Abstract
Painting a glass slide with branched or linear N,N-dodecyl methyl-polyethylenimines (PEIs) and certain other hydrophobic PEI derivatives enables it to kill influenza virus with essentially a 100% efficiency (at least a 4-log reduction in the viral titer) within minutes, as well as the airborne human pathogenic bacteria Escherichia coli and Staphylococcus aureus. For most of the coating polyions, this virucidal action is shown to be on contact, i.e., solely by the polymeric chains anchored to the slide surface; for others, a contribution of the polyion leaching from the painted surface cannot be ruled out. A relationship between the structure of the derivatized PEI and the resultant virucidal activity of the painted surface has been elucidated.
Keywords: bactericidal, hydrophobic polyions, virucidal coatings, polyethylenimine, flu
Influenza virus causes one of the most prevalent human infections: in a typical year, ≈15% of the U.S. population is infected, resulting in up to 40,000 deaths and 200,000 hospitalizations (www.cdc.gov/flu). Furthermore, an influenza pandemic (when a new strain of the virus, to which humans have no immunity, acquires the ability to readily infect people), assuming the estimated mortality rate of the 1918 Spanish flu pandemic (1), might kill some 75 million people worldwide.
Influenza (as many other diseases) typically spreads when aerosol particles containing the virus, exhaled or otherwise emitted by an infected person, settle onto surfaces subsequently touched by others (2). Hence, this spread of infection, in principle, could be prevented if common things encountered by people are coated with “paints” that inactivate influenza virus.
Recently, building on our prior studies with covalently derivatized surfaces (3), we discovered that certain water-insoluble, hydrophobic polycations, e.g., N,N-dodecyl methyl-polyethylenimine (PEI), when painted onto surfaces, kill bacteria on contact because of rupturing of bacterial cell membranes by erect fragments of the polycationic chains (“tentacles”) (4). Because influenza virus, belonging to a class of enveloped viruses, is protected from the outside by a lipid membrane (5, 6), we reasoned that the aforementioned hydrophobic polycations might damage it as well, thereby inactivating the virus. Indeed, in the present study we find that such painted (coated) surfaces, in addition to being extremely bactericidal, also lower the titer of the encountered influenza A virus [the most infectious type in humans (7)] at least 10,000-fold; a relationship between this virucidal activity and the coating polymer structure has been elucidated and rationalized.
Results and Discussion
To mimic a scenario whereby aerosolized aqueous droplets containing influenza virus settle onto surfaces and the virus then spreads (2), we adopted the following approach. A 10-μl droplet of a PBS-buffered solution containing (1.6 ± 0.3) × 103 pfu of the A/WSN/33 (H1N1) strain of influenza virus was placed in the center of a 2.5 × 2.5 cm glass slide (either coated or plain control). Then another plain glass slide of the same size was placed on top and pressed against the first to flatten the droplet. After a room temperature (r.t.) incubation for 30 min (unless stated otherwise), one edge of the upper slide was lifted and both virus-exposed glass surfaces were thoroughly washed with 1.99 ml of aqueous PBS. The resultant washings underwent five consecutive 2-fold dilutions with the same buffer, and 200-μl aliquots of the undiluted and the serially diluted samples each were added into a well of a six-well plate covered with a monolayer of Madin–Darby canine kidney (MDCK) cells. After a 1-h incubation, the solutions were removed, and 2 ml of plaque medium was placed in each well, followed by a 3-day incubation at 37°C in a humidified air. Finally, the cells were fixed with formaldehyde and stained after removal of the agar overlay, and the plaques were counted.
When this procedure was applied to uncoated slides, the concentration of the viable virus in the washings barely changed compared with the identically diluted droplets not exposed to the slide, 650 ± 150 versus 800 ± 150 pfu/ml, respectively. Thus, such a contact with a control glass slides results in no statistically significant decrease in the viral titer, i.e., influenza virus survives essentially unscathed this incubation at r.t. between two plain glass slides.
Next, we “painted” a glass slide with a solution of branched N,N-dodecyl methyl-PEI (1a) (synthesized by quaternizing a branched 750-kDa PEI as depicted in Fig. 1) in butanol and let the solvent evaporate. When the foregoing testing was used with this coated slide, not a single plaque was detected even by using the undiluted washings. To further quantify this apparent 100% virucidal activity, we carried out a separate experiment with a higher initial viral titer and also a lower dilution. Despite the greater sensitivity and assay range, still no plaques were observed, indicating that the exposure of the virus to the coated slides for 30 min lowers its titer at least some 10,000-fold (i.e., 4 logs).
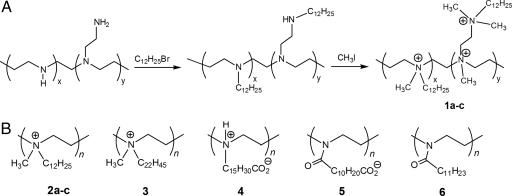
Fig. 1.
Hydrophobic PEI derivatives prepared in the study. (A) Schematic representation of the N-dodecylation and subsequent N-methylation of branched PEIs (see Materials and Methods for details). In the case of 1, the letters a, b, and c correspond to the N,N-dodecyl methyl-polycations prepared from 750-, 25-, and 2-kDa PEIs, respectively. (B) Chemical structures of linear PEI-based polymers synthesized in this work. In the case of 2, the letters a, b, and c correspond to the N,N-dodecyl methyl-polycations prepared from 217-, 21.7-, and 2.17-kDa PEIs, respectively. For 3 through 6, 217-kDa PEI was used.
When the PEI precursors of 1 with molecular masses of lower than 750 kDa, namely 25 kDa and 2 kDa, were used to make the hydrophobic polycationic coatings (1b and 1c, respectively), still very high but slightly incomplete virucidal efficiencies were observed, 98 ± 0.4% and 97 ± 0.2%, respectively. It is noteworthy that slides painted with these smaller N-alkylated PEI derivatives previously were found also to have incomplete bactericidal efficiencies (4). Thus, as in the case of bacteria, the polycations must be large enough, perhaps to allow their tentacles to penetrate and damage the viral lipid envelope.
For simple steric reasons, the chain-length constraints should be alleviated by replacing the branched polycations with their linear counterparts. To test this hypothesis, we examined the virucidal properties of three linear N,N-dodecyl methyl-PEIs: 2a, 2b, and 2c, synthesized from the 217-kDa, 21.7-kDa, and 2.17-kDa linear PEI precursors, respectively. Slides coated with all of these linear hydrophobic polycations indeed inactivated influenza virus with a 100% efficiency. Moreover, 2a (like 1a) was shown to reduce the viral titer by at least some 4 logs; it was used in most subsequent experiments.
To further investigate the effect of hydrophobicity of the polycation in virucidal action, we raised the latter by alkylating linear 217-kDa PEI with docosyl (C22) instead of dodecyl (C12) bromide (Fig. 1). A glass slide coated with resultant 3 was as completely lethal to influenza virus as that coated with 1a or 2a–c.
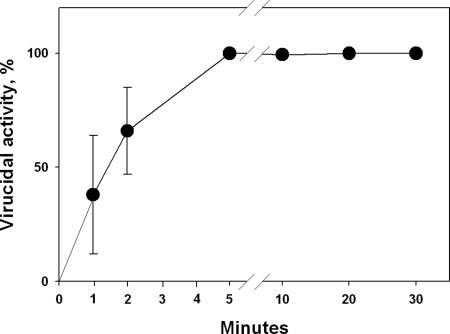
To determine how quick the virucidal action is in our experimental system, the time of exposure of influenza virus to a slide coated with 2a was varied from 1 min to 2 h. As seen in Fig. 2, a 100% virucidal efficiency already is achieved after as little as 5 min, albeit not after 1 or 2 min, possibly reflecting the time required for all viral particles present to reach the coated surface.
Fig. 2.
The time course of inactivation of influenza virus (WSN strain) by a glass slide painted with 2a at r.t. See Materials and Methods for details.
All of the coating paints examined thus far were polycationic. To ascertain the role of the charge, we also synthesized derivatives of linear 217-kDa PEI that were nominally zwitter-ionic (4), anionic (5), and electrostatically neutral (6) with otherwise roughly similar side chains as in 1 and 2 (Fig. 1). As shown in the second column of Table 1, zwitter-ionic 4, just as cationic 1a and 2a (and also 2b–c and 3, see above), is 100% virucidal after a 30-min exposure. In contrast, the anionic 5 is only partially virucidal, and the neutral 6 is not virucidal at all. The virucidal impotence of the last derivative presumably is owing to the lack of individual sticking-out tentacles, which, in the absence of significant charges, should strongly hydrophobically associate with each other. The fact that the polyanionic coating significantly inactivates influenza virus suggests that there are both positively and negatively charged sites attacked in the viral membrane; the latter ones appear predominant because 2a–c and even 4 are virucidally superior to 5.
Table 1.
Microbicidal activity of glass slides painted with 1a,2a, 4, 5, and 6
| PEI derivative | Virucidal activity after 30 min, % * | Bactericidal activity,% | |
|---|---|---|---|
| S. aureus | E. coli | ||
| 1a | 100 | 99±1 | 99±1 |
| 2a | 100 | 100 | 100 |
| 4 | 100 | 26±4 | 14±2 |
| 5 | 66±3 | 21±1 | 22±3 |
| 6 | 6±6 | 34±1 | 14±2 |
Glass slides used in these experiments were painted twice or more to attain the levels of activity indicated (presumably reflecting imperfections of our painting procedure)
*Virucidal activities were tested against the WSN strain of influenza virus.
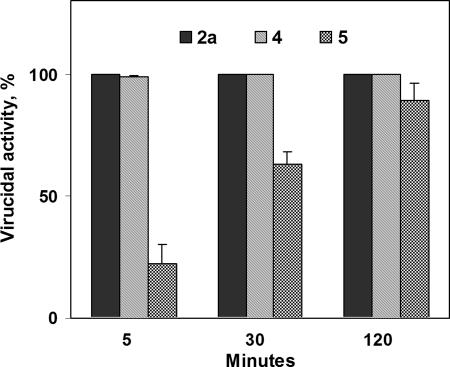
To gain further insights into these observations, we investigated the time course of the virucidal activity of slides coated with 4 and 5. Not only did zwitter-ionic 4, like cationic 2a, already inactivate the entirety of the exposed influenza virus after a 30-min incubation, but even after just 5 min the virucidal activity of 4 was as high as 98 ± 0.7% (Fig. 3). Interestingly, the virucidal activity of anionic 5 rose steadily with time (see the last bar at each time point in Fig. 3) to reach 89 ± 7% after a 2-h exposure. Thus, it seems that the differences in virucidal activities among the polymeric coatings are a matter of kinetics rather than ultimate degree, i.e., that the hydrophobic polycations merely inactivate the virus faster than other hydrophobic polyions.
Fig. 3.
The virucidal activity against influenza virus (WSN strain) of glass slides painted with 2a, 4, and 5 after different times of exposure at r.t. See Materials and Methods for details.
Our previous studies (4) revealed that the bactericidal activity of glass slides coated with 1a was not caused by the putative dissolved polycations leached into solution from the surface. To examine this possibility for the virucidal activities of the polyions observed herein, we conducted two sets of control experiments.
In one set, we conservatively approximated the leaching conditions into a 10-μl aqueous droplet squeezed between a coated and plain glass slides as follows. A coated slide was placed upside-down in a well of a six-well plate containing 2 ml of a PBS-buffered solution and incubated for 2 h (the longest exposure used in this study, e.g., see Fig. 3) with periodic agitation to facilitate mass transfer. Then, to 0.99 ml of this solution, 10 μl of an influenza virus solution was added, followed by a 30-min incubation at r.t., appropriate dilutions, and the standard viral assay. With glass slides coated with 1a, 1b, 2b, 3, 4, 5, and 6, the viral titers measured were statistically indistinguishable from that determined when the uncoated slide was subjected to the same procedure. In contrast, when the polycations 1c, 2a, and 2c were used as coatings, the viral titers obtained were 20% to 40% below that with the uncoated slide.
In the second set of controls, we deliberately inflated the possible extent of leaching of the polymers deposited onto the glass slide surface. To this end, 200 mg of a neat solid polymer was dispersed in 1 ml of an aqueous PBS by vortexing, followed by a 16-h incubation at r.t. and subsequent centrifugation to obtain a clear solution. To 390 μl of this solution, 10 μl of an influenza virus solution was added, incubated for 30 min at r.t., appropriately diluted, and titrated for the virus. Even in this exaggerated leaching test, with 1b, 2b, 3, 5, and 6 as coatings, the viral titer obtained was statistically indistinguishable from that observed when 390 μl of a fresh aqueous PBS was used instead of those putatively saturated with the polymers (with 1a, 1c, 2a, 2c, and 4, the viral titers were much lower).
On the basis of the results of the foregoing controls, we conclude that, at least for slides painted with 1a, 1b, 2b, 3, 4, and 5, the virucidal activity observed is attributable solely to the polyions remaining deposited on the slide's surface, i.e., the tentacles of these immobilized polyions inactivate the virus on contact. In contrast, in the case of 1c, 2a, and 2c coatings, contributions of the leached polycations to the virucidal activity of the painted slides cannot be ruled out.
We also compared bactericidal activities of the differently charged derivatives of linear 217-kDa PEI against two common human pathogenic bacteria: Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli. Slides painted with 1a and 2a killed both airborne bacteria on contact with a 100% efficiency or statistically indistinguishably from that level (see the last two columns of Table 1). In contrast, 4, 5, and 6 coatings were only marginally bactericidal (even though the first one is completely virucidal).
Finally, to ascertain the generality of the ability of 1 and 2 to inactivate influenza virus, we tested these coatings against A/Victoria/3/75 (H3N2), a strain distinct from the A/WSN/33 (H1N1) used thus far. Slides painted with 1a and 2a both exhibited 98 ± 0.5% virucidal activities after a 30-min exposure to the coated surfaces and 100% virucidal activities after 2 h. Therefore, although the Victoria strain appears more resistant than its WSN counterpart, given enough time, 1a and 2a coatings completely inactivate both of them.
In closing, we have demonstrated that certain hydrophobic polycations can be painted onto surfaces to render them not only highly bactericidal but also extremely virucidal against at least two distinct strains of influenza virus (and, hence, potentially other enveloped viruses). In terms of its virucidal and bactericidal efficiencies (as well as the lack of ambiguity in the virucidal mode of action), painting with 1a seems optimal. Given the simplicity of the coating procedure, it should be applicable to various common materials, thereby enabling them to interrupt the spread of both viral and bacterial infections.
Materials and Methods
Commercial Chemicals.
Branched PEIs (Mw values of 750, 25, and 2 kDa), poly(2-ethyl-2-oxazoline) (Mw values of 500, 50, and 5 kDa), organic solvents, and all low-molecular-weight chemicals were from Sigma-Aldrich Chemical Co. (St. Louis, MO) and were used without further purification.
Bacteria and Media.
The bacterial strains used were S. aureus (ATCC, 33807) and E. coli (E. coli genetic stock center, CGSC4401). Yeast-dextrose broth contained (per liter of deionized water): 10 g of peptone, 8 g of beef extract, 5 g of NaCl, 5 g of glucose, and 3 g of yeast extract (8). PBS contained 8.2 g of NaCl and 1.2 g of NaH2PO4·H2O per liter of deionized water. The pH of the PBS solution was adjusted to 7.0 with 1 M aqueous NaOH. Both solutions were autoclaved for 20 min before use.
Cells and Viruses.
MDCK cells were obtained from the ATCC (Manassas, VA). They were grown at 37°C in a humidified-air atmosphere (5% CO2/95% air) in Dulbecco's modified Eagle's (DME-Hepes) medium supplemented with 10% heat-inactivated FCS (GIRGO 614), 100 units/ml penicillin G, 100 μg/ml streptomycin, and 2 mM l-glutamine. Plaque-purified influenza A/WSN/33 (H1N1) strain was grown in a confluent monolayer of MDCK cells by infecting them with WSN at a multiplicity of infection (moi) of 0.001 at r.t. for 1 h. The virus then was incubated with a growth medium (E4GH) containing 0.3% BSA at 37°C in a humidified-air atmosphere (5% CO2/95% air) for 2 days. The supernatants were harvested from infected cultures, and the virus was stored at −80°C. Its titer was assayed by a plaque-forming assay in MDCK cells (9). Influenza A/Victoria/3/75 (H3N2) strain was obtained from Charles River Laboratories (Wilmington, MA) and used as such.
Syntheses.
Branched N,N-dodecyl methyl-PEIs (1a, 1b, and 1c).
Branched N,N-dodecyl methyl-PEIs (1a, 1b, and 1c) (prepared from branched PEIs with Mr of 750, 25, and 2 kDa, respectively) were synthesized (Fig. 1) and characterized as described in ref. 4.
Long, linear N,N-dodecyl methyl-PEI (2a).
Long, linear N,N-dodecyl methyl-PEI (2a) (from 217-kDa linear PEI) was prepared first by fully deacylating commercial poly(2-ethyl-2-oxazoline) as described in ref. 10. The resultant protonated PEI was dissolved in water and neutralized with excess of aqueous KOH to precipitate the polymer. The latter was isolated by filtration, washed with deionized water until the pH became neutral, and dried under vacuum. The yield was 1.25 g (97%). 1H NMR (CDCl3: δ = 2.72 (s, 4H, NCH2CH2N), 1.71 (s, 1H, NH) (NMR spectra here and henceforth were recorded with a Mercury 300-MHz NMR spectrometer; Varian, Palo Alto, CA). Next, 2.0 g (47 mmol of the monomeric units) of the PEI prepared was dissolved in 25 ml of tert-amyl alcohol, followed by the addition of 7.7 g (57 mmol) of K2CO3, and 33 ml (134 mmol) of 1-bromododecane, and the reaction mixture was stirred at 95°C for 96 h. After removing the solids by filtration under reduced pressure, 5.5 ml of iodomethane was added, followed by stirring at 60°C for 24 h in a sealed flask-condenser system. The resultant solution was added to excess of ethyl acetate; the precipitate formed was recovered by filtration under reduced pressure, washed with excess of ethyl acetate, and dried at r.t. under vacuum overnight. The yield was 7.0 g. 1H NMR for 2a (CDCl3): δ = 5.5–3.0 (NCH2CH2-(CH2)9CH3, NCH2CH2N, NCH3), 1.80 (NCH2CH2(CH2)9CH3), 1.6–1.0 (NCH2CH2(CH2)9CH3), 0.88 (NCH2CH2(CH2)9CH3).
Polycations 2b and 2c.
Polycations 2b and 2c from linear 21.7-kDa and 2.17-kDa PEIs, respectively, were synthesized as described in the preceding paragraph, except that after the N-methylation, the reaction mixture was poured into methanol to obtain the final product. 1H NMR (CDCl3) for 2b: δ = 5.5–3.0 (NCH2CH2-(CH2)9CH3, NCH2CH2N, NCH3), 1.83 (NCH2CH2(CH2)9CH3, 1.6–1.0 (NCH2CH2(CH2)9CH3), 0.88 (NCH2CH2(CH2)9CH3); for 2c: δ = 5.5–3.0 (NCH2CH2(CH2)9CH3, NCH2CH2N, NCH3), 1.83 (NCH2CH2(CH2)9CH3), 1.6–1.0 (NCH2CH2(CH2)9CH3), 0.88 (NCH2CH2(CH2)9CH3).
N,N-docosyl methyl-PEI (3).
N,N-docosyl methyl-PEI (3) was synthesized from linear 217-kDa PEI similarly to 2, except that 1-bromodocosane was used as the alkylating agent instead of 1-bromododecane. 1H NMR (CDCl3): δ = 5.5–3.0 (NCH2CH2-(CH2)19CH3, NCH2CH2N, NCH3), 1.85 (NCH2CH2(CH2)19-CH3), 1.6–1.0 (NCH2CH2(CH2)19CH3), 0.88 (NCH2CH2-(CH2)19CH3).
N-(15-carboxypentadecyl)-PEI (4) HCl salt
N-(15-carboxypentadecyl)-PEI (4) HCl salt was synthesized by dissolving 86 mg (2 mmol on the monomer basis) of linear 217-kDa PEI and 670 mg (2 mmol) of 16-bromohexadecanoic acid in 10 ml of tert-amyl alcohol, followed by addition of 0.61 g (4.4 mmol) of K2CO3, and the reaction mixture was stirred at 95°C for 96 h. After cooling to r.t., the reaction mixture was poured into 100 ml of acetone and filtered. The filter cake was suspended in 30 ml of CH2Cl2 and stirred with 30 ml of 1 M HCl for 2 h. The organic phase (containing undissolved solids) was separated and filtered, and the solid residue obtained was washed with CH2Cl2 and dried under vacuum. The product then was dissolved in 50 ml of CHCl3 and stirred with 40 ml of 1 M HCl for 3 h, followed by separation of the organic phase and solvent evaporation. The salt of 4 was obtained as a pale yellow solid. The yield was 0.39 g. 1H NMR (DMSO-d6): δ = 4.0–2.8 (NCH2CH 2N, NCH2(CH2)14CO2H), 2.17 (CH2CO2H), 1.8–1.4 (CH2CH2CO2H, NCH2CH2(CH2)13-CO2H), 1.4–1.1 (NCH2CH2(CH2)11CH2CH2CO2H).
N-(11-carboxyundecanoyl)-PEI (5)
Dodecanedioic acid (4.6 g, 20 mmol) was suspended in 100 ml of dry CH2Cl2, followed by addition of 2.16 g (20 mmol) of benzyl alcohol, catalytic amounts of 4-(dimethylamino)pyridine, and 4.12 g (20 mmol) of 1,3-dicyclohexylcarbodiimide. After stirring the mixture for 48 h at r.t., the solid was removed by filtration, and the filtrate was washed with 60 ml of 1 M HCl. The organic phase was dried with anhydrous Na2SO4, and the solvent was evaporated under reduced pressure. Silica gel column chromatography [2:3 (vol/vol) ethyl acetate/hexane as a mobile phase] resulted in 1.5 g (24% yield) of dodecanedioic acid mono-benzyl ester. 1H NMR spectrum (CDCl3) was consistent with the literature data (11). Then 1.28 g (5.2 mmol) of this product was dissolved in 10 ml of dry CH2Cl2, followed by the addition of 0.66 g (5.2 mmol) of oxalyl chloride and one drop of N,N-dimethylformamide. After stirring the reaction mixture at r.t. for 1 h, the solvent and excess of oxalyl chloride were removed under vacuum to give the corresponding carbonyl chloride used in the next step without further purification.
Linear 217-kDa PEI (86 mg, 2 mmol on the monomer basis) and N,N-diisopropylethylamine (0.52 g, 4 mmol) were dissolved in 10 ml of CH2Cl2, and the reaction mixture was chilled to 0°C by using an ice-water bath. To this solution, the carbonyl chloride made above in 10 ml of dry CH2Cl2 was added drop-wise, the ice-water bath was removed, and the reaction mixture was stirred at r.t. for 24 h. The reaction was quenched with 2 ml of methanol, and the solvent was evaporated. The residue obtained was washed with five 30-ml portions of methanol to remove soluble components and dried under vacuum to yield N-[(11-benzyloxycarbonyl)undecanoyl]-PEI as a white solid (0.6 g, 87%). 1H NMR (CDCl3): δ = 7.34 (m, 5H, C6H5), 5.10 (s, 2H, C6H5CH2), 3.43 (s, 4H, NCH2CH2N), 2.33 (m, 4H, CH2CO), 1.60 (m, 4H, CH2CH2CO), 1.26 (s, 12H, OCCH2CH2(CH2)6CH2- CH2CO). Finally, 60 mg (0.174 mmol on the monomer basis) of this compound was dissolved in 1 ml of THF and deprotected by adding 0.5 ml of 1 M NaOH and stirring for 24 h at r.t. The solution was neutralized with 0.2 ml of 2 M HCl, and the solvent was removed to give a solid residue, which was washed first with water to remove NaCl and then with CHCl3 to remove benzyl alcohol. The yield was 40 mg (90%). 1H NMR for 5 (CD3OD): δ = 3.43 (s, 4H, NCH2CH2N), 2.40–2.10 (m, 4H, CH2CO), 1.55 (m, 4H, CH2CH2CO), 1.26 (s, 12H, OCCH2CH2(CH2)6- CH2CH2CO).
N-(undecanoyl)-PEI (6).
N-(undecanoyl)-PEI (6) was synthesized by dissolving 1.08 g (25 mmol on the monomer basis) of 217-kDa linear PEI in 100 ml of chloroform, to which 6.46 g (50 mmol) of N,N-diisopropylethylamine was added. The reaction mixture was cooled to 0°C by using an ice-water bath, and 11.2 g (50 mmol) of lauroyl chloride was added drop-wise over 30 min. The ice-water bath then was removed, and the reaction mixture was stirred at r.t. for 24 h. Half of the solvent was removed under reduced pressure, and the remaining solution was poured into 350 ml of methanol. After standing overnight, the solid was separated by filtration and washed with five 50-ml portions of methanol. The yield was 4.87 g (86%). 1H NMR of 6 (CDCl3): δ = 3.43 (s, 4H, NCH2CH2N), 2.28 (d, 2H, COCH2), 1.59 (s, 2H, COCH2CH2), 1.4–1.2 (br s, 16H, (CH2)8CH3), 0.88 (t, 3H, CH3).
Preparation of Painted Slides.
Coating polymers were dissolved (50 mg/ml) in butanol for 1a–c and 2a–c, chloroform for 3, hot ethanol for 4, methanol-dichloromethane (1:1) for 5, and dichloromethane for 6. Commercial glass slides (VWR microscope slide; VWR International, Bridgeport, NJ), 2.5 × 7.5 cm for bactericidal tests and 2.5 × 2.5 cm for virucidal tests, were brush-coated with one of these solutions with a cotton swab, followed by air-drying.
Determination of Bactericidal Efficiency.
A 100-μl suspension of S. aureus or E. coli in 0.1 M PBS (≈1011 cells per ml) was added to 20 ml of the yeast-dextrose broth in a 50-ml sterile centrifuge tube, followed by shaking at 200 rpm and 37°C overnight (Innova 4200 Incubator Shaker; New Brunswick Scientific, Edison, NJ). The bacterial cells were harvested by centrifugation at 6,000 rpm for 10 min (Sorvall RC-5B; DuPont Instruments, Wilmington, DE), washed twice with PBS, and diluted to 5 × 106 cells per ml for S. aureus and to 3 × 107 cells per ml for E. coli. The bacterial suspensions in PBS were sprayed onto slides at a rate of ≈10 ml/min in a fume hood. After a 2-min r.t. drying under air, the resultant slide was placed in a Petri dish and immediately covered with a layer of solid growth agar (1.5% agar in the yeast-dextrose broth, autoclaved, poured into a Petri dish, and allowed to gel at r.t. overnight). The Petri dish was sealed and incubated at 37°C overnight, and the bacterial colonies grown on the slide surface were counted on a light box.
Plaque Assay.
Confluent MDCK cells in six-well cell-culture plates were washed twice with 5 ml of PBS and infected with 200 μl of a virus solution in PBS at r.t. for 1 h. The solution then was removed by aspiration, and the cells were overlaid with 2 ml of plaque medium (2 × F12 supplemented with 0.01% DEAE-dextran, 0.1% NaHCO3, 100 units/ml penicillin G, 100 μg/ml streptomycin, 4 μg/ml trypsin, and 0.6% agar (purified agar, L28; Oxoid Co., Hampshire, U.K.). After a 3-day incubation at 37°C in a humidified-air atmosphere (5% CO2/95% air), the cells were fixed with 1% aqueous formaldehyde for 1 h at r.t. The agar overlay was removed, and the cells were stained with 1% crystal violet in 20% (vol/vol) aqueous methanol for 2 min at r.t. After removing the excess of the dye by aspiration, the plaques were counted.
Virucidal Activity.
A glass slide coated with a polymer (or plain in a control experiment) was placed into a polystyrene Petri dish (6.0 × 1.5 cm), and then a 10-μl droplet of a virus solution [(1.6 ± 0.3) × 105 pfu/ml for the WSN strain and (1.2 ± 0.4) × 106 pfu/ml for the Victoria strain] in PBS was deposited in the center of the slide. Another, plain glass slide was put on top and pressed to spread the droplet between the slides. This sandwiched system was incubated at r.t. typically for 30 min. One edge of the top slide then was lifted, and virus-exposed sides of both slides were washed thoroughly with 1.99 ml of PBS. Finally, plaque assay was performed to determine the virucidal activity of the washing and of its 2-fold serial dilutions (five times).
Nonleaching Tests
No. 1. A glass slide coated with a polymer (or plain in a control experiment) was placed upside-down in a well of a six-well plate containing 2 ml of PBS and incubated for 2 h at r.t. with periodic agitation. Then 0.99 ml of the solution was withdrawn, mixed with 10 μl of a virus solution [(1.4 ± 0.1) × 107 pfu/ml of WSN], and incubated at r.t. for 30 min. After a 200-fold dilution and subsequent 2-fold serial dilutions (five times), the plaque assay was performed as described above.
No. 2.
Two hundred milligrams of a neat solid polymer was dispersed in 1 ml of PBS by vortexing for 5 min and then incubated at r.t. for 16 h, followed by centrifugation at 9,000 rpm (Galaxy 7; VWR International) for 30 min thrice and then passing through a glass wool to obtain a clear solution. Then, 0.39 ml of this solution was mixed with 10 μl of a virus solution [(8.7 ± 1.4) × 106 pfu/ml of WSN] and incubated at r.t. for 30 min. After a 300-fold dilution and subsequent 2-fold serial dilutions (five times), the plaque assay was performed as described above.
Acknowledgments
We thank members of J.C.'s group for help with handling influenza virus. Special thanks to Dr. Ching-Hung Shen for helpful discussions. L.A.d.C. is grateful to Fundacion Ramon Areces of Spain for a postdoctoral fellowship. This work was supported financially by the U.S. Army through the Institute for Soldier Nanotechnologies at Massachusetts Institute of Technology under Contract DAAD-19-02-D-0002 with the Army Research Office (to A.M.K.) and National Institutes of Health Grants AI56267 and AI69208 (to J.C.).
Abbreviations
- PEI
polyethylenimine
- r.t.
room temperature
- MDCK
Madin–Darby canine kidney.
Footnotes
The authors declare no conflict of interest.
References
- 1.Wood JM, Robertson JS. Nat Rev Microbiol. 2004;2:842–847. doi: 10.1038/nrmicro979. [DOI] [PubMed] [Google Scholar]
- 2.Wright PF, Webster RG. Fields Virology. 4th Ed. Lippincott, Philadelphia, PA: Knipe DM, Howley PM; 2001. pp. 1533–1579. [Google Scholar]
- 3.Lewis K, Klibanov AM. Trends Biotechnol. 2005;23:343–348. doi: 10.1016/j.tibtech.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Park D, Wang J, Klibanov AM. Biotechnol Progr. 2006;22:584–589. doi: 10.1021/bp0503383. [DOI] [PubMed] [Google Scholar]
- 5.Choppin PW, Compans RW. The Influenza Viruses and Influenza. Academic, New York: Kilbourne ED; 1975. pp. 15–51. [Google Scholar]
- 6.Steihauer DA, Wiley DC, Skehel JJ. Principles of Medical Biology. 9B. JAI Press, Greenwich, CT: Bittar EE, Bittar N; 1997. pp. 329–351. [Google Scholar]
- 7.Lüscher-Mattli M. Arch Virol. 2000;145:2233–2248. doi: 10.1007/s007050070017. [DOI] [PubMed] [Google Scholar]
- 8.Cunliffe D, Smart CA, Alexander C, Vulfson EN. Appl Environ Microbiol. 1999;65:4995–5002. doi: 10.1128/aem.65.11.4995-5002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge Q, McManus MT, Nguyen T, Shen C-H, Sharp PA, Eisen HN, Chen J. Proc Natl Acad Sci USA. 2003;100:2718–2723. doi: 10.1073/pnas.0437841100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas M, Lu JJ, Ge Q, Zhang C, Chen J, Klibanov AM. Proc Natl Acad Sci USA. 2005;102:5679–5684. doi: 10.1073/pnas.0502067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prata CAH, Zhao Y, Barthelemy P, Li Y, Luo D, McIntosh TJ, Lee SJ, Grinstaff MW. J Am Chem Soc. 2004;126:12196–12197. doi: 10.1021/ja0474906. [DOI] [PubMed] [Google Scholar]