) | No. (%) of zygotes developed to two-cell | No. of two-cells transferred (no. of recipients) | No. (%) of live normal offspring at term |
|---|---|---|---|---|---|
| A | Intact | 108 (7) | 102 (94) | 102 (9) | 41 (40.2)* |
| B | Isolated heads | 142 (10) | 137 (96) | 137 (12) | 80 (58.3)† |
| C | Lysolecithin-treated, individually | 123 (7) | 123 (100) | 123 (9) | 88 (71.5)‡ |
| D | Lysolecithin-treated, as a group | 158 (7) | 149 (94) | 149 (13) | 70 (46.9)§ |
| E | Triton X-100-treated, individually | 118 (7) | 115 (97) | 98 (7) | 58 (59.2)¶ |
| F | Triton X-100-treated, as a group | 120 (9) | 118 (98) | 118 (11) | 43 (36.4)‖ |
Experiment (Exp.) A, a motile spermatozoon with intact head and tail was injected into each oocyte; B, only the head was injected after separation of the head and tail by piezo pulses; C and E, spermatozoa were individually treated for 1 min with 0.02% LL or 0.02% Triton X-100 and washed in Hepes-CZB containing 12% PVP for 1 min before injection of a single sperm head isolated from the tail by piezo pulses; D and F, ≈105 spermatozoa were treated for 1 min with 0.02% LL or 0.02% Triton X-100, washed by centrifugation for 3 min, and left in Hepes-CZB for 5–60 min before injection of a single sperm head isolated from the tail by piezo pulses. ∗ vs. †, P < 0.01; † vs. ‡, P < 0.01; ‡ vs. §, P < 0.01; ¶ vs. ‖, P < 0.01.
Discussion
This study confirmed that mouse oocytes developed into live offspring after ICSI regardless of introduction or nonintroduction of sperm plasma membrane into oocytes. However, the proportion of live offspring produced was considerably higher after injection of membrane-free spermatozoa than injection of membrane-intact ones (Table 2). How does this happen? This study showed that plasma membrane-free spermatozoa activated oocytes earlier than membrane-intact ones (Figs. 1 and 3). This result was particularly evident for human spermatozoa (Figs. 1D and 3 E–G). It should be noted that human spermatozoa have more stable plasma membranes than the spermatozoa of other species tested (Table 1). If the sperm plasma membrane of a given species is stable and the ability of an oocyte's cytoplasm to “digest” the sperm plasma membrane is low, the membrane will disintegrate slowly or does not disintegrate at all within the oocyte. Quick disintegration of the sperm plasma membrane within the oocyte is important because oocyte activation depends on sperm-borne oocyte-activating factor (SOAF). The strongest candidate for SOAF in mammals thus far is phospholipase C-ζ (17–20). At least part of SOAF is localized in the perinuclear theca in the postacrosomal region (21–24) and under the plasma membrane over the equatorial segment of the acrosome (25). When an intact spermatozoon is injected, SOAF would not be exposed to the oocyte's cytoplasm until the sperm plasma membrane in these two regions disintegrates. Membrane disintegration may occur earlier in some oocytes than in others, and this fact may explain earlier activation in some oocytes than in others. When spermatozoa are freed from plasma membranes before ICSI, soluble cytosolic SOAF will leak out of the spermatozoon and be lost in the medium. However, the SOAF bound to the perinuclear theca will remain and be exposed to the ooplasm upon injection, resulting in an immediate initiation of Ca2+ oscillations. This situation is close to normal fertilization in which SOAF comes into an immediate contact with the ooplasm upon sperm–oocyte membrane fusion.
One of the reasons why development of embryos after ICSI has not been very successful in many animal species (2) could be delayed, erratic, or failed Ca2+ oscillations as recorded in bovine and equine oocytes after ICSI (26, 27), likely because of a delayed or disordered exposure of SOAF to the ooplasm. Conversely, earlier and synchronous oocyte activation brought by removal of sperm plasma membrane may lead to better embryonic development, culminating in the birth of many live offspring. According to Ajduk et al. (28), pretreatment of mouse spermatozoa with 20 μM Ca2+ ionophore synchronizes pronuclear development of mouse oocytes after ICSI. Because such high concentrations of Ca2+ ionophore immobilizes (“kills”) most spermatozoa (29), the synchronization of pronuclear development Ajduk et al. (28) observed is likely because of extensive damage or loss of sperm plasma membranes by influx of excessive Ca2+.
Mouse ICSI using LL-treated spermatozoa resulted in a high percentage of normal live offspring (Table 2). Although Triton X-100-treated spermatozoa can produce live offspring as well, we prefer LL because it is not “alien” to spermatozoa. It is a product of hydrolysis of membrane phospholipids by phospholipase A. LL plays important roles in various biological processes, including sperm acrosome reaction (30, 31). A brief exposure of spermatozoa to LL apparently did not damage sperm nuclei, as evidenced by the birth of many normal offspring. Although LL was washed away before ICSI and was not injected into oocytes, a small amount (≈1.0 pg) of LL we intentionally injected into each oocyte neither activated oocytes nor affected embryonic and fetal development of fertilized mouse eggs (K.M. and T.S., unpublished data). Another reason for better embryonic development after injection of plasma membrane-free spermatozoa could be the absence of the acrosome in an injected spermatozoon. We had reported that the contents of the acrosome are potentially harmful to zygotes and perhaps to developing embryos (4). Triton X-100 and LL remove not only sperm plasma membrane but also the acrosome, which contains a spectrum of powerful hydrolyzing enzymes. Even though oocytes injected with acrosome-intact spermatozoa may appear to be undamaged, acrosomal enzymes released into the cytoplasm of oocytes may have lasting effects on the development of embryos. This possibility must be taken into consideration for species with large sperm acrosomes. The golden hamster is an extreme example. Injection of an acrosome-intact hamster spermatozoon “kills” the oocyte (3).
The importance of minimizing the time between sperm membrane disruption and ICSI cannot be overemphasized. The longer the interval, the more deterioration of sperm nuclei we must expect. Endogenous nucleases, which are activated by Ca2+ (32), may cleave sperm DNA when the sperm nucleus is exposed directly to artificial media. Until media or conditions are developed that keep sperm DNA intact for hours or days, sperm plasma membrane should not be removed until immediately before ICSI.
Materials and Methods
Reagents and Media.
All inorganic and organic reagents were purchased from Sigma (St. Louis, MO) unless otherwise stated. The medium used for culturing oocytes after ICSI was a bicarbonate-buffered Chatot, Ziomet, and Bavister medium (CZB) supplemented with 5.56 mM d-glucose and 4 mg/ml BSA (33). The medium used for oocyte collection and ICSI was a modified CZB with 20 mM Hepes-Na, 5 mM NaHCO3, and 0.1 mg/ml polyvinyl alcohol (cold water-soluble) instead of BSA. CZB was used under 5% CO2 in air, and Hepes-buffered CZB solution (Hepes-CZB) was used under 100% air. The pH of these media was ≈7.4. The reason for our using CZB instead of a currently popular KSOM medium is that 10 mM SrCl2, which we used to activate mouse oocytes, tends to precipitate in the KSOM medium. As long as oocytes and embryos are from hybrid mice, we do not see any differences between these two media in their ability to support preimplantation development of embryos.
Animals.
Mice, Syrian hamsters, and rats were maintained in accordance with the guidelines of the Laboratory Animal Service at the University of Hawaii and the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources National Research Council (DHEW publication [NIH] 80–23, revised in 1985).
Preparation of Mouse Oocytes.
Female B6D2F1 mice, 7–15 weeks of age, were superovulated by i.p. injection of 7.5 IU of equine chorionic gonadotropin, followed 48 h later by i.p. injection of 7.5 IU of human chorionic gonadotropin (hCG). Mature oocytes were collected from oviducts 14–16 h after hCG injection. They were freed from cumulus cells by a 3-min treatment with 0.1% (wt/vol) bovine testicular hyaluronidase (300 USP units/mg; ICN, Costa Mesa, CA) in Hepes-CZB. The cumulus-free oocytes were thoroughly rinsed and kept in CZB before ICSI for up to 3 h at 37°C.
Preparation of Spermatozoa.
A drop (≈5 μl) of dense sperm mass from a cauda epididymis of the mouse, Syrian hamster, or rat was placed at the bottom of a 1.5-ml centrifuge tube containing 300 μl of CZB for 5–20 min at 37°C to allow spermatozoa to swim up into the medium. Frozen bull semen in plastic straws (CRI, Shawano, WI) were thawed in a 37°C water bath immediately before use. Boar semen stored at 17°C (International Boar Semen, Eldora, IA) was brought to room temperature. A 500-μl aliquot of bovine or boar semen was placed at the bottom of a 1.5-ml plastic centrifuge tube containing 500 μl of CZB to allow spermatozoa to swim up for 20 min at 37°C. One to 2 ml of liquefied human semen was placed at the bottom of a 5-ml tube containing 3 ml of CZB to allow spermatozoa to swim into the medium for 30–60 min at 37°C. Some spermatozoa that swam into the medium were treated with plasma membrane-disrupting agents, Triton X-100 (Sigma) or LL (Avanti, Birmingham, AL), by mixing a 500-μl sperm suspension with an equal volume of Hepes-CZB containing 0.04–2.0% (vol/vol) Triton X-100 or 0.04–0.4% (wt/vol) LL. The mixture was vortexed or sonicated for 1 min at 0°C. Spermatozoa were washed in Hepes-CZB by centrifugation (2,000 × g for 3 min) before ICSI. Some spermatozoa were picked up individually and demembranated by treating with 0.02% Triton X-100 or 0.02% LL for 1 min, washed, and injected into oocytes immediately.
ICSI.
ICSI was carried out according to Kimura and Yanagimachi (34) and Szczygiel and Yanagimachi (35) with some modifications. An aliquot (25 μl) of sperm suspension was mixed thoroughly with 50 μl of Hepes-CZB containing 8–12% (wt/vol) polyvinylpyrrolidone (Mr 360,000). A drop of this suspension was transferred under paraffin oil [either Squibb & Sons (Princeton, NJ) or Merck (Tokyo, Japan)] in a plastic dish (100 × 100 mm) previously placed on the stage of an inverted microscope equipped with a micromanipulation system. Three types of spermatozoa were injected into oocytes: (i) the entire body of a single, live spermatozoon, (ii) a sperm head isolated from the tail by applying a single or a few piezo pulses to the neck region, and (iii) the head of a single spermatozoon previously treated with either Triton X-100 or LL. Because the heads of human spermatozoa, unlike those of spermatozoa of other species, were unable to be separated from the tail by piezo pulses, the entire body of a single spermatozoon was injected regardless of whether it was treated with Triton X-100 or LL. Approximately 15 oocytes in a group were operated within 5 min. ICSI was completed within 2 h after collection of oocytes from the oviduct.
Comparision of Oocyte Activation After ICSI Using Spermatozoa With or Without Plasma Membranes.
Four types of spermatozoa were prepared: (i) motile spermatozoa, (ii) spermatozoa immobilized by a piezo pulse, (iii) spermatozoa treated for 1 min with 0.02–1.0% Triton X-100, and (iv) spermatozoon treated for 1 min with 0.02–0.2% LL. The latter two had either extensively disrupted or no plasma membranes (ref. 21 and Fig. 2). Application of a single or a few piezo pulses (36) to the neck region separated sperm heads from tails in all species except for the human. As the rule, only the heads of mouse, bovine, and boar spermatozoa were injected into oocytes.
To assess the timing of oocyte activation, the extrusion of the second polar body was observed. ICSI oocytes were maintained at 37°C, examined every 30 min, fixed, and stained (37) to verify the completion of the meiotic divisions of oocytes. As the second indication of oocyte activation, the onset of intracellular Ca2+ rises after ICSI was determined. Repetitive intracellular Ca2+ rises are known to trigger oocyte activation (7, 8, 19) Oocytes were loaded with the Ca2+-sensitive fluorescent dye fura-2 acetoxymethyl ester (fura-2 AM; Dojindo Laboratories, Kumamoto, Japan) by incubating them in Hepes-CZB containing 2.5 μM fura-2 AM for 7 min at 24°C. After washing, a group of 10–15 eggs were subjected to intracellular calcium ion concentration ([Ca2+]i) measurement by a conventional Ca2+ imaging method (11) using an image processor (Arugas 200; Hamamatsu Photonics, Hamamatsu, Japan). Three to five oocytes were consecutively injected with spermatozoa at 23–24°C and transferred to a dish of M2 medium (38) previously placed on a warmed stage (32–33°C) of the UV microscope. Some ICSI was performed on a warmed stage (32–33°C) of the UV microscope (Fig. 3D). Intracellular calcium ion concentration measurement started immediately or within several minutes after onset of ICSI and continued at about 32°C because lowering the temperature at this magnitude slows down aging of oocytes and reduces damages by UV illumination without changing the Ca2+ response.
Transfer of ICSI Embryos to Surrogate Mothers.
Two-cell embryos developed from ICSI oocytes were transferred into oviducts of pseudopregnant CD1 (albino) females that had been mated during the previous night with vasectomized males of the same strain. Surrogate females were killed on day 19 of pregnancy, and their uteri were examined for the presence of live fetuses. Live fetuses, if any, were collected by Cesarean section and raised by lactating CD1 foster mothers.
Statistical Analysis.
The difference between the experimental group and matched control group was compared by using Fisher's exact probability test or the χ2 test. Differences were considered significant at the P < 0.05 level.
Acknowledgments
We thank Mrs. Tina Carvalho and Mrs. Hiroko Yanagimachi for preparing micrographs of mouse and human spermatozoa, Dr. Chin N. Lee and Dr. Halina M. Zaleski for their arrangements of importation of bovine and porcine semen, Dr. Thomas T. F. Huang (University of Hawaii) for providing us with the semen samples used in this study, and Dr. Stefan Moisyadi, Dr. Vincent De Feo, and Mrs. Charlotte Oser for reading and revising the original manuscript. This study was supported by the Research and Training Revolving Fund of the University of Hawaii.
Abbreviations
- CZB
Chatot, Ziomet, and Bavister medium
- Hepes-CZB
Hepes-buffered CZB solution
- ICSI
intracytoplasmic sperm injection
- LL
lysolecithin
- SOAF
sperm-borne oocyte-activating factor.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 17585.
References
- 1.Nagy ZP, Liu J, Joris H, Verheyen G, Tournaye H, Camus M, Derde MC, Devroey P, Van Steirteghem AC. Hum Reprod. 1995;10:1123–1129. doi: 10.1093/oxfordjournals.humrep.a136104. [DOI] [PubMed] [Google Scholar]
- 2.Yanagimachi R. Reprod Biomed Online. 2005;10:247–288. doi: 10.1016/s1472-6483(10)60947-9. [DOI] [PubMed] [Google Scholar]
- 3.Yamauchi Y, Yanagimachi R, Horiuchi T. Biol Reprod. 2002;67:534–539. doi: 10.1095/biolreprod67.2.534. [DOI] [PubMed] [Google Scholar]
- 4.Morozumi K, Yanagimachi R. Proc Natl Acad Sci USA. 2005;102:14209–14214. doi: 10.1073/pnas.0507005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deguchi R, Shirakawa H, Oda S, Mohri T, Miyazaki S. Dev Biol. 2000;218:299–313. doi: 10.1006/dbio.1999.9573. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki S, Shirakawa H, Nakada K, Honda Y. Dev Biol. 1993;158:62–78. doi: 10.1006/dbio.1993.1168. [DOI] [PubMed] [Google Scholar]
- 7.Jones KT. Int J Dev Biol. 1998;42:1–10. [PubMed] [Google Scholar]
- 8.Miyazaki S. Semin Cell Dev Biol. 2006;17:233–243. doi: 10.1016/j.semcdb.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence Y, Whitaker M, Swann K. Development (Cambridge, UK) 1997;124:233–241. doi: 10.1242/dev.124.1.233. [DOI] [PubMed] [Google Scholar]
- 10.Nakano Y, Shirakawa H, Mitsuhashi N, Kuwabara Y, Miyazaki S. Mol Hum Reprod. 1997;3:1087–1093. doi: 10.1093/molehr/3.12.1087. [DOI] [PubMed] [Google Scholar]
- 11.Sato MS, Yoshitomo M, Mohri T, Miyazaki S. Cell Calcium. 1999;26:49–58. doi: 10.1054/ceca.1999.0053. [DOI] [PubMed] [Google Scholar]
- 12.Tesarik J, Sousa M, Testart J. Hum Reprod. 1994;9:511–518. doi: 10.1093/oxfordjournals.humrep.a138537. [DOI] [PubMed] [Google Scholar]
- 13.Yanagida K, Katayose H, Hirata S, Yazawa H, Hayashi S, Sato A. Hum Reprod. 2001;16:148–152. doi: 10.1093/humrep/16.1.148. [DOI] [PubMed] [Google Scholar]
- 14.Kasai T, Hoshi K, Yanagimachi R. Zygote. 1999;7:187–193. doi: 10.1017/s0967199499000568. [DOI] [PubMed] [Google Scholar]
- 15.Katayama M, Sutovsky P, Yang BS, Cantley T, Rieke A, Farwell R, Oko R, Day BN. Reproduction. 2005;130:907–916. doi: 10.1530/rep.1.0680. [DOI] [PubMed] [Google Scholar]
- 16.Tian JH, Wu ZH, Liu L, Cai Y, Zeng SM, Zhu SE, Liu GS, Li Y, Wu CX. Theriogenology. 2006;66:439–448. doi: 10.1016/j.theriogenology.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. Development (Cambridge, UK) 2002;129:3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- 18.Swann K, Larman MG, Saunders CM, Lai FA. Reproduction. 2004;127:431–439. doi: 10.1530/rep.1.00169. [DOI] [PubMed] [Google Scholar]
- 19.Swann K, Ozil JP. Int Rev Cytol. 1994;152:183–222. doi: 10.1016/s0074-7696(08)62557-7. [DOI] [PubMed] [Google Scholar]
- 20.Yoda A, Oda S, Shikano T, Kouchi Z, Awaji T, Shirakawa H, Kinoshita K, Miyazaki S. Dev Biol. 2004;268:245–257. doi: 10.1016/j.ydbio.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 21.Kimura Y, Yanagimachi R, Kuretake S, Bortkiewicz H, Perry AC, Yanagimachi H. Biol Reprod. 1998;58:1407–1415. doi: 10.1095/biolreprod58.6.1407. [DOI] [PubMed] [Google Scholar]
- 22.Fujimoto S, Yoshida N, Fukui T, Amanai M, Isobe T, Itagaki C, Izumi T, Perry AC. Dev Biol. 2004;274:370–383. doi: 10.1016/j.ydbio.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 23.Perry AC, Wakayama T, Cooke IM, Yanagimachi R. Dev Biol. 2000;217:386–393. doi: 10.1006/dbio.1999.9552. [DOI] [PubMed] [Google Scholar]
- 24.Knott JG, Kurokawa M, Fissore RA. Dev Biol. 2003;260:536–547. doi: 10.1016/s0012-1606(03)00251-3. [DOI] [PubMed] [Google Scholar]
- 25.Sutovsky P, Manandhar G, Wu A, Oko R. Microsc Res Tech. 2003;61:362–378. doi: 10.1002/jemt.10350. [DOI] [PubMed] [Google Scholar]
- 26.Bedford SJ, Kurokawa M, Hinrichs K, Fissore RA. Biol Reprod. 2004;70:936–944. doi: 10.1095/biolreprod.103.021485. [DOI] [PubMed] [Google Scholar]
- 27.Malcuit C, Maserati M, Takahashi Y, Page R, Fissore RA. Reprod Fertil Dev. 2006;18:39–51. doi: 10.1071/rd05131. [DOI] [PubMed] [Google Scholar]
- 28.Ajduk A, Yamauchi Y, Ward MA. Biol Reprod. 2006;75:442–451. doi: 10.1095/biolreprod.106.053223. [DOI] [PubMed] [Google Scholar]
- 29.Tanphaichitr N, Hansen C. Mol Reprod Dev. 1994;37:326–334. doi: 10.1002/mrd.1080370312. [DOI] [PubMed] [Google Scholar]
- 30.Lessig J, Glander HJ, Schiller J, Petkovic M, Paasch U, Arnhold J. Andrologia. 2006;38:69–75. doi: 10.1111/j.1439-0272.2006.00713.x. [DOI] [PubMed] [Google Scholar]
- 31.Roldan ER. Front Biosci. 1998;3:D1109–D1119. doi: 10.2741/a348. [DOI] [PubMed] [Google Scholar]
- 32.Sotolongo B, Huang TT, Isenberger E, Ward WS. J Androl. 2005;26:272–280. doi: 10.1002/j.1939-4640.2005.tb01095.x. [DOI] [PubMed] [Google Scholar]
- 33.Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. J Reprod Fertil. 1989;86:679–688. doi: 10.1530/jrf.0.0860679. [DOI] [PubMed] [Google Scholar]
- 34.Kimura Y, Yanagimachi R. Biol Reprod. 1995;52:709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- 35.Szczygiel MA, Yanagimachi R. In: Intracytoplasmic Sperm Injection. Nagy A, Gertsenstein M, Vintersten K, Behringer RR, editors. Woodbury, NY: Cold Spring Harbor Lab Press; 2003. pp. 1797–1924. [Google Scholar]
- 36.Kuretake S, Kimura Y, Hoshi K, Yanagimachi R. Biol Reprod. 1996;55:789–795. doi: 10.1095/biolreprod55.4.789. [DOI] [PubMed] [Google Scholar]
- 37.Yanagida K, Yanagimachi R, Perreault SD, Kleinfeld RG. Biol Reprod. 1991;44:440–447. doi: 10.1095/biolreprod44.3.440. [DOI] [PubMed] [Google Scholar]
- 38.Fulton BP, Whittingham DG. Nature. 1978;273:149–151. doi: 10.1038/273149a0. [DOI] [PubMed] [Google Scholar]