Abstract
Lentivirus-derived vectors are among the most promising viral vectors for gene therapy currently available, but their use in clinical practice is limited by the associated risk of insertional mutagenesis. We have overcome this problem by developing a nonintegrative lentiviral vector derived from HIV type 1 with a class 1 integrase (IN) mutation (replacement of the 262RRK motif by AAH). We generated and characterized HIV type 1 vectors carrying this deficient enzyme and expressing the GFP or neomycin phosphotransferase transgene (NEO) under control of the immediate early promoter of human CMV. These mutant vectors efficiently transduced dividing cell lines and nondividing neural primary cultures in vitro. After transduction, transient GFP fluorescence was observed in dividing cells, whereas long-term GFP fluorescence was observed in nondividing cells, consistent with the viral genome remaining episomal. Moreover, G418 selection of cells transduced with vectors expressing the NEO gene showed that residual integration activity was lower than that of the intact IN by a factor of 500–1,250. These nonintegrative vectors were also efficient in vivo, allowing GFP expression in mouse brain cells after the stereotactic injection of IN-deficient vector particles. Thus, we have developed a generation of lentiviral vectors with a nonintegrative phenotype of great potential value for secure viral gene transfer in clinical applications.
Keywords: HIV-1-derived vector, integrase deficient, stable transgene expression
Some of the viral vectors used for gene transfer integrate into the host cell chromatin, whereas others remain episomal. The most commonly used nonintegrative vectors are derived from adenoviruses (AdV), herpes virus (HSV) or adeno-associated virus (AAV). The integrative vectors in current use are derived from type C retroviruses, such as murine leukemia virus (MLV), or lentiviruses such as HIV.
Vectors derived from AdV and HSV have already proved to be efficient in many experimental models, but technical limitations make it extremely difficult to obtain vector stocks devoid of replication-competent particles. In general, the production systems for these vectors require helper viruses or genomes that cannot be totally eliminated from stocks, accounting for 0.01–1% of all of the viral particles present (1, 2). Stocks may therefore be strongly immunogenic, thereby hindering their general use in clinical applications.
Lentivirus-derived vectors appear to be among the most promising alternatives: they can transport up to 8 kb of DNA of interest, they can be pseudotyped so that they present the chosen tropism (broad or specific), they transduce both dividing and nondividing cells, they are produced easily without the need for helper particles, and they are only weakly immunogenic (3, 4). However, like all integrative systems, lentivirus-derived vectors present a risk of insertional mutagenesis, which also limits their clinical application. This risk was recently highlighted in an X-linked severe combined immunodeficiency ex vivo gene therapy trial based on a murine leukemia virus (MLV)-derived vector (5). Several studies have since pointed out that integration is not totally random for MLV (6) and lentiviral vectors (6, 7), which preferentially integrate into or close to transcriptionally active genome sequences. Moreover, macrophage cancers have been associated with HIV integration in some AIDS patients (8). Actually, AIDS has also been correlated with various types of cancer (9). Recently, Themis et al. (10) have reported the oncogenic potential of equine infectious anemia virus (EIAV) lentiviral vectors after the transduction of fetal and neonatal tissues. These findings clearly demonstrate the need to find means to prevent deleterious effects of integration of lentiviral vectors.
Two strategies for reducing the risk of insertional mutagenesis could be considered: the integration process could be rendered site-specific, or novel nonintegrative vectors could be developed. The development of new nonintegrative vectors, for targeting nondividing cells or for transient transgene expression in cycling cells, is simpler and could take advantage of an interesting feature of lentivirus biology. These viruses are normally present in various forms in the nucleus of infected cells: as integrated proviruses and as nonintegrated linear molecules and circular molecules with one or two long terminal repeat (LTR) sequence (11). Class 1 HIV integrase (IN) mutations prevent the IN reaction without disturbing other Gag-Pol functions, thereby favoring the circularization of the genome by host enzymes (12). These circular genomes have been shown to be functional templates for transcription by host cell machinery (refs. 13–; see ref. 17 for a review).
In this article, we investigate the efficiency of nonintegrative HIV vectors for expressing transgenes in vitro and in vivo. We have developed a HIV type 1 (HIV-1)-derived vector carrying a class 1 IN mutation (18), and we have shown that this vector drives efficient transgene expression in dividing and nondividing cells in vitro. These vectors are episomal and retain only a negligible integration capacity. We have estimated that the mutant vectors integrated 500–1,250 times less frequently than the WT vectors. Furthermore, in contrast to previous reports (19–23), we have shown that these vectors efficiently drove transgene expression in vivo after intrastriatal injection into mice. Thus, the generation of lentiviral vectors described has great potential to overcome insertional mutagenesis, the main limitation to the clinical application of gene therapy with retroviral vectors.
Results
Design of HIV1-Derived Vectors with a Class I IN Mutation.
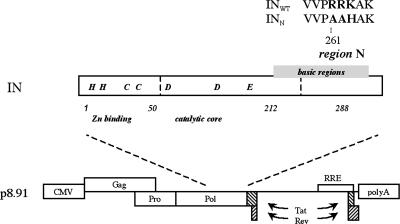
We used a class 1 IN mutation for the development of a nonintegrative lentiviral vector. This mutation does not prevent reverse transcription, nuclear import of the preintegration complex, or circularization of the viral genome in the cell nucleus (18). The mutation consists of replacement by AAH of the 262RRK motif, which is part of the N region of the HIV IN. For the production of lentiviral vectors containing this mutant IN, the mutant allele (INN) was introduced into the encapsidation plasmid in place of the WT allele (INWT). Two types of vector were produced: one containing an expression cassette for the GFP (INWT GFP and INN GFP) and the other containing an expression cassette of for the neomycin phosphotransferase (NEO) gene (INWT NEO and INN NEO) (Fig. 1).
Fig. 1.
Schematic representation of the intact (WT) and mutant (N) INs and of the encapsidation plasmids. (Upper) Schematic representation of the HIV-1 IN domains and the N region containing the modified amino acids (bold). Numbers correspond to amino acid position. (Lower) Schematic representation of the encapsidation plasmid encoding the intact IN (p8.91 INWT) or the N mutant IN (p8.91 INN).
Class 1 IN Mutation Does Not Prevent Transgene Expression.
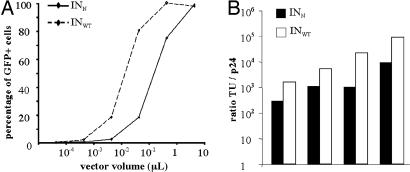
We evaluated the capacity of the mutant HIV-vector to transduce cells by titrating it on 293T cells with increasing doses of INWT GFP or INN GFP. After 72 h, the transduction rate was shown to be dose-dependent. Up to 90% of transduction efficiency is obtained with the highest volume of concentrated stock (5 μl) of both the control and the mutant vectors (Fig. 2A). This finding indicates that the selected IN mutation does not affect particle production or transduction process (encapsidation, reverse transcription, and nuclear translocation) or prevent transgene expression from vectors.
Fig. 2.
Compared transduction efficiency of mutant and WT vectors. (A) GFP expression in 293T cells transduced with the mutant (INN GFP) or the WT (INWT GFP) vector. The 293T cells were transduced with serial dilutions of either mutant vector (INN GFP) or WT vector (INWT GFP). At 72 h after transduction, cells were fixed in PFA, and GFP expression was analyzed by FACS. (B) Ratio of titers expressed in TU/ng p24 of INWT GFP (black) and INN GFP (white) of four separate stocks of vectors produced simultaneously.
With a lower dose (0.5 μl) of the mutant vector, we observed 29% of GFP-positive cells, whereas the same volume of the control vector transduced 87% of cells. These results suggest that the mutant vector expresses transgenes less efficiently than the control vector. This hypothesis was confirmed by comparing the p24 and transducing units (TU) titers of various stocks of mutant and WT vectors produced simultaneously (Fig. 2B). In simultaneous INWT and INN vector productions, the TU/p24 ratio was higher for WT vectors than for mutant vectors by a factor of 4.6–20.4 (mean factor: 9.9 ± 7.3), showing that the WT vector allows a more efficient transgene expression than does the mutant vector in 293T cells.
We then ascertained that the observed fluorescence resulted from de novo transcription rather than from a pseudotransduction mechanism. The 293T cells were transduced with various doses of mutant vector in the presence of 3′-azido-3′-deoxythymidine (AZT), a reverse-transcriptase inhibitor. AZT treatment resulted in a drastic reduction of GFP expression (data not shown). Thus, the GFP fluorescence observed with the mutant vector was clearly due to genuine transcription from newly formed vector genomes.
Transgene Expression from the Mutant Vector Is Transient in Dividing Cells.
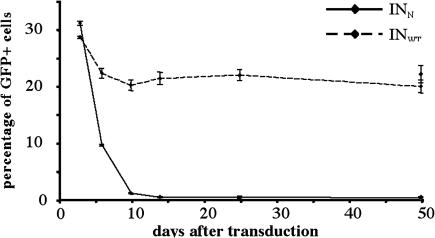
We then verified that GFP expression from INN vectors did not result from integrated provirus. If this were the case, then GFP fluorescence levels would remain stable through successive passages. Cells initially transduced with equal TU amounts of the INWT GFP and INN GFP vectors were cultured and passaged for up to 50 days. The cells were regularly analyzed by FACS to evaluate the stability of GFP expression through divisions. The percentage of GFP-positive cells was stable after transduction with the WT integrative vector: 28.42 ± 0.21% after 3 days and 19.74 ± 1.11% after 50 days of growth (Fig. 3). In contrast, GFP fluorescence rapidly decreased in cells transduced with the mutant INN GFP vector: from 30.91 ± 0.35% after 3 days, to 0.9 ± 0.11% after 10 days, and 0.26 ± 0.10% after 50 days of growth. This progressive loss of transgene expression in dividing cells observed with the mutant vector is consistent with a nonintegrative phenotype.
Fig. 3.
GFP expression in time in dividing cells. The 293T cells were transduced with equivalent TU amounts of the INN GFP (32 ng of p24 per μl) or the INWT GFP (8 ng of p24 per μl) vector. Cells were cultured and analyzed by FACS at various times after transduction to determine the percentage of GFP-positive cells. Cells were transduced in three replicate wells for each condition, and results are expressed as the mean of the three measurements ±SD. At day 47 after transduction, cells were passaged into two wells and left untreated or treated with 5 mM sodium butyrate for 24 h before the last harvesting, 50 days after transduction.
To confirm that this disappearance of transgene expression results from the dilution of the vector genome through cell division rather than from promoter silencing, we treated the cells with sodium butyrate, an inhibitor of histone deacetylases commonly used to reverse transgene silencing. This treatment, performed 50 days after transduction, did not significantly affect GFP expression level in INN-transduced cells, because 0.17 ± 0.05% of untreated cells expressed GFP, whereas 0.26 ± 0.10% of the treated cells were GFP positive (Fig. 3). Promoter silencing is, therefore, unlikely to be responsible for the disappearance of transgene expression.
These results strongly suggest that the IN mutation used in this study prevented integration of the vector genome and allowed the formation of episomes and their efficient transcription.
The Residual Integration Activity of INN Is Lower than That of INWT by a Factor of 500–1,250.
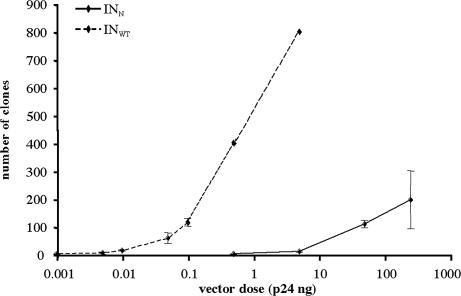
We precisely evaluated the residual integration activity of the mutant IN by means of an approach based on the expression of a NEO gene, conferring G418 resistance on cells. Proliferative HeLa cells were transduced with various amounts (normalized for p24) of mutant or control vectors expressing the NEO gene and were grown in the presence of G418. Thus, the number of clones formed after several days of selection reflects the frequency of integration events of the proviral vector. Because we could not determine the TU titer of the NEO vectors, stocks were evaluated by RNA dot blot analysis showing that equivalent amounts of p24 corresponded to equivalent numbers of RNA genomes (data not shown), as described for simultaneously produced stocks of lentiviral vectors (24). Transduced cells were cultured for 3 weeks in the presence of G418, and the number of clones obtained for each dose was determined (Fig. 4). The minimal dose required for clones to grow with the mutant vector was 0.5 ng of p24 (1 ± 1 clone), whereas the minimal dose required to observe clones with the WT vector was 0.001 ng of p24 (0.7 ± 0.6 clone). With 50 ng of p24 of the mutant vector, we observed 109 ± 12.2 clones. This dose was equivalent to 0.1 ng of p24 of the control vector (115 ± 13.5 clones). The mutant enzyme is, therefore, at least 500 times less efficient at integration than is the WT enzyme. This difference in efficiency was even greater for the higher doses tested, because we could estimate, by extrapolation of the dose–response curve, that the highest dose tested for the mutant vector (196 ± 104.5 clones with 250 ng of p24) was equivalent to 0.2 ng of p24 of the WT vector. At this dose, the mutant vector integrates 1,250 times less frequently than does the WT vector.
Fig. 4.
Evaluation of residual IN activity of the mutant enzyme. HeLa cells were transduced with increasing amounts of mutant vector INN NEO or WT vector INWT NEO and plated in T25 flasks. Cells were grown in the presence of G418 for 3 weeks, with the medium replaced every 3 days. Cells were transduced in three replicate wells for each condition, and results are expressed as the mean of the three measurements ±SD. For the highest doses tested with the integrative vector (0.5 and 5 ng of p24), the number of clones per flask was ≈400 and 800, respectively. Precise evaluation was not possible because of the confluence of the clones.
Episomal Genomes Allow Stable Transgene Expression in Nondividing Cells.
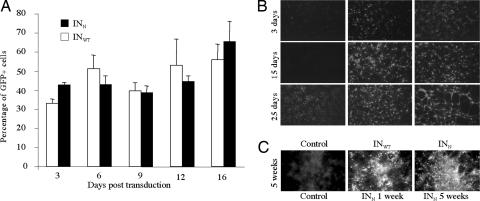
We investigated the stability of extrachromosomal forms of HIV vector genomes and their ability to drive long-term transgene expression by studying GFP expression in nondividing primary neurons and astrocytes after transduction with equivalent TU amounts of mutant or control vectors. GFP expression was observed for at least 16 days after transduction in neurons, with no evident decrease. Moreover, no significant difference was observed between the percentages of GFP-expressing cells after transduction with the mutant INN or the control INWT vectors (Fig. 5A) at any time point analyzed (two-way repeated measures ANOVA, P = 0.9321), indicating that transgene expression levels were equivalent for control and mutant vectors at the doses used. GFP expression was observed in such cultures for up to 25 days (Fig. 5B). Similar results were obtained for postmitotic astrocytes in which GFP expression was shown to be stable for 5 weeks (Fig. 5C). We can, therefore, conclude that the nonintegrative lentiviral vectors developed are stable in vitro in nondividing cells and allow sustained transgene expression for at least 5 weeks.
Fig. 5.
GFP expression in nondividing primary neural cells in vitro. GFP expression in cortical primary neurons and astrocytes was evaluated after transduction with equal TU amounts of mutant vector INN GFP or control vector INWT GFP. Primary neurons were transduced and fixed with PFA at various times after transduction. GFP expression was visualized by immunocytochemistry. (A) The percentage of GFP-expressing cells in neurons was evaluated up to 16 days. Three replicate wells were analyzed for each condition, and results are expressed as the mean of the three measurements ±SD. (B and C) Immunocytofluorescence analyses of GFP expression up to 25 days in neurons (B) and 5 weeks in astrocytes (C). Control wells (untransduced) at 25 days for neurons and 5 weeks for astrocytes show high background because of cell death. The endogenous fluorescence is easily differentiated from the high GFP fluorescence by looking at the cells through the red filter highlighting dying cells but not true GFP expression.
IN-Mutant Lentiviral Vectors Are Able to Transduce Neural Cells in Vivo.
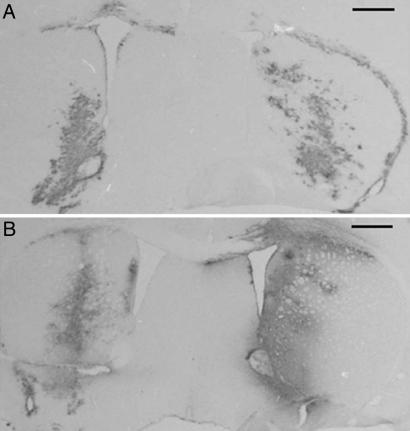
Control INWT GFP vector (10 × 104 TU) and mutant INN GFP vector (5 × 104 TU) were injected into the mouse brain, and GFP expression was analyzed by immunohistochemistry (Fig. 6). One week after injection (n = 2) transgene expression with the mutant vector was significant and could be compared with expression from the control vector, according to the difference of doses. GFP expression was observed for 1 month after injection of the nonintegrative vector (n = 2). Levels of expression at this time appeared to be equivalent to those observed 1 week after injection, with both the mutant INN or control and the control INWT vectors. Thus, the nonintegrative vector efficiently and stably directed transgene expression in the brain.
Fig. 6.
GFP expression in vivo. Immunocytochemical analysis of GFP expression in mouse brain 1 week (A) and 4 weeks (B) after stereotactical injection of 5 × 104 TU of the mutant INN GFP vector (left) or 10 × 104 TU of the control INWT GFP vector (right). (Scale bars, 0.5 mm.)
Discussion
Safety concerns are the major obstacle to the use of viral vectors for gene therapy in clinical practice. Lentiviral vectors present a promising alternative to the vectors currently used, because they transduce many types of cells efficiently and can be produced in the absence of replication-competent particles. However, their potential to induce insertional mutagenesis continues to limit their clinical use.
In this study, we show that lentiviral vectors containing a class I IN mutation integrate only very poorly into the target cell chromatin and, yet, can transduce cells both in vitro and in vivo.
Gene Expression from Circular Forms of HIV.
Although efficient viral replication seems to be associated with integration events, HIV transcription was shown to be supported by circular molecules. Expression of viral proteins, e.g., tat, nef, or reverse transcriptase as well as indicator genes linked to the LTR promoter, have been reported in many assays using virus particles containing class 1 nonpleiotropic IN mutations (13–16).
In contrast, lentivirus-derived vectors containing a class I IN mutation have been described in several studies that reported an absence of transgene expression. The first such studies (19–21) described an HIV-1-derived vector containing a substitution in the catalytic domain of the IN (D64V mutant IN) that transduced only rare cells in vitro and in vivo. These studies concluded that lentiviral-vector genome integration was necessary for transgene expression. Two recent articles described feline immunodeficiency virus-derived vectors containing a D66V substitution in the IN sequence (22, 23), the D66 residue being equivalent to the D64 residue of HIV IN. Surprisingly, these vectors allowed transgene expression only in nondividing cells in vitro (aphidicolin cell cycle-arrested cell lines or primary postmitotic neurons). Very little or no transgene expression was observed in dividing cells in vitro or in vivo in the rat eye, after subretinal injection. The authors thus concluded that transduction efficiency with feline immunodeficiency virus vectors containing a class 1 IN mutant is cell-cycle dependent.
Although class 1 IN mutant lentivirus-derived vectors were used in the above studies, none of the results obtained are consistent with what we observed with HIV vectors or what was observed with HIV viruses containing class 1 IN mutant (13–16). These discrepancies may be accounted for by the absence of the central flap (cPPT-CTS) in the HIV vectors used. This sequence is now known to be critical for nuclear import of the preintegration complex in lentiviruses and lentivirus-derived vectors (24–26). This element is present in the feline immunodeficiency virus vectors, but its implication in nuclear translocation has not yet been precisely described. However, it may not be as efficient as in HIV counterparts, because the cPPT sequence is not a repetition of the PPT (27). Indeed, both PPT and cPPT sequences have to be similar for efficient HIV nuclear transport (24). HIV integrative vectors lacking this element express transgenes less efficiently because most of the reverse-transcribed genomes are not imported into the nucleus of the transduced cell. Thus, expression from episomal genomes, which is weaker than from integrated proviruses, may not have been detected in these experiments.
We tested this hypothesis by producing INN lentiviral vectors devoid of the central flap and comparing them with INN vectors containing the central flap. Although we observed that the absence of the central flap resulted in a decrease in transgene expression by a factor of 2–4 in vitro, GFP expression remained significant in dividing cells. However, it should be noted that the GFP-expression cassette in this experiment was driven by a strong promoter (CMV) and contained the WPRE element that has been shown to increase transgene expression by a factor of ≈5 (28, 29).
We thus conclude that the discrepancy between our results, showing efficient expression from the HIV-1 derived INN vector, regardless of the cell cycle, and those of studies from other groups can be explained by the optimization of our vectors. This optimization involved (i) incorporation of the flap sequence, (ii) a deleted U3 region in the LTR, shown to improve transgene expression (30, 31), and/or (iii) a very strong expression cassette through combination of the CMV promoter and the WPRE sequence (28, 29). In other words, the absence of one or several of these elements in the vectors used in previous studies may have resulted in a transgene expression under the threshold of detection.
After this manuscript was submitted, a study describing nonintegrative lentiviral vectors was published by Yanez-Munoz et al. (32). In contrast to the 262RRK mutated IN vector, this group used a D64V mutated IN. In line with the current study, the D64V vectors exhibit an episomal phenotype, a negligible residual IN-mediated recombination, and efficient in vivo transduction.
The transduction efficiency of the D64V vector was reported by Yanez-Munoz et al. (32) to be equivalent to the WT integrative vector, whereas the 262RRK mutant vectors described in this study exhibited lower transduction efficiency. Although further comparison studies are needed, this discrepancy may be explained by a pleiotropic effect of the 262RRK IN mutation. Indeed, in addition to catalyzing integration reactions, the IN enzyme is involved in various steps of the virus life cycle. Thus, the IN mutation we used may impair transduction efficiency at any step upstream of the recombination (e.g., virion maturation, uncoating, or nuclear import).
Residual Integrase Activity.
We have shown that INN vectors retain a very weak integration capacity. Using a NEO-expression cassette, we showed that this activity was weaker than that of similar vectors containing a WT IN by a factor up to ≈1,000. This method has the advantage of taking into account integration events that can arise only after extensive cell divisions. INN vector integration may result from both illegitimate recombination of the linear and circular forms of the vector genomes and/or residual catalytic activity of the mutant IN. In all retrovirus integration processes, IN-mediated catalysis is characterized by the deletion of two base pairs at the extremities of the LTRs. Thus, if integration is mediated by IN activity, the sequence at the end of the LTR is CA rather than CAGT. We analyzed the extremities of integrated INN vectors (selected by G418) by the linear amplification-mediated PCR technique [adapted from Schmidt et al. (33)], and showed that four of five clones had a CA sequence at the extremity, demonstrating that integration resulted from residual activity of the mutated IN (data not shown). However, the possibility that integration also occurs through illegitimate recombination of the circular forms of the vector is not excluded. Moreover, this finding suggests that the residual integration frequency observed could be further reduced, e.g., by alteration of att sequences of the LTR (34).
Potential Use of Episomal Lentiviral Vectors.
These newly developed INN vectors represent a step forward in the clinical application of lentiviral vectors for gene transfer. Because these vectors retain very weak, almost negligible, integration activity, the risk of insertional mutagenesis is almost totally abolished. Moreover, the combined use of a class I mutant IN and deletion of the U3 region (SIN vectors) decreases the risk of activation or deregulation of nearby genes if integration occurs. This may, therefore, ensure safe gene transfer. These vectors can thus be used both for stable transgene expression in nondividing cells and for transient expression in proliferating cells. We recently observed that stable expression over a 6-month period could be obtained in the dog eye after the subretinal injection of an IN mutant vector encoding GFP and could be monitored by angioretinography (S. Bonnel, S.P., C. Vetu, M. Abitbol, J.M., and C. Sarkis, unpublished data). Stable transgene expression in the retina by using a D64V nonintegrative lentiviral vector was also reported by Yanez-Munoz et al. (32), confirming the ability of episomal forms of HIV genomes to stably drive transgene expression.
Expression from these vectors could be increased, making them more suitable for clinical gene transfer, by incorporating regulatory cis sequences. A few studies have demonstrated the possibility of enhancing transgene expression from an episome by the incorporation of an insulator into the vector genome (35–37). In combination with other sequences, such as the APP 5′ UTR or TH 3′ UTR, as described for integrative lentiviral vectors (29), these modifications may result in very strong transgene expression from INN vectors, if required.
Conclusion
We have developed an IN-mutant lentivirus-derived vector that is nonintegrative and allows the formation of circular episomal genomes in the nucleus of transduced cells. We have shown that these nonintegrated forms are efficiently transcribed by the cellular machinery, consistent with previous reports based on virus observation and in contrast to vector-based studies. However, transgene expression with the mutant vector did not appear to be as efficient as with its WT counterparts. This transgene expression was transient in dividing cells and stable in nondividing cells. We have confirmed an absence of integration and, more importantly, we have demonstrated that these vectors drove transgene expression in the mouse striatum in vivo as efficiently as an integrative vector. This newly developed nonintegrative lentiviral vector retains all of the interesting features of lentiviral vectors but does not present the most important drawback to the clinical application of these vectors: the risk of insertional mutagenesis. Moreover, the IN mutation also increases the safety of this vector by reducing the risk of formation of replication-competent recombinant particles. Because the viruses containing such a mutation are defective for replication (18, 38), recombinant particles would also be expected to be replication-defective. This generation of lentivirus-derived vectors thus overcomes a major hurdle to gene therapy and offers many application perspectives. It could be used in gene therapy strategies requiring transient transgene expression in dividing cells and in long-term treatments targeting nondividing cells, such as all treatments of CNS diseases.
Methods
Plasmids.
Two plasmids encoding an epitope-flagged IN, described by Petit et al. (18), were used to generate encapsidation plasmids: the plasmid BRU-INWT, in which the IN is functional, and the plasmid BRU-INN, in which the 262RRK motif of the N region of the IN coding sequence is replaced by AAH, the equivalent motif of the Moloney murine leukemia virus IN. The BstZ17I-SalI fragments of these two plasmids were inserted into the transcomplementation plasmid p8.91 described in ref. 39, replacing the original IN sequence and generating two plasmids, p8.91 INWT and p8.91 INN, encoding INs with and without the N substitution, respectively.
The plasmid pTrip-CMV-GFP-WPRE is derived from the plasmid pΔ500 Trip-CMVmin-WPRE described by Vogel et al. (40). The CMV-GFP fragment was amplified by PCR from the pEGFP N1 (Clontech, Mountain View, CA) with primers modified to add a MluI restriction site in 5′ of the CMV (5′-GGGACGCGTATTAATAGTAATCAATTACGG-3′) and a SpeI restriction site in 3′ of the GFP (5′-CCCACTAGTTATGGCTGATTATGATCTAGA-3′) (restriction sites are italicized). The PCR product was subcloned into the plasmid pΔ500-Trip-CMVmin-WPRE in place of the CMVmin fragment by using MluI and SpeI restriction enzymes to generate the plasmid pTrip-CMV-GFP-WPRE.
The plasmid pTrip-CMV-NEO-WPRE is derived from the plasmid pTrip-CMV-GFP-WPRE. The NEO coding sequence was amplified by PCR from the pCDNA 3.0 (Invitrogen, Carlsbad, CA) with primers modified to add a AflII restriction site in 5′ (5′-GAGGACTTAAGCGCATGATTGAACAAGATGGATTGCAC-3′) and a EcoRI restriction site in 3′ (5′-CCCAAGAATTCCGCTCAGAAGAACTCGTCAAGG-3′). This fragment was subcloned into the plasmid pIRES-EGFP (Clontech) in 3′ of the CMV by using the AflII and EcoRI restriction enzymes. The expression cassette CMV-NEO-IRES-GFP was then subcloned into the pTrip-CMV-GFP-WPRE in place of the GFP coding sequence by using the SnaBI and BsrGI restriction enzymes, generating the pTrip-CMV-NEO-IRES-GFP-WPRE. Finally, because the GFP appeared to be expressed very weakly, the IRES-GFP fragment was removed by using the SpeI restriction sites flanking this region to obtain the plasmid pTrip-CMV-NEO-WPRE.
Cell Lines and Primary Cultures.
Human epithelial HeLa and 293T cells were grown in Dulbecco's modified medium supplemented with antibiotics and 10% FCS.
Primary cultures of cortical cells were set up from embryonic day 17 Sprague–Dawley rats (Janvier, Le Genest-St-Isle, France). Female rats were killed by CO2 asphyxiation, and embryos were removed from the uterine horns. Cortical tissue was dissected and rinsed in PBS supplemented with 0.6% glucose. For astrocyte culture, the PBS–glucose solution was removed and replaced with pyruvate-free DMEM containing 4.5 g/liter glucose and 0.58 g/liter l-glutamine supplemented with 10% FBS. The cells were plated and cultured in a humidified incubator at 37°C in a 10% CO2/90% air atmosphere. For neuron culture, the PBS–glucose solution was replaced with pyruvate-free DMEM containing 4.5 g/liter glucose and 0.58 g/liter l-glutamine supplemented with 100 g/ml transferrin, 25 g/ml insulin, 10 g/ml putrescin, 5 ng/ml sodium selenite, and 6.3 ng/ml progesterone (all from Sigma, St. Louis, MO). Tissue was mechanically dissociated in the serum-free culture medium, and cells were counted by trypan blue exclusion, plated at a density of 100,000 cells per cm2 in polyornithine-coated cell-culture dishes, and cultured in a humidified incubator at 37°C, in a 5% CO2/95% air atmosphere.
Lentiviral Vectors.
Lentiviral vectors were generated by the transient transfection of 293T cells by using the calcium phosphate precipitation method. For all experiments, the INN vector and the corresponding control INWT vector were produced simultaneously. Cells were cotransfected with the vector plasmid (pTrip-CMV-GFP, pTrip-CMV-GFP-WPRE, or pTrip-CMV-NEO-WPRE), the transcomplementation plasmid (p8.91 INWT or p8.91 INN), and the plasmid encoding the vesicular stomatitis virus envelope glycoprotein (pMD-G). The medium was replaced 12 h after transfection and collected 36 h later. Supernatants were either used directly to transduce cell lines or concentrated. Supernatants were treated with DNase and passed through a filter with 0.45-μm pores. Viral particles were then concentrated by ultracentrifugation (90 min, 22,000 rpm, rotor SW28) and resuspended in 0.1 M PBS.
The HIV p24 Gag antigen was quantified for each stock by ELISA (HIV-1 P24 antigen assay; Beckman Coulter, Fullerton, CA) for titration.
In addition, GFP-expressing vectors were titered by transducing 80,000 293T cells in 24-well plates with serial dilution (5 μl, 5 × 10−1 μl, 5 × 10−2 μl, 5 × 10−3 μl, and 5 × 10−4 μl). Cells were harvested by centrifugation and resuspended in a fixative solution of 1% PFA 72 h after transduction. The number of GFP-positive cells was determined in a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
For NEO stocks, RNA genomes were evaluated by dot blot assay. Genomic RNA was extracted from NEO-expressing vectors with the RNeasy Mini kit (Qiagen, Valencia, CA). Serial dilutions of RNA were blotted on a Hybond membrane (Amersham Biosciences, Piscataway, NJ), hybridized with a 32P-labeled vector-specific DNA probe and quantified with a PhosphorImager using AIDA 2.43 software.
Evaluation of Residual Integration Activity.
HeLa cells were seeded at a density of 50,000 cells per dish (3-cm diameter dish). The cells were transduced 24 h later with serial dilutions of INN NEO vector (250, 50, 5, and 0.5 ng of p24), and of the INWT NEO vectors (5, 5 × 10−1, 10−1, 5 × 10−2, 10−2, 5 × 10−3, and 10−3 ng of p24). The medium was removed 24 h later and replaced with medium supplemented with 1 mM G418. The medium was replaced every 3 days. Cells were grown until clones developed and were then stained with neutral red and fixed with ethanol. Clones on each flask were counted. We transduced cells in three replicate dishes for each condition, and results are expressed as the mean of three measurements.
Transduction of Primary Cortical Cultures and Evaluation of the Rate of Transduction.
Confluent cells were transduced with equivalent numbers of TU of INN GFP and INWT GFP vectors. The cells were fixed at various times after transduction with 1% PFA in PBS. GFP-expressing cells were immunostained by using a rabbit polyclonal anti-GFP primary antibody (1:3,000; Abcam, Cambridge, U.K.) and a goat anti-rabbit FITC-conjugated secondary antibody (1:100; Tebu-Bio, Le Perray en Yvelines, France). Both nonimmunoreactive and immunoreactive cells were counted in triplicate, in three representative fields per well. The results were expressed as a mean of the three experiments ±SEM. The statistical significance of the results was evaluated by one-way ANOVA.
Stereotactic Injections and Histological Analysis.
We sterotactically injected 2 μl of INWT GFP (10 × 104 TU) or INN GFP (5 × 104 TU) into the brain of C3H adult mice (Janvier). Injections were performed in two subsites for each vector (from bregma: anteriority, +0.5; laterality, ±1.6; ventrality, −3.5/−4). Animals were anesthetized with pentobarbital and intracardially perfused with 4% PFA 1 week and 4 weeks after inoculation. Brains were removed, cryopreserved in 15% sucrose, and frozen in cold isopentane. Immunohistochemical analysis was performed on 20-μm cryostat sections by using a rabbit polyclonal primary antibody (1:3,000; Abcam). Primary antibodies were detected with biotinylated goat anti-rabbit secondary antibody coupled with an avidin–biotin amplification system (Vectastain ABC kit) and the VIP kit from Vector Laboratories (Burlingame, CA). All animals were maintained and treated according to the European Community guidelines.
Acknowledgments
We thank Pierre Charneau for his useful comments and advice and Pierre Sonigo for his contribution. We thank the Centre National de la Recherche Scientifique, Université Pierre et Marie Curie, the Institut pour la Recherches sur la Moëlle Épinière (IRME), and Retina France for supporting this work. S.P. was supported by a fellowship from Université Denis Diderot Paris 7, H.M. by a fellowship from IRME, and C. Serguera by France Alzheimer.
Abbreviations
- IN
integrase
- LTR
long terminal repeat
- HIV-1
HIV type 1
- NEO
neomycin phosphotransferase
- TU
transducing unit.
Footnotes
The authors declare no conflict of interest.
References
- 1.Sakhuja K, Reddy PS, Ganesh S, Cantaniag F, Pattison S, Limbach P, Kayda DB, Kadan MJ, Kaleko M, Connelly S. Hum Gene Ther. 2003;14:243–254. doi: 10.1089/10430340360535797. [DOI] [PubMed] [Google Scholar]
- 2.Logvinoff C, Epstein AL. Hum Gene Ther. 2001;12:161–167. doi: 10.1089/104303401750061221. [DOI] [PubMed] [Google Scholar]
- 3.Kafri T, Blomer U, Peterson DA, Gage FH, Verma IM. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- 4.Abordo-Adesida E, Follenzi A, Barcia C, Sciascia S, Castro MG, Naldini L, Lowenstein PR. Hum Gene Ther. 2005;16:741–751. doi: 10.1089/hum.2005.16.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, Radford I, Villeval JL, Fraser CC, Cavazzana-Calvo M, Fischer A. N Engl J Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, Li Y, Crise B, Burgess SM. Science. 2003;300:1749–1751. doi: 10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
- 7.Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F. Cell. 2002;110:521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 8.Killebrew DA, Troelstrup D, Shiramizu B. Cell Mol Biol. 2004;50:581–589. [PubMed] [Google Scholar]
- 9.Gallagher B, Wang Z, Schymura MJ, Kahn A, Fordyce EJ. Am J Epidemiol. 2001;154:544–556. doi: 10.1093/aje/154.6.544. [DOI] [PubMed] [Google Scholar]
- 10.Themis M, Waddington SN, Schmidt M, von Kalle C, Wang Y, Al-Allaf F, Gregory LG, Nivsarkar M, Holder MV, Buckley SM, et al. Mol Ther. 2005;12:763–771. doi: 10.1016/j.ymthe.2005.07.358. [DOI] [PubMed] [Google Scholar]
- 11.Coffin JM. In: Fields Virology. Fields NB, Knipe DM, Howley PM, Chanock RM, Melnick JL, Monath TP, Roizman B, Straus SE, editors. Philadelphia: Lippincott-Raven; 1996. pp. 1767–1847. [Google Scholar]
- 12.Farnet CM, Haseltine WA. J Virol. 1991;65:6942–6952. doi: 10.1128/jvi.65.12.6942-6952.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevenson M, Haggerty S, Lamonica CA, Meier CM, Welch SK, Wasiak AJ. J Virol. 1990;64:2421–2425. doi: 10.1128/jvi.64.5.2421-2425.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelman A, Englund G, Orenstein JM, Martin MA, Craigie R. J Virol. 1995;69:2729–2736. doi: 10.1128/jvi.69.5.2729-2736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiskerchen M, Muesing MA. J Virol. 1995;69:376–386. doi: 10.1128/jvi.69.1.376-386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakajima N, Lu R, Engelman A. J Virol. 2001;75:7944–7955. doi: 10.1128/JVI.75.17.7944-7955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Y. Retrovirology. 2004;1:13. doi: 10.1186/1742-4690-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petit C, Schwartz O, Mammano F. J Virol. 2000;74:7119–7126. doi: 10.1128/jvi.74.15.7119-7126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 20.Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blomer U, Naldini L, Verma IM, Trono D, Gage FH. Hum Mol Genet. 1996;5:1397–1404. doi: 10.1093/hmg/5.supplement_1.1397. [DOI] [PubMed] [Google Scholar]
- 22.Loewen N, Leske DA, Chen Y, Teo WL, Saenz DT, Peretz M, Holmes JM, Poeschla EM. J Gene Med. 2003;5:1009–1017. doi: 10.1002/jgm.447. [DOI] [PubMed] [Google Scholar]
- 23.Saenz DT, Loewen N, Peretz M, Whitwam T, Barraza R, Howell KG, Holmes JM, Good M, Poeschla EM. J Virol. 2004;78:2906–2920. doi: 10.1128/JVI.78.6.2906-2920.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zennou V, Petit C, Guetard D, Nerhbass U, Montagnier L, Charneau P. Cell. 2000;101:173–185. doi: 10.1016/S0092-8674(00)80828-4. [DOI] [PubMed] [Google Scholar]
- 25.Zennou V, Serguera C, Sarkis C, Colin P, Perret E, Mallet J, Charneau P. Nat Biotechnol. 2001;19:446–450. doi: 10.1038/88115. [DOI] [PubMed] [Google Scholar]
- 26.Follenzi A, Ailles LE, Bakovic S, Geuna M, Naldini L. Nat Genet. 2000;25:217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- 27.Whitwam T, Peretz M, Poeschla E. J Virol. 2001;75:9407–9414. doi: 10.1128/JVI.75.19.9407-9414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zufferey R, Donello JE, Trono D, Hope TJ. J Virol. 1999;73:2886–2892. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brun S, Faucon-Biguet N, Mallet J. Mol Ther. 2003;7:782–789. doi: 10.1016/s1525-0016(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 30.Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM. J Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, Trono D. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanez-Munoz RJ, Balaggan KS, MacNeil A, Howe SJ, Schmidt M, Smith AJ, Buch P, MacLaren RE, Anderson PN, Barker SE, et al. Nat Med. 2006;12:348–353. doi: 10.1038/nm1365. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt M, Zickler P, Hoffmann G, Haas S, Wissler M, Muessig A, Tisdale JF, Kuramoto K, Andrews RG, Wu T, et al. Blood. 2002;100:2737–2743. doi: 10.1182/blood-2002-02-0407. [DOI] [PubMed] [Google Scholar]
- 34.Chen H, Engelman A. J Virol. 2000;74:8188–8193. doi: 10.1128/jvi.74.17.8188-8193.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ehrhardt A, Peng PD, Xu H, Meuse L, Kay MA. Hum Gene Ther. 2003;14:215–225. doi: 10.1089/10430340360535779. [DOI] [PubMed] [Google Scholar]
- 36.Chancham, P van Tjerperen T, McDoom I, Hughes JA. J Drug Target. 2003;11:205–213. doi: 10.1080/10611860310001603823. [DOI] [PubMed] [Google Scholar]
- 37.Papapetrou EP, Ziros PG, Micheva ID, Zoumbos NC, Athanassassiadou A. Gene Ther. 2006;13:40–51. doi: 10.1038/sj.gt.3302593. [DOI] [PubMed] [Google Scholar]
- 38.Cannon PM, Byles ED, Kingsman SM, Kingsman AJ. J Virol. 1996;70:651–657. doi: 10.1128/jvi.70.1.651-657.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 40.Vogel R, Amar L, Thi AD, Saillour P, Mallet J. Hum Gene Ther. 2004;15:157–165. doi: 10.1089/104303404772679968. [DOI] [PubMed] [Google Scholar]