Abstract
Neotropical poison frogs (Dendrobatidae) contain a variety of lipophilic alkaloids in their diffusely distributed cutaneous glands, including a major class of compounds known as pumiliotoxins. Pumiliotoxins are highly toxic and are believed to protect frogs against predators. Their potential activity against ectoparasites, however, has not been investigated. We tested female yellow fever mosquitoes (Aedes aegypti) for responses to 8-hydroxy-8-methyl-6-(2′-methylhexylidene)-1-azabicyclo[4.3.0]nonane, designated pumiliotoxin 251D [PTX (+)-251D], a skin alkaloid present in all genera of dendrobatids and in other anurans, and to its unnatural enantiomer, PTX (−)-251D. Both enantiomers of PTX 251D presented on silicone feeding membranes reduced landing and feeding by A. aegypti, but PTX (+)-251D did so at lower concentrations. PTX (+)-251D also induced toxicosis, shown when mosquitoes failed to fly off membranes. Similarly, mosquitoes confined with copper wires coated with PTX (+)-251D exhibited greater latencies to fly off the substrate and a higher incidence of leg autotomy than did those confined with the (−)-enantiomer. Our results on the contact toxicities of PTX 251D enantiomers parallel those reported for mice injected with them. The presentation of serial dilutions of PTX (+)-251D to A. aegypti revealed a minimum toxic concentration of 0.1 μg/cm2. This value is substantially lower than that estimated for the cutaneous abundance of this compound in some frogs, an observation consistent the function of PTX 251D in anuran chemical defense against ectoparasitic arthropods.
Keywords: alkaloids, chemical defense, dendrobatid poison frogs, insecticidal activity, skin secretions
Analyses of skin extracts of neotropical poison frogs (Dendrobatidae) reveal nearly 500 lipophilic alkaloids representing over 20 structural classes (1). Cutaneous alkaloids are believed to protect dendrobatids, as reflected by the many potential predators that avoid or reject them presumably in response to noxious skin secretions (2–7). Field experiments in Costa Rica, for example, reveal that ants (5) and spiders (6) typically release Dendrobates pumilio after biting it, suggesting that this frog's skin contains contact deterrents.
The responses to dendrobatid skin chemicals by ectoparasitic arthropods, such as mosquitoes and other biting flies, however, has not been investigated. Biting flies attack anurans (8–11), but apparently none has been reported to feed on dendrobatids. Nor have we (J.W.D., R. Saporito, and M. Donnelly, unpublished observations) observed flies attacking dendrobatids during field studies on these frogs, spanning >40 years, throughout Central and South America.
Pumiliotoxins/allopumiliotoxins (7-hydroxypumiliotoxins), represented by ≈80 compounds, comprise a major class of alkaloids found in most poison frogs (1, 12). We tested mosquitoes for responses to 8-hydroxy-8-methyl-6-(2′-methylhexylidene)-1-azabicyclo[4.3.0]nonane, designated pumiliotoxin 251D (PTX 251D) (Scheme 1). PTX 251D often is present in major or minor amounts in frogs of the genera Dendrobates, Epipedobates, and Minyobates, and in trace amounts in Phyllobates aurotaenia (12, 13) (Fig. 1). This compound also occurs in the skins of Madagascan frogs (Mantella spp.) (14) and South American toads (Melanophryniscus spp.) (15).
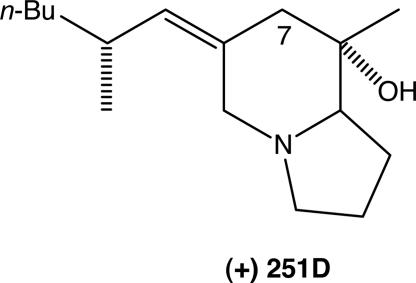
Scheme 1.
PTX (+)-251D.
Fig. 1.
Representative dendrobatid frogs that contain PTX (+)-251D in their skin (12). (Upper Left) D. pumilio from Isla Cayo Agua, Bocas, Panama. (Upper Right) E. tricolor from Azuay, Ecuador. (Lower Left) Minyobates bombetes from Valle, Colombia. (Lower Right) P. aurotaenia from Chocó, Colombia. The sizes are not to scale.
PTX 251D and synthetic analogs of it were shown to be insecticidal in a study of the tobacco budworm (Heliothis virescens) (16). When injected into budworm larvae, PTX 251D was among the most toxic of the compounds tested, causing convulsions with only a dose of 10 ng per larva and exhibiting an LD50 of 150 ng per larva. These compounds also were toxic to larvae when applied topically. Interestingly, pumiliotoxin B [now designated PTX (+)-323A], which is highly toxic to mice (17), was weakly toxic to budworms (16).
Our subject was the yellow fever mosquito (Aedes aegypti), a circumtropical vector of viral, bacterial, and filarial diseases (11). A. aegypti feeds primarily on mammals, but it accepts anurans (8, 11). Thus, this species is appropriate to test with frog-derived compounds.
We first examined mosquitoes' responses to the naturally occurring skin alkaloid enantiomer, PTX (+)-251D, and the unnatural enantiomer, PTX (−)-251D, by using a module and membrane system designed to evaluate landing and feeding deterrents (18). We then confined mosquitoes with PTX 251D-coated wires to examine their responses to contact with this compound. Our results demonstrate that PTX 251D deters feeding A. aegypti and induces contact toxicosis, as evidenced by impaired flight and leg autotomy. We also show that the naturally occurring enantiomer, PTX (+)-251D, is more toxic to mosquitoes than is PTX (−)-251D, and that PTX (+)-251D is effective at levels observed naturally in dendrobatid frogs.
Results
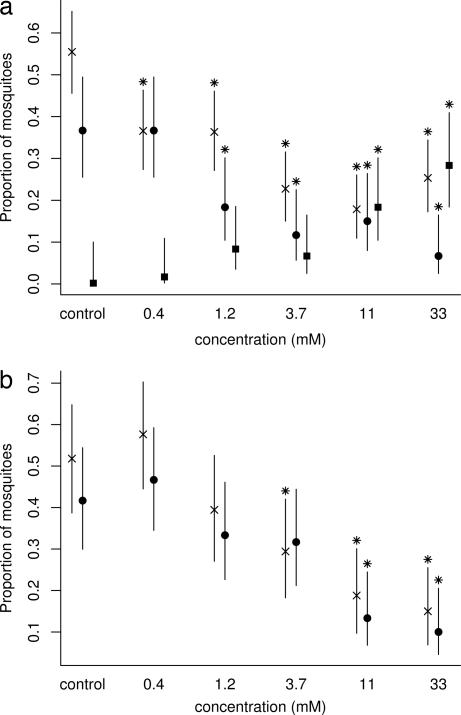
In the membrane-feeding tests, mosquitoes landed on membranes treated with natural PTX (+)-251D less frequently than on control membranes at all doses (P = 0.0085, 0.0077, <0.0001, <0.0001, and <0.0001, in increasing concentration, t test) (Fig. 2). For membranes treated with PTX (−)-251D, there was significantly less landing than for controls only at the three highest doses (P = 0.5314, 0.1879, 0.0164, 0.0003, and <0.0001, in increasing concentration, t test). Compared with controls, fewer mosquitoes fed on membranes treated with 1.2, 3.7, 11.0, and 33.0 mM solutions of the (+)-enantiomer (P = 1.0000, 0.0268, 0.0022, 0.0083, and 0.0003, in increasing concentration, χ2 test) or on membranes treated with the 11.0 and 33.0 mM solutions of the (−)-enantiomer (P = 0.5815, 0.3465, 0.2568, 0.0009, and 0.0002, in increasing concentration, χ2 test) (Fig. 2).
Fig. 2.
Effect of PTX 251D on A. aegypti in membrane-feeding tests. (a) Mean proportions and 95% confidence intervals of mosquitoes (n = 60) landing (×), feeding (●), and immobile (■) on silicone membranes treated with methanol (control) or PTX (+)-251D methanolic solutions. (b) Mean proportions and 95% confidence intervals of mosquitoes (n = 60) landing (×) and feeding (●) on silicone membranes treated with methanol (control) or methanolic solutions of PTX (−)-251D. An asterisk indicates a significant difference from control.
Some mosquitoes exposed to PTX (+)-251D-treated membranes turned over on their backs, slowly flailed or autotomized their legs, and, at the end of tests, failed to fly off membranes; they appeared moribund or dead. The numbers of mosquitoes that failed to fly off membranes at the two highest doses of this enantiomer were significantly higher than for the control membranes, from which all mosquitoes departed (P = 1.000, 0.1307, 0.2044, 0.0150, and 0.0027, in increasing concentration, t test) (Fig. 2). No mosquitoes were disabled on membranes during tests of the (−)-enantiomer.
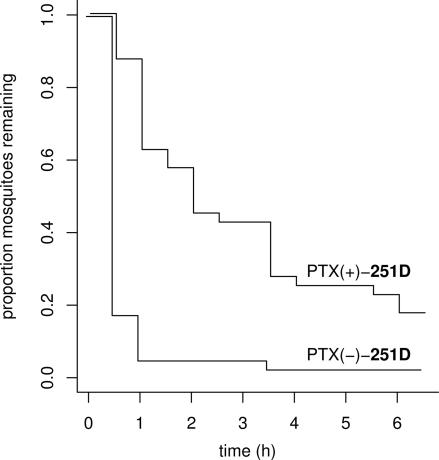
In the wire-contact tests, mosquitoes exhibited significantly greater latencies to fly off the substrate after exposure to the (+)-enantiomer (P < 0.0001, χ2 statistic, likelihood ratio test) (Fig. 3). Although the numbers of dead mosquitoes did not differ statistically between the PTX (+)-251D versus PTX (−)-251D treatments (5/40 versus 1/40, respectively; P = 0.1014, one-sided χ2 test with continuity correction), the proportions of legs autotomized did differ (0.175 versus 0.0125, respectively, P = 0.0174, one-sided χ2 test with continuity correction).
Fig. 3.
Effect of PTX 251D on A. aegypti in wire-contact tests. Shown are proportions of mosquitoes (n = 80) remaining on the floor of a plastic container during 6 h after a 3-min exposure to wire treated with 1.2 mM methanolic solutions of PTX (+)-251D or PTX (−)-251D.
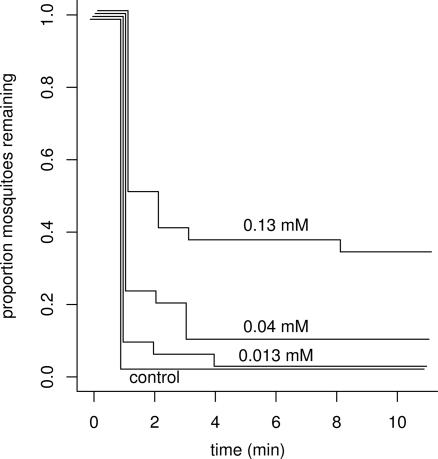
In our experiment with serial dilutions of PTX (+)-251D, significantly greater latencies to fly off the substrate were observed after exposure to the 0.04 mM and 0.13 mM treatments than to the control (P = 0.0380 and <0.0001, respectively, two-sided χ2 test on contrast) (Fig. 4). However, no significant difference was detected in the responses to the 0.013 mM treatment versus the control (P = 0.413).
Fig. 4.
Effect of PTX 251D on A. aegypti in wire-contact tests. Shown are proportions of mosquitoes (n = 120) remaining on the floor of a plastic container during 10 min after a 3-min exposure to a wire treated with methanol (control) or with 0.013, 0.04, and 0.13 mM methanolic solutions of PTX (+)-251D.
Discussion
The results of our tests with PTX 251D are consistent with studies showing that the skin secretions of frogs deter predatory and ectoparasitic arthropods (19). In addition to the field tests cited earlier on the responses of ants (5) and spiders (6) to D. pumilio, laboratory studies show that the skin secretions of Australian frogs (Litoria spp. and/or Uperoleia mjobergi) kill blow-flies on contact (20) or repel mosquitoes (21). The mosquito repellents emanating from these frogs are believed to be volatile terpenes (21).
PTX 251D is moderately volatile; however, our methods do not indicate whether mosquitoes detect it by olfaction. Nonetheless, compared with controls, both enantiomers of PTX 251D presented on silicone membranes reduced landing and feeding by A. aegypti. PTX (+)-251D deterred landing and feeding at lower concentrations than did the (−)-enantiomer, and it induced toxicosis, shown when mosquitoes failed to fly off experimental membranes. Toxicosis was not observed when mosquitoes were exposed to membranes treated with comparable doses of PTX (−)-251D. Similarly, mosquitoes confined with PTX (+)-251D-coated wires exhibited greater latencies to fly and a higher incidence of leg autotomy than did those confined with the (−)-enantiomer. Mosquitoes are reported to autotomize their legs in response to other noxious compounds, including volatile repellents (22).
The minimum concentration of PTX (+)-251D that induced toxicosis in A. aegypti in our experiments was 0.1 μg/cm2. Precisely how this value relates to the amounts of PTX (+)-251D encountered on the skin surface of frogs is unclear because this and other skin alkaloids are concentrated in granular glands embedded in the epidermis. The cutaneous concentrations of PTX (+)-251D also vary greatly among dendrobatid populations and species (13).
With these caveats in mind, we consider the plausibility of PTX (+)-251D as a defensive agent for dendrobatids by referring to the abundance of this compound reported in Epipedobates tricolor from Ecuador. The average skin concentration of PTX (+)-251D from one population of this frog was 37 μg per individual (23). Assuming the total skin area of an adult E. tricolor, which attains a snout-vent length of ≈2 cm, to be 4–6 cm2, we estimate cutaneous concentrations of PTX (+)-251D ranging from 6 to 9 μg/cm2. This range of values greatly exceeds the minimum toxic concentration of PTX (+)-251D that we observed for A. aegypti. Thus, we surmise that the amounts of this compound in the skin of E. tricolor are sufficient to deter biting mosquitoes. Further studies assessing the defensive value of cutaneous alkaloids should consider the combined and possibly synergistic effects of the diverse compounds present on various anuran species. In addition, deterrence of mosquitoes, such as Uranotaenia spp. (10), known routinely to feed on anurans could be tested with selected dendrobatid skin alkaloids.
Our results on the contact toxicity of PTX (+)-251D enantiomers with mosquitoes parallel those reported for mice injected with them. A 10 mg/kg dose of PTX (+)-251D administered s.c. to mice elicited convulsions, hyperalgesia, and death, whereas PTX (−)-251D at the same dose had no detectable effect (13). Pumiliotoxins are hypothesized to cause cardiac stimulation and, at high doses, convulsions, by prolonging the opening of voltage-sensitive sodium channels (13, 24). Other sites of action or selectivity at different sodium channel subtypes may underlie the diverse pharmacological activities of PTX (+)-251D. Whatever the mode of action, PTX (+)-251D appears to exhibit broad spectrum toxicity and, against arthropods, at least, contact toxicity consistent with a function in anuran chemical defense.
Materials and Methods
Mosquitoes.
Laboratory colonies of A. aegypti were reared at 28°C and 80% relative humidity and kept on a 12:12 (light/dark) photoperiod. Adult female mosquitoes, 5–16 days old, were maintained on a 10% sucrose solution presented on cotton pads. For the membrane-feeding experiments, the sucrose-soaked pads were replaced with water-moistened pads 48 h before mosquitoes were tested.
Chemicals.
PTXs (+)-251D and (−)-251D were synthesized as described in ref. 13.
Membrane-Feeding Tests.
All experiments were conducted at 0900–2100 hours in a walk-in incubator (26°C, 63–80% relative humidity) illuminated by fluorescent lights. In membrane-feeding experiments, mosquitoes were tested in a two-piece Plexiglas module (18). The top piece of the module consisted of six 4.5 × 4.0 × 5.0-cm chambers, the floors of which were fitted with a sliding door positioned over a circular opening (diameter, 3.5 cm). For each test, five female mosquitoes were aspirated into each chamber through an aperture, which then was plugged with corks wrapped in plastic film.
The bottom piece of the module was a 40 × 7 × 4-cm hollow platform supporting six circular wells (diameter, 3.8 cm; depth, 6 mm). Water (40°C) flowed through the central cavity of the platform at a rate of 215 ml/min. The wells were filled with 7 ml of 10% sucrose solution containing ATP (2.9 mg/ml). Fifty microliters of green dye (McCormick Food Color, Hunt Valley, MD) was added to each well so that the mosquitoes imbibing the sugar solution through the membranes could be identified when crushed at the end of a test.
Twenty-five microliters of methanol or PTX (+)-251D or PTX (−)-251D in 0.4, 1.2, 3.7, 11.0, and 33.0 mM methanolic solutions was applied by pipette to a 9.6-cm2 circular area on 0.1-mm-thick silicone membranes reinforced by nylon mesh (1.0, 3.1, 9.4, 28.6, and 85.9 nmol/cm2, respectively). The treated area of each membrane was laid over a well. The top piece of the module then was placed with floor openings of each chamber aligned with an underlying membrane-covered well. The sliding floors of the chambers then were opened, allowing access to the membranes. The number of mosquitoes landing on membranes was recorded each minute for 5 min.
At the conclusion of each test, the module was transferred to a freezer for 20 min. Mosquitoes were removed one at a time and crushed on white paper towels with forceps to determine whether their remains contained green dye.
Wire-Contact Tests.
Mosquitoes were aspirated, five at a time, through the main aperture (diameter, 0.5 cm) of a glass pipette, which then was plugged with cotton to create a 7.5-cm-long chamber. The tip of the pipette was broken off, leaving an aperture 0.2 cm in diameter at its narrow end. Mosquitoes acclimated in the pipette for 1 min.
Ten microliters of methanol or 1.2 mM methanolic solutions of PTX (+)-251D or PTX (−)-251D were applied by micropipette to a 7-cm segment of a 16-cm-long copper wire (diameter, 1 mm; area, 220 mm2). The wire was allowed to air-dry for 1 min and then was gently inserted through the narrow aperture of the pipette, thus forcing mosquitoes to contact it. After exposure for 3 min to PTX 251D enantiomers, the treated wires were gently withdrawn from the pipettes, the cotton plugs were removed, and the end of the pipette was inserted through a septum of a plastic cylindrical carton (height, 19 cm; diameter, 17 cm) covered with nylon mesh. Mosquitoes were lightly tapped out of the pipette into a plastic cup (height, 7 cm; diameter, 9 cm) placed inside the carton. The number of mosquitoes on the floor of the cup was monitored every 30 min for 6 h and then examined for dead mosquitoes and detached legs after 24 h.
In our last experiment, we attempted to estimate the concentration threshold of PTX (+)-251D that induces toxicosis in mosquitoes. Mosquitoes were tested with methanol and 0.013, 0.04, and 0.13 mM methanolic solutions of PTX (+)-251D as described in the preceeding experiment. The latencies of mosquitoes to fly off the substrate were observed for 10 min.
Statistics.
The proportions of mosquitoes feeding per trial in membrane-feeding tests were analyzed by using a standard generalized linear model with a logit link (25) with Proc Genmod in SAS (26). The χ2 statistics are contrasts with the methanol control. The number of mosquitoes landing on the membrane were repeated measures; five readings were obtained for each group of five mosquitoes. Thus, to avoid problems of dependencies arising from repeated measures, we summed the five readings, then divided each sum by 25 (maximum score) to create one proportion representing each group of five mosquitoes. The proportions then were transformed by using the standard variance-stabilizing transformation for proportions (sin−1 , where y is the proportion). The P values reported are contrasts with the methanol control (two-tailed t tests, 66 df). Data on flying mosquitoes were treated in the same manner as those landing on membranes. For these results, we tested 60 mosquitoes per dosage group (720 total for the two enantiomers).
The latencies of mosquitoes to fly off the substrate in our recovery and threshold tests were analyzed with Proc Phreg in SAS (26), which allows for censored (truncated) survival time, provides overall χ2 statistics, and can produce χ2 statistic contrasts with the control. We set statistical significance at α = 0.05 for all tests.
Acknowledgments
M. A. Donnelly, R. A. Saporito, and C. W. Myers shared field observations and/or photographs of frogs; J. P. Benante, R. Coleman, W. Dheranetra, M. Dowler, L. Jones, N. McLean-Cooper, E. Rowton, and J. Williams (Walter Reed Army Institute of Research) facilitated tests of mosquitoes; H. Brooks (Gorgas Memorial Library, Walter Reed Army Institute of Research) and D. T. Steere, Jr., (Smithsonian Libraries) provided valuable references; and H. M. Garraffo (National Institute of Diabetes and Digestive and Kidney Diseases) created Fig. 1. Work at the National Institutes of Health was supported by intramural funds from the National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviation
- PTX 251D
pumiliotoxin 251D.
Footnotes
The authors declare no conflict of interest.
References
- 1.Daly JW, Spande TF, Garraffo HM. J Nat Prod. 2005;68:1556–1575. doi: 10.1021/np0580560. [DOI] [PubMed] [Google Scholar]
- 2.Lüling K-H. Bonner Zool Beitr. 1971;22:161–174. [Google Scholar]
- 3.Wassersug RJ. Am Midl Nat. 1971;86:101–109. [Google Scholar]
- 4.Silverstone PA. Bull Nat Hist Mus Los Angeles County. 1975;21:1–55. [Google Scholar]
- 5.Fritz G, Rand AS, dePamphilis CW. Biotropica. 1981;13:158–159. [Google Scholar]
- 6.Szelistowski WA. Biotropica. 1985;17:345–346. [Google Scholar]
- 7.Darst CR, Cummings ME. Nature. 2006;440:208–211. doi: 10.1038/nature04297. [DOI] [PubMed] [Google Scholar]
- 8.Heatwole H, Shine R. Aust Zool. 1976;19:69–75. [Google Scholar]
- 9.Van Beurden E. Aust Zool. 1980;20:501–504. [Google Scholar]
- 10.Cupp EW, Zhang D, Yue X, Cupp MS, Guyer C, Sprenger TR, Unnasch TR. Am J Trop Med Hyg. 2004;71:272–276. [PMC free article] [PubMed] [Google Scholar]
- 11.Christophers SR. Aedes aegypti (L.), The Yellow Fever Mosquito: Its Life History, Bionomics, and Structure. Cambridge, UK: Cambridge Univ Press; 1960. [Google Scholar]
- 12.Daly JW, Myers CW, Whittaker N. Toxicon. 1984;25:1023–1095. doi: 10.1016/0041-0101(87)90265-0. [DOI] [PubMed] [Google Scholar]
- 13.Daly JW, Garraffo HM, Spande TF, Clark VC, Ma J, Ziffer H, Cover JF., Jr Proc Natl Acad Sci USA. 2003;100:11092–11097. doi: 10.1073/pnas.1834430100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daly JW, Andriamaharavo NR, Andriantsiferana M, Myers CW. Am Mus Novitates. 1996;3177:1–34. [Google Scholar]
- 15.Garraffo HM, Spande TF, Daly JW, Baldessari A, Gros EG. J Nat Prod. 1993;56:357–373. doi: 10.1021/np50093a008. [DOI] [PubMed] [Google Scholar]
- 16.Bargar TM, Lett RM, Johnson PL, Hunter JE, Chang CP, Pernich DJ, Sabol MR, Dick MR. J Agric Food Chem. 1995;43:1044–1051. [Google Scholar]
- 17.Daly JW, Myers CW. Science. 1967;196:970–973. doi: 10.1126/science.156.3777.970. [DOI] [PubMed] [Google Scholar]
- 18.Weldon PJ, Aldrich JR, Klun JA, Oliver JE, Debboun M. Naturwissenschaften. 2003;90:301–304. doi: 10.1007/s00114-003-0427-2. [DOI] [PubMed] [Google Scholar]
- 19.Weldon PJ, Carroll JF. In: Insect Repellents: Principles, Methods, and Uses. Debboun M, Frances SP, Strickman D, editors. Boca Raton, FL: CRC; 2007. pp. 45–73. [Google Scholar]
- 20.Williams CR, Wallman JF, Tyler MJ. Austral J Entomol. 1998;37:85–89. [Google Scholar]
- 21.Williams CR, Smith BPC, Best SM, Tyler MJ. Biol Lett. 2006;2:242–245. doi: 10.1098/rsbl.2006.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudolfs W. Bull NJ Agric Exp Station. 1930;496:1–24. [Google Scholar]
- 23.Daly JW, Tokuyama T, Fujiwara T, Highet RJ, Karle IL. J Am Chem Soc. 1980;102:830–836. [Google Scholar]
- 24.Daly JW, Gusovsky F, McNeal ET, Secunda S, Bell M, Creveling CR, Nishizawa Y, Overman LE, Sharp MJ, Rossignol DP. Biochem Pharmacol. 1990;40:315–326. doi: 10.1016/0006-2952(90)90694-g. [DOI] [PubMed] [Google Scholar]
- 25.McCullagh P, Nelder JA. Generalized Linear Models. London: Chapman & Hall; 1989. [Google Scholar]
- 26.SAS Institute. SAS/STAT User's Guide, Version 8. Cary, NC: SAS; 2000. [Google Scholar]