Abstract
One hundred fifty years ago glial cells were discovered as a second, non-neuronal, cell type in the central nervous system. To ascribe a function to these new, enigmatic cells, it was suggested that they either glue the neurons together (the Greek word “γλια” means “glue”) or provide a robust scaffold for them (“support cells”). Although both speculations are still widely accepted, they would actually require quite different mechanical cell properties, and neither one has ever been confirmed experimentally. We investigated the biomechanics of CNS tissue and acutely isolated individual neurons and glial cells from mammalian brain (hippocampus) and retina. Scanning force microscopy, bulk rheology, and optically induced deformation were used to determine their viscoelastic characteristics. We found that (i) in all CNS cells the elastic behavior dominates over the viscous behavior, (ii) in distinct cell compartments, such as soma and cell processes, the mechanical properties differ, most likely because of the unequal local distribution of cell organelles, (iii) in comparison to most other eukaryotic cells, both neurons and glial cells are very soft (“rubber elastic”), and (iv) intriguingly, glial cells are even softer than their neighboring neurons. Our results indicate that glial cells can neither serve as structural support cells (as they are too soft) nor as glue (because restoring forces are dominant) for neurons. Nevertheless, from a structural perspective they might act as soft, compliant embedding for neurons, protecting them in case of mechanical trauma, and also as a soft substrate required for neurite growth and facilitating neuronal plasticity.
Keywords: biomechanics, elasticity, viscosity, retina, hippocampus
In his search for a “connective tissue” in the brain, the pathologist Rudolf Virchow (1) discovered a non-neuronal cell type that he termed glial cells (“Nervenkitt”), stemming from his designation of their function as a glue or putty for the already known neurons. Another equally prominent historic view implies that glial cells provide a supportive scaffold (“Stützzellen”) to which the neurons are attached like Christmas ornaments to a tree (2). Motivated by this perspective the term “support cells,” support as in structural support, is generally used for glial cells of sensory organs such as the retina.
To recognize the difference in these two viewpoints, one needs to envisage that putty at the times of Virchow was rather soft and viscous (i.e., nonelastic), similar to honey, whereas the term support cell implies glial cells to be stiff and dominantly elastic elements like the piers of a bridge. Thus, these two hypotheses, assuming either a more fluid or solid character of glial cells, are mutually exclusive. Nevertheless, both of them are still widely accepted, despite the fact that they must be considered mere speculations. There is a nearly complete lack of data on glial cell mechanics because current glial cell research has been focused on their electrophysiological and biochemical properties.
Recently, it has been shown that neurons are capable of sensing the stiffness of their environment in vitro and prefer soft substrates (3). However, little is known about the mechanical properties of their physiological environment, i.e., particularly the glial cells that fill most of the space between the neuronal elements. Interestingly, glial cells in vitro grow better on hard materials (4). Rheological studies on brainstem preparations have been carried out to understand posttraumatic alterations in tissue of the CNS (5). However, even the normal mechanical properties at the level of individual neural cells are still poorly understood. For the retina as an ontogenetically evolved part of the CNS, biomechanics may play an especially important role. This thin, fragile tissue sheet is held in place by a subtle balance between the intraocular pressure and suction from retinal pigment epithelium (6). Several retinal disorders such as retinoschisis, malignant myopia, and retinal detachment are thought to be caused by mechanical stress facilitated by anomalous mechanical properties of retinal (glial) cells (7).
Results
We assessed the mechanical properties of both neurons and glial cells from two different areas of the mammalian CNS, the hippocampus (a part of the brain) and the retina. As representative cell “pairs” of the two tissues, we investigated (i) pyramidal neurons and astrocytes (glial cells) of the hippocampus, and (ii) retinal interneurons (i.e., bipolar and amacrine cells) and Müller (glial) cells. To characterize the local viscoelastic properties of individual cells, we measured the complex Young's modulus E* = E′ + iE″ in a defined range of deforming frequencies (30, 100, and 200 Hz) using a scanning force microscope (SFM) (8, 9). A detailed frequency scan of Müller cells is shown in Fig. 5, which is published as supporting information on the PNAS web site. The real part E′ reflects the elastic storage (energy storage) response and the imaginary part E″ reflects the viscous loss (energy dissipation) response of the tissue. Thus, the resulting storage modulus is a measure of a tissue's elastic stiffness and the loss modulus measures the tissue's viscous properties. Furthermore, we measured the Poisson's ratio ν, which describes the tendency of a material to contract in two dimensions when it is stretched in the third one (Fig. 6, which is published as supporting information on the PNAS web site). In the case of both types of CNS tissues studied, all of the enzymatically acutely dissociated cells remained vital during measurement, and most even maintained their characteristic morphology (Fig. 1a-c).
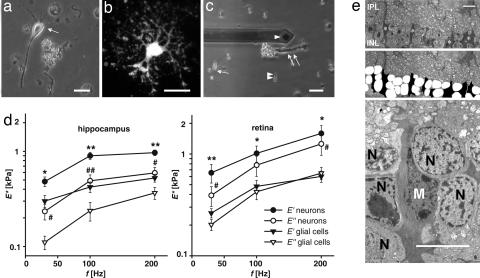
Fig. 1.
Comparison of viscoelastic properties of neurons and glial cells. (a–c) Acutely dissociated cells from hippocampus (a, arrow, pyramidal neuron; b, GFAP-EGFP-fluorescent astrocyte) and retina (c, single arrow, bipolar neuron; double arrow, Müller glial cell; double arrowhead, photoreceptor cell) are shown. The tip of the cantilever is also visible in c (single arrowhead). (a and c) Phase-contrast images. (b) Fluorescence image. (d) Elastic storage (E′) and viscous loss modulus (E″) of somata of hippocampal (n = 43) and retinal (bipolar cells, n = 6; amacrine cells, n = 5) neurons and glial cells (hippocampal astrocytes, n = 40; retinal Müller cells, n = 40) (mean ± SEM) is shown. In both types of CNS tissues, glial cells are softer than neurons because E′glia is statistically significant smaller than E′neuron (∗, P < 0.05; ∗∗, P < 0.01, E′neurons vs. E′glia; #, P < 0.05; ##, P < 0.01, Eneurons vs. Eglia), and for all cell types E′/E″ > 1, which means that they have an allover elastically restoring force. (e) (Top) Transmission electron micrograph of the inner nuclear layer (INL) of a guinea pig retina, close to the optic disk. Asterisks indicate Müller cell somata. (Middle) The same image; in the INL, neuronal cell somata are labeled in white, Müller cell somata are in black. Note the more irregular outline of the latter. (Bottom) The image at higher magnification. IPL, inner plexiform layer; M, Müller cell nucleus; N, nuclei of retinal interneurons. (Scale bars: a–c, 20 μm; e, 10 μm.)
Two general features became obvious. First, in all evaluated cell types the elastic response was dominant as compared with the viscous response (i.e., E′ was greater than E″). Thus, CNS cells displayed the rheological characteristics of elastic solids. Second, glial cells were found to be about twice as soft as neurons.
A detailed comparison between neurons (pyramidal cells) and glial cells (astrocytes) from the hippocampus revealed distinct differences. As shown in Fig. 1d, the elastic storage modulus E′ and the viscous loss modulus E″ of neurons were significantly higher than corresponding values of glial cells. The somata of pyramidal neurons had an elastic modulus between ≈480 Pa at 30 Hz and ≈970 Pa at 200 Hz. E′ of astrocyte somata was between ≈300 Pa at 30 Hz and ≈520 Pa at 200 Hz. The same situation was found for the retina. Data from somata of retinal neurons, i.e., bipolar and amacrine cells, which displayed similar values, were compared with data from somata of Müller glial cells. The E′ value of the somata of investigated neurons ranged from ≈650 Pa at 30 Hz to ≈1,590 Pa at 200 Hz; that of the somata of Müller glial cells was between ≈260 Pa at 30 Hz and ≈600 Pa at 200Hz (Fig. 1d).
Bipolar and amacrine neurons, situated in the same retinal layer as the Müller glial cell somata, are stiffer than the latter (Fig. 1d). Because in situ the somata of these three cell types are closely packed together, this mechanical difference should result in different degrees of deformation. We examined this hypothesis by using electron microscopy (Fig. 1e). The cytoplasm of guinea-pig Müller cells is known to be electron-denser than that of the neurons (10), allowing an easy identification. The somata of bipolar cells were smoothly rounded on average and seen to indent the neighboring, irregular-shaped Müller cell somata; this finding was consistent with our finding that Müller cells were softer.
Müller cells are typical glial cells with an extended morphology that lends itself easily to a detailed study of the spatial distribution of viscoelastic properties within individual cells. They have a characteristic bipolar shape with two distinct stem processes emanating from the soma, one terminating in a wide endfoot and the other one in thin branches and microvilli (Fig. 2a) (11). We separately characterized the wide endfoot, densely packed with smooth endoplasmatic reticulum, the adjacent inner stem process, containing mainly bundles of intermediate filaments, the bulged soma with the nucleus and the perinuclear cytoplasm, and the thin outer process, containing microtubules (12, 13). Both of the Müller cell processes were significantly softer than soma and endfoot, whereas the soma was even stiffer than the endfoot. The elastic moduli of the inner and outer processes were ≈130 and ≈100 Pa at 30 Hz and ≈160 and ≈210 Pa at 200 Hz, respectively. In comparison, we measured ≈220/370 Pa at 30/200 Hz for endfeet and ≈260/600 Pa at 30/200 Hz for cell somata (Fig. 2b).
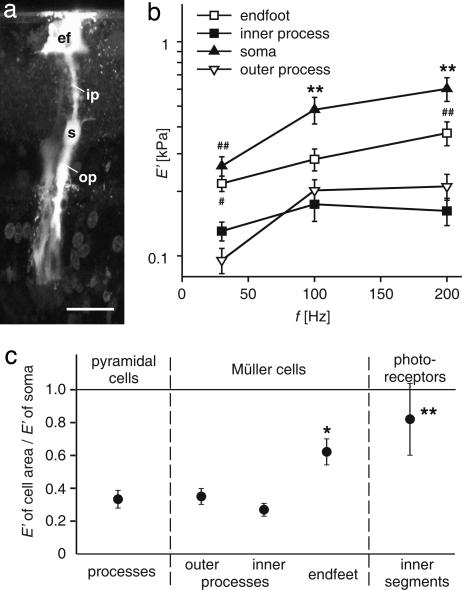
Fig. 2.
Spatial variation of the cells' mechanical properties. (a) Fluorescence microphotograph of a dye-injected Müller cell (Lucifer Yellow) in a guinea pig retinal slice preparation. ef, endfoot; ip, inner process; s, soma; op, outer process of the Müller cell. (Scale bar: 20 μm.) (b) Comparison of the stiffness of different parts of the radial glial (Müller) cells of the retina (#, P < 0.05; ##, P < 0.01 vs. processes; ∗∗, P < 0.01 vs. all other parts of Müller cells; mean ± SEM). Both cell processes were significantly softer than somata and endfeet, which might be attributed to a different distribution of cell organelles rather than of cytoskeletal elements. (c) Relative stiffness of the respective cellular areas compared with the soma of the according cell (E′cell area/E′soma) at 200 Hz (∗, P < 0.05; ∗∗, P < 0.01 vs. cell processes; mean ± SEM). Processes of Müller cells and pyramidal cells displayed a similar relative stiffness. The endfeet of Müller cells and the inner segments of photoreceptor cells were stiffer with respect to the other cellular compartments, but softer than their somata. Similar relations were observed at 30 and 100 Hz.
The observation that cell processes were softer than the somata was also made for pyramidal neurons. Furthermore, the inner segments of photoreceptor cells proved to be softer than their somata. The storage moduli of both Müller (glial) cell and pyramidal cell processes amounted to about one-third of their respective somata, whereas the storage moduli of Müller cell endfeet and photoreceptor inner segments amounted to about two-thirds of their respective somata (Fig. 2c).
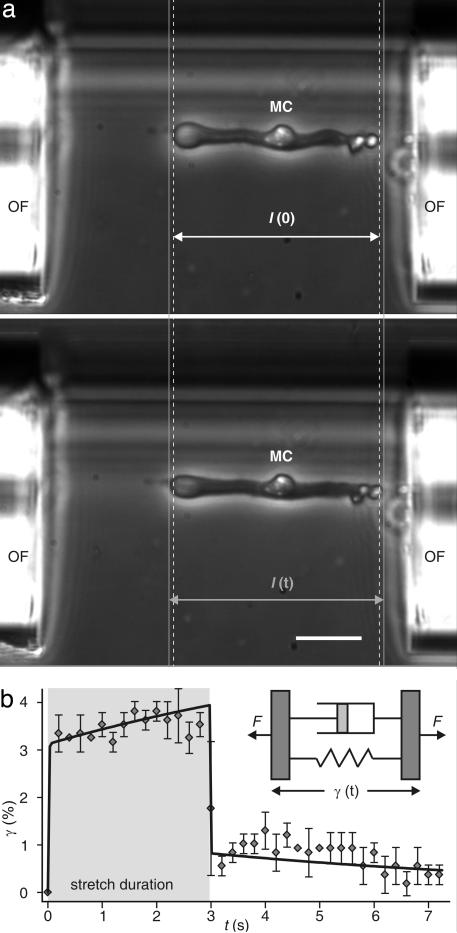
Our results show that glial cells act as soft compliant structures surrounding the neurons, which may be comparable to cushioning material in common packaging. Because this means that they could protect the neurons from mechanical trauma, we analyzed the reaction of whole glial cells to a global deformation. For this purpose we used an optical cell stretcher (Fig. 3) (14). After trapping the cells in this dual-beam infrared laser trap at low power, a 10-fold increase in the laser power led to a deforming force on the surface of the cells on the order of hundreds of piconewtons. The exact cell stretching and subsequent relaxation behavior was found to be analogous to the behavior of two Voigt elements in series (Fig. 3b). The Voigt element is a viscoelastic object composed of a restoring spring (elastic part) and a dissipative dashpot (viscous part), connected in parallel (Fig. 3b Inset). The resulting overdamped viscoelastic behavior of the cells is comparable to that of shock absorbers (a detailed description of the calculation is given as Supporting Text, which is published as supporting information on the PNAS web site).
Fig. 3.
Mechanical deformation and relaxation behavior of a whole Müller (glial) cell (MC) deformed with an optical stretcher. Cells with a length l(0) are aligned with the forces induced on their surface by two infrared laser beams emanating from the optical fibers (OF), whose ends are visible on both sides of the image. (a Upper) Increasing the laser power leads to a stretching of the cells along the laser beam axes. (a Lower) That process results in a cell length l(t). The gray lines help to compare the length before and during the stretching. (Scale bar: 25 μm.) (b) Axial cell strain γ = [I(t) − I(0)]/I(0) as a function of time (mean ± SD). The viscoelastic response of a stretched cell can be modeled by two viscoelastic Voigt elements in series (solid line), resembling a shock absorber. The gray area indicates the duration of the stretching. (Inset) The Voigt model considers a material to be composed of a spring and a dashpot connected in parallel, which represent its elastic and viscous components, respectively. F, forces acting on the viscoelastic object.
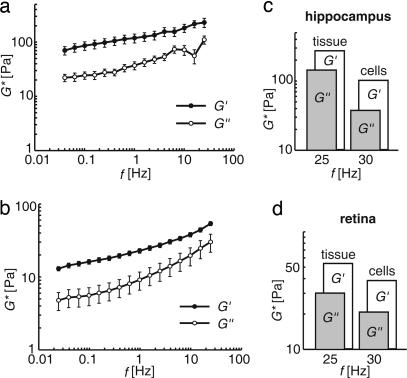
The measurements on the cellular level were accompanied by bulk rheological measurements on slices of hippocampi and retinal wholemount preparations (Fig. 4). In analogy to the complex Young's modulus E* we determined the complex shear modulus G* = G′ + iG″ [G* = E*/2(1 + ν)], where ν is the Poisson's ratio as a function of frequency. We found that for intact tissue the elastic behavior was dominant over the whole frequency range, i.e., G′ > G″, in agreement with our data on isolated cells. Both tissues were comparatively soft with a storage modulus not exceeding a few hundred Pascal in the case of the hippocampus (Fig. 4a) and even <100 Pa in the case of the retina (Fig. 4b). Finally, a conversion of the mechanical storage modulus E′ measured for single cells (compare Figs. 1 and 2) into the storage component of the shear modulus G′ measured for tissues (compare Fig. 4 a and b) revealed that the elastic moduli are of the same magnitude (Fig. 4 c and d).
Fig. 4.
Bulk rheology on CNS tissue. (a and b) Measurements of the complex frequency-dependent shear modulus G* = G′ + iG″ of bovine hippocampal brain slices (n = 9) (a) and retinae (n = 13) (b) (mean ± SEM). Note that the storage modulus G′ always exceeds the loss modulus G″ meaning that both tissues are elastic rather than viscous. (c and d) Comparison between the viscoelastic properties of whole tissues and individual glial cells. There is a good agreement between the data obtained for tissue samples and those calculated for isolated cells [G* = E*/2(l + ν)] even though different animal species were used for methodological reasons.
Discussion
In summary, we used SFM to study local viscoelastic properties of the CNS's principal individual building blocks, neurons and glial cells. Because the environment of cell cultures differs considerably from that existing in vivo, alterations of cultured cells, especially of their mechanical properties, are very likely to occur. To avoid any culture artifacts (15), our SFM measurements were performed on acutely isolated cells. The results of these measurements corresponded well to the rheological data that were obtained from intact tissue samples (Fig. 4 c and d). It should be noted, however, that in intact tissues not only the cells but also structures that bind them together (such as the extracellular matrix and intercellular adhesion molecules) and the associated (inter)cellular forces will contribute to the tissues' complex viscoelastic behavior (16).
Several distinct cellular areas of Müller glial cells were investigated. The viscoelastic properties vary along a given cell although the submembranous actin cortex appears to be rather homogenously distributed throughout cells (17). The actin cytoskeleton is a key contributor to the mechanical properties of many cells such as fibroblasts, epithelial cells, and neutrophils (18–20). There was no significant difference in stiffness between inner and outer processes (Fig. 2b), although they differ in their dominant cytoskeletal elements; the inner process is rich in intermediate filament bundles, whereas the outer process contains mainly microtubules (12, 13). Both cell processes were softer than the endfoot and the soma (Fig. 2b). Thus, it seems that the typical cytoskeletal elements are not solely responsible for intracellular viscoelasticity. The cells' observed “biomechanical segmentation” may be caused by differences in contents of cellular organelles. The soma contains abundant rough endoplasmic reticulum, and the dense nucleus is known to add a relatively stiff characteristic (21). The endfoot was also stiffer than the processes but softer than the soma (Fig. 2b); it contains a very dense agglomerate of smooth endoplasmic reticulum (12, 13). Further work is needed to elucidate how these subcellular inhomogeneities are generated. Although it is generally thought that lipid membranes cannot contribute to a cell's stiffness (20), it has been shown that tubes of membranes do resist pulling (22). Consequentially, there might be a stiffening of the cells because of the elevated turgor within the ER, caused by its solid (proteins) or fluid (water) contents, but it remains to be proven. The spatial variation of viscoelastic properties in Müller cells (compare Fig. 2b) may generally occur within CNS cells, as we also found the processes of the hippocampal neurons to be softer than their somata. The inner segments of photoreceptor cells (which are densely packed with mitochondria) were significantly stiffer than the neuronal processes (Fig. 2c), but still softer than their own somata.
A fundamental result of our study is that the CNS consists of very soft cells if compared with cells from other tissues. Studies on fibroblasts, for example, revealed E′ and E″ values at 200 Hz of ≈3 and 2 kPa, respectively (8). These cells are about twice as stiff as the stiffest structures, i.e., neuronal somata, we found in the CNS. This finding corresponds well to the experience of neuroscientists and surgeons that both the brain and the retina are very soft tissues. There is also evidence from literature that nervous tissue, composed mainly of neurons and glial cells, is softer than other tissues (23).
For all cell types examined, the storage modulus exceeded the loss modulus (Fig. 1d), meaning that their elastic properties are more important than their viscous aspects. Thus, individual cells of the CNS as its fundamental building blocks display mechanical features similar to elastic solids rather than to fluids. There are earlier reports favoring the view that nervous tissue shows fluid-like characteristics (24). However, within the frequency range investigated here, which, for example covered the impact of mechanical trauma, the cells behaved as elastic, restoring but highly compliant soft solids.
It was surprising that the CNS glial cells were even softer than neurons. This finding is in strong contradiction to the idea of glial cells acting as a rigid scaffold. Obviously, these extremely soft cells are no “support pillars,” as, for example, Müller cells are often called. Virchow's original prediction that glial cells are some kind of a putty or glue in which the neurons are embedded comes closer to the truth. However, his view certainly neglected the dominant elastic restoring forces of glial cells; in the times of Virchow, the modern elastic glues (such as nowadays used to repair the tubes of bikes) were unknown. In this context, it should be recalled that there is no need to glue neurons together (in the sense of preventing their dispersion): the available space for their distribution is well limited by hard envelopes (the skull, the ocular bulb, etc.), and the cells are pressed together by both their very high package density and the secretion of fluids (cerebrospinal fluid and aqueous humor). Specific cellular compartments of adjacent neurons (such as the presynaptic and postsynaptic elements), or layered sheaths of neural cells (such as the photoreceptor inner segments at the “outer limiting membrane” of the retina) are fixed together by certain adhesion molecules or even by zonulae adherentes, but this is a different matter. Under normal circumstances both brain and retina are exposed to a pressure exceeding the barometric pressure (both the intracranial and the intraocular pressure amount to ≈10–20 mm/Hg) (25, 26). In this regard, glial cells provide a soft embedding for the neurons, which is flexible enough to accommodate swelling of neuronal compartments during neuronal activity (27).
In the cases of mechanical trauma, brain and retina may be exposed to large deforming forces. In these cases, and even during frequent trivial events such as every footstep we make, the soft spring-like glial cells might form a protective soft compliant matrix around the neurons. In a structural sense this matrix may appear as a redundant feature because most eukaryotic cells (including neurons themselves) behave viscoelastically and, thus, behave similar to shock absorbers. In the CNS, nevertheless, a soft matrix as buffer between the fragile neurons and the rigid skull and sclera might be advantageous.
Finally, another aspect of CNS physiology should be considered that benefits from very soft glial cells. It has been recently shown that in culture neuronal growth is favored on soft substrates. On these substrates neurons form significantly more branches than on stiffer ones (3, 4). This observation leads to the appealing hypothesis that the softness of glial cells facilitates neurite growth or is even a precondition during ontogenetic development and in the course of physiological (e.g., activity dependent) and pathophysiological neuronal plasticity (e.g., during neuronal regeneration). The hippocampus exhibits a particularly high degree of wiring plasticity (28) and is known to generate new neurons and glial cells throughout adulthood; the processes of the newly born cells must penetrate the adult tissue to contact target cells and become functional (29). Furthermore, extensive (anomalous) growth of neuronal cell processes has been demonstrated in the retina after cellular degeneration (30, 31); in accordance with our hypothesis, the sites of such growth (and the normal developmental establishment of synaptic contacts) are the plexiform layers where Müller cells are softest (Fig. 7, which is published as supporting information on the PNAS web site; compare Fig. 2b). In summary, the biomechanical properties of glial cells may enable them to perform even more neuron-supportive functions than hitherto elucidated by electrophysiological and biochemical work.
Materials and Methods
All experiments were carried out in accordance with applicable German laws of animal protection and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Sample Preparation.
Retinal cells.
Retinal cells of adult guinea pigs were acutely isolated as described (32). Briefly, guinea pigs were deeply anesthetized and killed by an overdose of urethane (2 g/kg, i.p.). After enucleation of the eyes and excision of the retina, retinal pieces were incubated in Ca2+- and Mg2+-free PBS (pH 7.4; Seromed Biochrom, Berlin, Germany) containing papain (0.03–0.1 mg/ml; Boehringer Mannheim, Mannheim, Germany) for 30 min at room temperature or at 37°C. After washing with PBS containing DNase I (200 units/ml; Sigma, Deisenhofen, Germany), the tissue pieces were gently triturated by a wide-bore pipette to obtain suspensions of isolated cells. The cell suspensions were stored at 4°C up to 4 h (33) in extracellular solution until use. The main retinal cell types found in the suspensions (amacrine cells, bipolar cells, photoreceptor cells, and Müller glial cells) were easily distinguished by their morphology (compare Fig. 1a).
Extracellular solution consisted of 136 mM NaCl, 3 mM KCl, 1 mM MgCl2, 10 mM Hepes, 2 mM CaCl2, and 10 mM d-glucose. The pH of the solution was adjusted to 7.4 by using Tris (1 M; pH 10.42).
Hippocampal cells.
Cells were acutely isolated as described (34, 35). Briefly, Tg(hGFAP/EGFP) mice (36) from postnatal days 25–30 were decapitated, and their brains were dissected and cut into 300-μm-thick slices in frontal orientation by using a vibratome (HM 650V; Microm International, Walldorf, Germany). Slice preparation was performed in ice-cold, carbogen-saturated (95% O2/5% CO2) artificial cerebrospinal fluid (ACSF) containing sucrose (pH 7.4). After incubation for 30 min at 35°C in ACSF containing sucrose, the slices were stored for 20 min in ACSF at room temperature. Then the slices were incubated for 12–15 min either in pronase- (2 mg/ml; Roche, Indianapolis, IN) or papain-containing solution (2 mg/ml, supplemented with 0.3 mg/ml l-cysteine; Sigma) at room temperature for the isolation of neurons and glial cells, respectively. After washing, the CA1 region of the hippocampus was dissected, and cells were isolated in Hepes-buffered solution by using wide-bore pipettes. The cell suspensions contained many pyramidal neurons (identified by their characteristic morphology) and astrocytes (identified by their inherent EGFP fluorescence).
ACSF with sucrose consisted of 87 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 7 mM MgCl2, 0.5 mM CaCl2, 25 mM NaHCO3, 25 mM d-glucose, and 75 mM sucrose gassed with 95% O2/5% CO2.
ACSF without sucrose consisted of 126 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 2 mM MgSO4, 2 mM CaCl2, 26 mM NaHCO3, and 10 mM d-glucose gassed with 95% O2/5% CO2.
Hepes-buffered solution consisted of 150 mM NaCl, 5 mM KCl, 2 mM MgSO4, 2 mM CaCl2, 10 mM Hepes, and 10 mM d-glucose; gassed with 100% O2. The pH of the solution was adjusted to 7.4 by using NaOH or HCl.
Tissue slices.
Fresh bovine eyeballs and brains were obtained from a local slaughterhouse and stored in ice-cold PBS. Retinae and hippocampi were excised immediately before the experiments. Circular retinal pieces were obtained by using a stamp-like tool; hippocampal pieces were first “stamped out,” and subsequently sliced with a scalpel blade.
Electron Microscopy.
Excised guinea pig eyes were opened at their corneal margin, the vitreous body was removed, and the eyecups were then fixed in a buffered mixture of 0.5% glutaraldehyde and 4% paraformaldehyde overnight and postfixed in 1% osmium tetroxide for 2 h. Subsequently, eyecups were rinsed and dehydrated in ethanol. The tissue was stained overnight in 70% ethanol saturated with uranylacetate. After further dehydration in absolute ethanol and propylene oxide, the samples were embedded in a Araldite 502 Kit (Sigma) and sectioned on a Reichert FCR Ultracut ultramicrotome (Leica, Bensheim, Germany). Ultrathin sections were stained with uranylacetate and lead citrate, and then studied by using an EM 10 electron microscope (Zeiss, Oberkochen, Germany).
Biophysical Measurements.
SFM.
SFM measurements were taken with a NanoWizard SFM (JPK Instruments, Berlin, Germany), which was placed on an inverted microscope DM IRB (Leica Microsystems, Wetzlar, Germany). Commercial cantilevers (NANOSENSORS; Nano World, Schaffhausen, Switzerland) with spring constants of ≈0.02–0.06 N/m were modified as described (8, 9) by gluing polystyrene beads (Seradyn Particle Technology, Indianapolis, IN; radius ≈3 μm) to the tip, to avoid damage of the sample and any nonlinear deforming stresses. The oscillatory drive signal necessary to perform frequency-dependent viscoelasticity measurements was fed to the scanner signal through a lock-in amplifier SR850 (Stanford Research Systems, Sunnyvale, CA) (8). Phase and amplitude differences between the applied modulation and the cantilever response signal were recorded. Cells were placed on commercial glass slides, Superfrost-Plus (Menzel-Gläser, Braunschweig, Germany). A detailed description of the force measurements on acutely isolated cells is published as Supporting Text.
Bulk rheology.
Circular pieces of 25- and 8-mm diameter were cut out of bovine retinal whole mounts and hippocampal slices, respectively. These pieces were then placed on a RFS 3 Rheometer (Scientific Rheometric, Piscataway, NJ). Subsequently, a stainless-steel plate of according size was brought into contact with the upper surface of the tissue. The adhesion of the tissues to the steel plates was strong for frequencies between 0.1 and 150 rad/s; in control experiments with the tissues fixed to the plates with fibrinogen/thrombin gels similar results were obtained. The frequency sweeps, i.e., examination of G′ and G″ at different frequencies, were carried out with controlled applied strain of 2%.
Optical stretcher.
Isolated guinea pig Müller cells were trapped and aligned in a dual beam infrared laser trap (λ = 1,064 nm) which was mounted on an inverted microscope (DMIL, Leica) (trapping power 0.1 W in each beam). Increasing the laser power (1.0 W) led to a stretching of the cells along the laser axis (14, 19, 37). Cells were recorded with a digital camera (Basler A202k; Basler Vision, Ahrensburg, Germany). Changes in cell length were obtained by using custom-built software (based on LabView, National Instruments, Austin, TX), and data analysis was done by using Igor Pro (WaveMetrics, Lake Oswego, OR) software.
Supplementary Material
Acknowledgments
We thank Peter Rudolf (Institut für Klassische Philologie und Komparatistik, Universität Leipzig) for inspiring discussions about the terminology of glia; Jens Grosche and Katja Rillich for providing images; and Bernd Kohlstrunk for technical help. This study was supported by a stipend from the Deutscher Akademischer Austauschdienst through the Sandwich Program (to Y.-B.L.), Deutsche Forschungsgemeinschaft Research Training School “InterNeuro” Grant GRK 1097 (to J.K. and A.R.), and Deutsche Forschungsgemeinschaft Priority Program “Glia and Synapse” Grant SPP 1172 (to G.S., C.S., F.K., and A.R.).
Abbreviations
- SFM
scanning force microscope(microscopy)
- ACSF
artificial cerebrospinal fluid.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Virchow R. Gesammelte Abhandlungen zur Wissenschaftlichen Medicin. Frankfurt, Germany: von Meidinger Sohn; 1856. [Google Scholar]
- 2.Schultze M. Zur Anatomie und Physiologie der Retina. Sohn, Bonn, Germany: von Max Cohen; 1866. [Google Scholar]
- 3.Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. NeuroReport. 2002;13:2411–2415. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Georges PC, Miller WJ, Meaney DF, Sawyer E, Janmey PA. Biophys J. 2006;90:3012–3018. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbogast KB, Margulies SS. J Biomech. 1998;31:801–807. doi: 10.1016/s0021-9290(98)00068-2. [DOI] [PubMed] [Google Scholar]
- 6.Yao XY, Hageman GS, Marmor MF. Invest Ophthalmol Visual Sci. 1994;35:744–748. [PubMed] [Google Scholar]
- 7.Reid SN, Yamashita C, Farber DB. J Neurosci. 2003;23:6030–6040. doi: 10.1523/JNEUROSCI.23-14-06030.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahaffy RE, Shih CK, MacKintosh FC, Kas J. Phys Rev Lett. 2000;85:880–883. doi: 10.1103/PhysRevLett.85.880. [DOI] [PubMed] [Google Scholar]
- 9.Mahaffy RE, Park S, Gerde E, Kas J, Shih CK. Biophys J. 2004;86:1777–1793. doi: 10.1016/S0006-3495(04)74245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Germer A, Biedermann B, Wolburg H, Schuck J, Grosche J, Kuhrt H, Reichelt W, Schousboe A, Paasche G, Mack AF, Reichenbach A. J Neurocytol. 1998;27:329–345. doi: 10.1023/a:1006934724566. [DOI] [PubMed] [Google Scholar]
- 11.Reichenbach A, Schneider H, Leibnitz L, Reichelt W, Schaaf P, Schumann R. Anat Embryol (Berl) 1989;180:71–79. doi: 10.1007/BF00321902. [DOI] [PubMed] [Google Scholar]
- 12.Reichenbach A, Hagen E, Schippel K, Bruckner G, Reichelt W, Leibnitz L. Z Mikrosk Anat Forsch. 1988;102:897–912. [PubMed] [Google Scholar]
- 13.Reichenbach A, Hagen E, Schippel K, Eberhardt W. Z Mikrosk Anat Forsch. 1988;102:721–755. [PubMed] [Google Scholar]
- 14.Guck J, Ananthakrishnan R, Mahmood H, Moon TJ, Cunningham CC, Kas J. Biophys J. 2001;81:767–784. doi: 10.1016/S0006-3495(01)75740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidry C. Invest Ophthalmol Visual Sci. 1996;37:740–752. [PubMed] [Google Scholar]
- 16.Moore SW, Keller RE, Koehl MA. Development (Cambridge, UK) 1995;121:3131–3140. doi: 10.1242/dev.121.10.3131. [DOI] [PubMed] [Google Scholar]
- 17.Vaughan DK, Fisher SK. Exp Eye Res. 1987;44:393–406. doi: 10.1016/s0014-4835(87)80173-2. [DOI] [PubMed] [Google Scholar]
- 18.Rotsch C, Radmacher M. Biophys J. 2000;78:520–535. doi: 10.1016/S0006-3495(00)76614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wottawah F, Schinkinger S, Lincoln B, Ananthakrishnan R, Romeyke M, Guck J, Kas J. Phys Rev Lett. 2005;94:098103. doi: 10.1103/PhysRevLett.94.098103. [DOI] [PubMed] [Google Scholar]
- 20.Ananthakrishnan R, Guck J, Wottawah F, Schinkinger S, Lincoln B, Romeyke M, Moon T, Kas J. J Theor Biol. 2006;242:502–516. doi: 10.1016/j.jtbi.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Caille N, Thoumine O, Tardy Y, Meister JJ. J Biomech. 2002;35:177–187. doi: 10.1016/s0021-9290(01)00201-9. [DOI] [PubMed] [Google Scholar]
- 22.Leduc C, Campas O, Zeldovich KB, Roux A, Jolimaitre P, Bourel-Bonnet L, Goud B, Joanny JF, Bassereau P, Prost J. Proc Natl Acad Sci USA. 2004;101:17096–17101. doi: 10.1073/pnas.0406598101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Discher DE, Janmey P, Wang YL. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 24.Bilston LE, Liu Z, Phan-Thien N. Biorheology. 1997;34:377–385. doi: 10.1016/s0006-355x(98)00022-5. [DOI] [PubMed] [Google Scholar]
- 25.Czosnyka M, Pickard JD. J Neurol Neurosurg Psychiatry. 2004;75:813–821. doi: 10.1136/jnnp.2003.033126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin XD. Ophthalmologica. 1992;205:57–63. doi: 10.1159/000310313. [DOI] [PubMed] [Google Scholar]
- 27.Uckermann O, Vargova L, Ulbricht E, Klaus C, Weick M, Rillich K, Wiedemann P, Reichenbach A, Sykova E, Bringmann A. J Neurosci. 2004;24:10149–10158. doi: 10.1523/JNEUROSCI.3203-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pittenger C, Kandel ER. Philos Trans R Soc London B. 2003;358:757–763. doi: 10.1098/rstb.2002.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ming GL, Song H. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 30.Peichl L, Bolz J. Science. 1984;223:503–504. doi: 10.1126/science.6691162. [DOI] [PubMed] [Google Scholar]
- 31.Lewis GP, Sethi CS, Linberg KA, Charteris DG, Fisher SK. Mol Neurobiol. 2003;28:159–175. doi: 10.1385/MN:28:2:159. [DOI] [PubMed] [Google Scholar]
- 32.Francke M, Weick M, Pannicke T, Uckermann O, Grosche J, Goczalik I, Milenkovic I, Uhlmann S, Faude F, Wiedemann P, et al. Invest Ophthalmol Visual Sci. 2002;43:870–881. [PubMed] [Google Scholar]
- 33.Gefen A, Margulies SS. J Biomech. 2004;37:1339–1352. doi: 10.1016/j.jbiomech.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 34.Weber M, Dietrich D, Grasel I, Reuter G, Seifert G, Steinhauser C. J Neurochem. 2001;77:1108–1115. doi: 10.1046/j.1471-4159.2001.00340.x. [DOI] [PubMed] [Google Scholar]
- 35.Matthias K, Kirchhoff F, Seifert G, Huttmann K, Matyash M, Kettenmann H, Steinhauser C. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nolte C, Matyash M, Pivneva T, Schipke CG, Ohlemeyer C, Hanisch UK, Kirchhoff F, Kettenmann H. Glia. 2001;33:72–86. [PubMed] [Google Scholar]
- 37.Guck J, Ananthakrishnan R, Moon TJ, Cunningham CC, Kas J. Phys Rev Lett. 2000;84:5451–5454. doi: 10.1103/PhysRevLett.84.5451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.