Abstract
The ability of synapses to sustain signal propagation relies on rapid recycling of transmitter-containing presynaptic vesicles. Clathrin- and dynamin-mediated retrieval of vesicular membrane has an undisputed role in synaptic vesicle recycling. There is also evidence for other modes of vesicle retrieval, including bulk retrieval and the so-called kiss-and-run recycling. Whether dynamin in required for these other modes of synaptic vesicle endocytosis remains unclear. Here, we have tested the role of dynamin in synaptic vesicle endocytosis by using a small molecule called dynasore, which rapidly inhibits the GTPase activity of dynamin with high specificity. Endocytosis after sustained or brief stimuli was completely and reversibly blocked by dynasore in cultured hippocampal neurons expressing the fluorescent tracer synaptopHluorin. By contrast, dynasore had no effect on exocytosis. In the presence of dynasore, low-frequency stimulation led to sustained accumulation of synaptopHluorin and other vesicular proteins on the surface membrane at a rate predicted from net exocytosis. These vesicular components remained on surface membranes even after the stimulus was terminated, suggesting that all endocytic events rely on dynamin during low-frequency activity as well as in the period after it. Ultrastructural analysis revealed a reduction in the density of synaptic vesicles and the presence of endocytic structures only at synapses that were stimulated in the presence of dynasore. In sum, our data indicate that dynamin is essential for all forms of compensatory synaptic vesicle endocytosis including any kiss-and-run events.
Keywords: clathrin, hippocampal, vesicle recycling, kiss-and-run
Despite substantial progress in understanding the mechanistic basis of the synaptic vesicle cycle, several fundamental questions remain. Evidence for a “classical” pathway of vesicle recycling through clathrin-mediated endocytosis comes from a variety of studies and techniques, and many molecular players in this process have been identified (1–4). There is also evidence for other modes of vesicle retrieval, including bulk retrieval (5, 6) and the so-called kiss-and-run recycling (7–12). Mechanistic studies of kiss-and-run recycling have been difficult, in part, because of a lack of agreement on the exact definition of this process.
The final mechanical step of the separation of a budding clathrin-coated vesicle from the plasma membrane relies on the GTPase dynamin (13, 14). Studies using a temperature-sensitive dynamin ortholog shibire in Drosophila have indicated that dynamin function is critical for synaptic vesicle endocytosis (15, 16). If and how dynamin is involved in alternate modes of vesicle recycling such as kiss-and-run is unclear (17–22), especially at small central synapses where these modes have been proposed (8, 12, 23, 24). At several giant synapses, the role of dynamin in synaptic vesicle endocytosis has been assayed by using nonhydrolyzable analogs of GTP such as guanosine 5′-[γ-thio]triphosphate (GTPγS) or peptides that interfere with the interaction of dynamin with critical protein partners (20, 22, 25). Both approaches suffer from the problem of irreversible effects and lack of specificity: GTPγS will affect numerous GTPases at a synapse, and peptides can have nonspecific or indirect effects such as squelching. In addition, similar studies have not been done in small central synapses (but see ref. 26), which contain a small pool of vesicles and might rely on fast recycling (8, 23, 27).
Here, we have tested the role of dynamin in synaptic vesicle endocytosis at hippocampal synapses by using dynasore, a highly specific, cell-permeable inhibitor of dynamin GTPase function that blocks clathrin-mediated endocytosis in nonneuronal cells (28). We have found that all forms of compensatory synaptic vesicle endocytosis in rodent hippocampal neurons are blocked by dynasore.
Results
Dose-Dependent and Reversible Block of Endocytosis by Dynasore.
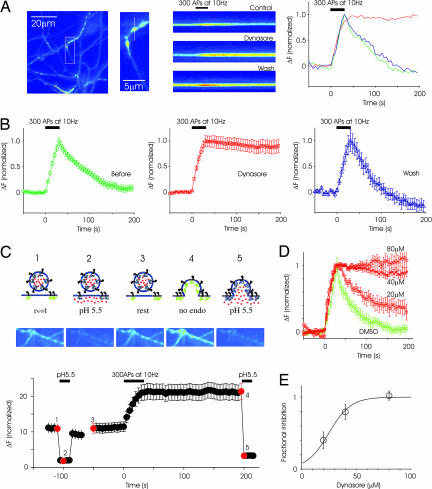
We used the genetically encoded pH-sensitive fluorescent probe synaptopHluorin (spH; ref. 29) as a reporter of exocytosis and endocytosis in transiently transfected neurons. In synapses expressing spH, exocytosis triggered by a stimulus of 300 action potentials (APs) at 10 Hz caused an increase in fluorescence intensity that returned to resting levels as vesicles were endocytosed and reacidified (Fig. 1A). In the presence of 40 μM dynasore, these same synapses exhibited a normal stimulus-triggered increase in fluorescence intensity, but the fluorescence remained elevated even after the termination of stimulus. This result suggested that synaptic vesicle endocytosis was blocked by reduction of the GTPase activity of dynamin. To test whether the block was reversible, the preparation was washed with fresh medium for 25 min. Another round of stimulation resulted in an increase in fluorescence intensity followed by a decline (Fig. 1B), demonstrating the reversibility of the effect of dynasore.
Fig. 1.
Dynasore reversibly blocks endocytosis in hippocampal synapses. (A Left) Image of axons and presynaptic terminals labeled with spH. (A Center) Higher magnification image of a region from which a single bouton was chosen for linescan display. The line scan shows the time course of fluorescence intensity at the same bouton in control condition, after applying dynasore and after wash. A stimulus of 300 APs at 10 Hz was applied at the time indicated by the black bar. (A Right) Normalized fluorescence intensity at this bouton is plotted in graphical form as a function of time. (B) From the same experiment as in A, spH responses were averaged across 45 boutons. (C) Sustained poststimulus increase in fluorescence is caused by higher levels of surface spH rather than blocking vesicle reacidification. The logic of the experiment is illustrated at the top. Neurons were briefly exposed to the acidic solution before stimulation (C 1 and 2), resulting in a reversible reduction in baseline fluorescence (C3). Stimulation caused an increase in fluorescence that failed to return to baseline. Subsequent addition of acidic medium led to a decrease in fluorescence similar to that seen before stimulation (C 4 and 5). Images corresponding to the red dots on the response plot (Bottom) are displayed above. (D) The block of endocytosis is dose-dependent. DMSO alone does not block endocytosis. Each graph is from a single experiment (at least 25 boutons each). (E) Average data from multiple experiments (n = 3 separate experiments at each concentration, >50 boutons each). The fraction of inhibition was calculated as the remaining fluorescence intensity at 200 s after stimulation. The half-maximal effectiveness was ≈30 μM.
Because spH reports pH and not endocytosis per se, it is possible that some of the effects of dynasore may be related to impaired vesicle reacidification; that is, vesicles could have been endocytosed normally but not reacidified. To rule out this possibility, we used a cell-impermeant acid to quench surface fluorescence. After treating synapses with 80 μM dynasore for 5 min, we exposed synapses to the acidic solution, which quenched the surface spH reversibly (Fig. 1C2). Then, we stimulated neurons with 300 APs, and we confirmed the block of fluorescence recovery. If the sustained increase in fluorescence intensity in the poststimulus period was the result of an accumulation of spH in the plasma membrane, this fluorescence should be quenched by reexposure to acidic extracellular solution. Indeed, poststimulus replacement of normal solution with acidic media led to a decrease in fluorescence similar to that seen before stimulation (Fig. 1C5). The reduction in fluorescence after lowering the pH in the poststimulus period was 95 ± 1.4% of that expected from acid application in the prestimulus period. This observation indicated that spH remains largely on the surface in the poststimulus period, and it confirms that dynasore leads to a block of vesicle endocytosis. These results with spH were further corroborated by experiments with the stryryl dye FM4-64, which could not be internalized in the presence of dynasore (Fig. 6, which is published as supporting information on the PNAS web site).
To characterize further the efficacy of the endocytic block by dynasore, we stimulated neurons in the presence of different concentrations of dynasore. The block was dose-dependent, with full inhibition seen at 80 μM and a half-maximal inhibition of ≈30 μM (Fig. 1D). Control experiments indicated that DMSO alone at the maximum concentration used (0.4%) did not block endocytosis (Fig. 1D). These results are in close agreement with extensive experiments in nonneuronal cells as well as cell-free in vitro assays of GTP hydrolysis (28).
Dynasore Has No Immediate Effect on Exocytosis.
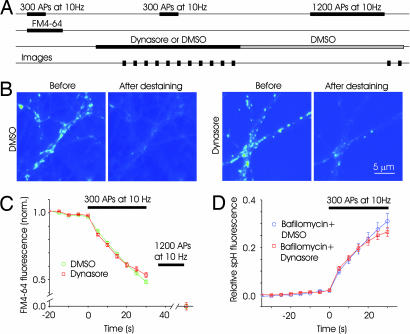
To confirm that the observed effects of dynasore were the result of its perturbation of the endocytic machinery rather than an indirect reflection of its effect on exocytosis, we performed experiments with FM4-64 (30). In this method, measures of exocytosis and endocytosis are obtained by examining the rate of uptake or release of dye by synaptic vesicles (31, 32). We chose to use FM labeling rather than more classical electrophysiological experiments for two reasons. First, it offers a more direct index of exocytosis because presynaptic terminals are examined directly. Second, we wanted to avoid any effect that a dynamin inhibitor may have on postsynaptic receptor recycling, which might alter the amplitude of synaptic currents. We prelabeled synapses with FM4-64 by using a stimulus of 300 APs at 10 Hz in control solution (31, 32). After removing surface dye by washing, we stimulated the preparation with 1,200 stimuli at 10 Hz in the presence of dynasore or DMSO, causing release of FM4-64 because of vesicle exocytosis (Fig. 2A). The loss of dye from dynasore-treated synapses was very similar to that from control synapses until ≈300 stimuli, indicating that dynasore had no immediate effect on exocytosis (Fig. 2 B and C).
Fig. 2.
Dynasore has no immediate effect on exocytosis. (A) Schematic of the time course of FM-labeling experiments. A loading stimulus of 300 APs at 10 Hz was used to label synaptic vesicles with FM4-64. After the wash step, medium containing either 0.4% DMSO or 80 μM dynasore was added. After a 5–10-min wait, a destaining stimulus of 300 APs at 10 Hz was given, and images were obtained every 5 s. Normal solution containing DMSO was then perfused for 25 min to allow reversal of the effects of dynasore. A final round of 1,200 APs was delivered to release any remaining dye and to obtain baseline values of fluorescence. (B) Fluorescence image from sample experiments depicting FM-labeled boutons before and after destaining stimulus in the two conditions. (C) Time course of fluorescence change for synapses in DMSO and dynasore. The decay of fluorescence, an index of exocytosis, was similar for the two conditions in the early phase, indicting that dynasore had no immediate effect on exocytosis (n = 4 experiments, 200 boutons each). (D) Exocytosis measured with spH was unaffected by dynasore. Synapses expressing spH were stimulated (300 APs) in the presence of bafilomycin to get an index of net exocytosis. Fluorescence change was normalized to a total value obtained after neutralizing all vesicles with NH4Cl. Fluorescence changes in the presence of dynasore were similar to those in bafilomycin (dynasore: n = 3 experiments, 80 boutons; bafilomycin: n = 3 experiments, 70 boutons).
We also used the method of alkaline trapping at spH-expressing synapses (33, 34) to rule out a direct effect of dynasore on exocytosis. Alkaline trapping of vesicles by bafilomycin allows for the estimation of cumulative exocytosis throughout the stimulation; endocytosed vesicles do not contribute to additional changes in fluorescence because they cannot be reacidified. We compared the fluorescence response of spH synapses to a stimulus of 300 APs at 10 Hz in the presence of bafilomycin with the response in the presence of dynasore. Fluorescence changes were normalized to the total pool of vesicles, which was estimated by neutralizing all vesicles with ammonium chloride (34). As shown in Fig. 2D, the fluorescence changes in the two conditions were indistinguishable until near the end of the stimulus. Together, the FM4-64 and spH experiments indicate that dynasore has minimal effect on exocytosis for the length of stimuli used here.
Dynasore Blocks Endocytosis During Mild Activity.
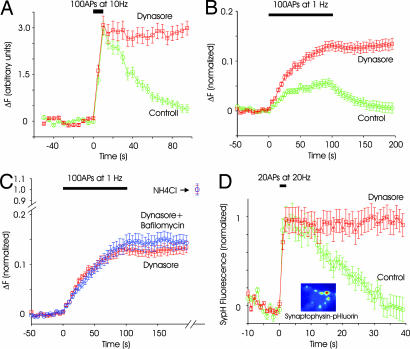
Endocytosis triggered by brief stimulation is faster than that triggered by longer stimuli (33, 35, 36). We stimulated synapses at 10 Hz for a brief period of 10 s, releasing <30% of the releasable pool of vesicles. Endocytosis after this briefer stimulus was completely blocked by dynasore, with minimal effect on exocytosis (Fig. 3A). We also asked whether vesicle reuptake would be inhibited when stimulation occurred at low frequencies, a stimulus regime in which kiss-and-run is thought to predominate (8, 23, 24). Because the signal-to-noise ratio was not sufficient to detect exocytosis of individual vesicles, we relied on the cumulative increase in fluorescence resulting from many stimuli (37). In control synapses, 1-Hz stimulation resulted in only a slight increase in fluorescence intensity, which reached a plateau in ≈20 s (Fig. 3B), presumably because endocytosis can match exocytosis at low frequencies (37). By contrast, 1-Hz stimulation caused a continual increase in fluorescence intensity in the presence of dynasore (Fig. 3C). A likely reason for this difference is that dynamin-dependent endocytosis is the main pathway of vesicle retrieval at low frequencies, and blocking endocytosis causes an irreversible rise in fluorescence.
Fig. 3.
Endocytosis is blocked by dynasore during mild stimulation. (A) Endocytosis after a brief stimulation of 100 APs at 10 Hz is blocked by dynasore (n = 2 experiments, 36 boutons). (B) In control conditions, low-frequency stimulation of synapses (100 APs at 1 Hz) causes little change in fluorescence intensity (green circles) because endocytosis and exocytosis are balanced. In the presence of dynasore, fluorescence intensity increases steadily during stimulation, and it remains steady after stimulation (red squares). Fluorescence intensity was normalized to the total fluorescence measured after neutralizing all vesicles with NH4Cl (see C). n = 3 experiments, 110 boutons. (C) Stimulating synapses 100 times at 1 Hz in the presence of both dynasore and bafilomycin (blue circles) led to an increase in fluorescence intensity that was similar to that with dynasore alone (red squares). For each condition, fluorescence was normalized to the value obtained by neutralizing all vesicles with NH4Cl (n = 3 experiments, 110 boutons for dynasore only and 60 boutons for bafilomycin and dynasore). (D) Retrieval of a different vesicle protein, spH, was also blocked by dynasore. A brief stimulus of 20 APs at 20 Hz gives rise to an increase in fluorescence, followed by decay to baseline. The addition of dynasore blocked the return without affecting the rising phase. (Inset) Example image of synapses expressing sypH. Boutons were more clearly defined than for spH because the surface fraction was much lower for spH (n = 3 experiments, 90 boutons).
To estimate the proportion of endocytic events that were dynamin-dependent during 1-Hz stimulation, we again used the method of the alkaline trapping (33). If some endocytosis continued to occur in the presence of dynasore, the addition of bafilomycin should increase the rate of increase of fluorescence during 1-Hz stimulation. We found that the rise in fluorescence intensity at synapses in the presence of both dynasore and bafilomycin was indistinguishable from that in synapses treated with dynasore alone (Fig. 3C). This result offers strong evidence that all of synaptic vesicle endocytosis during 1-Hz stimulation is blocked by dynasore.
Dynasore Blocks Reuptake of Different Vesicular Proteins.
In all of the experiments above, we probed endocytosis by using synaptobrevin-2 fused to pHluorin as the cargo protein. To determine whether other vesicle proteins behave similarly, we used a fusion protein of synaptophysin and pHluorin (sypH). Synaptophysin is thought to be better sorted to synaptic vesicles that synaptobrevin (38), and therefore it may have different routes of endocytosis. SypH had a better signal-to-noise ratio than spH because there was less surface fluorescence (Fig. 3D Inset). A robust increase in fluorescence was observed even after a brief stimulus (20 APs at 20 Hz), which then relaxed back to baseline with a t½ of ≈15 s. Endocytosis was again completely blocked in the presence of dynasore (Fig. 3D). A third vesicle protein synaptotagmin fused to pHluorin yielded identical results, exhibiting complete block of endocytosis after the addition of dynasore (Fig. 7, which is published as supporting information on the PNAS web site). These data also show that the recapture of different vesicular cargo proteins is equally impaired by dynasore, and it is therefore unlikely that certain forms of endocytosis would have escaped notice because of the choice of a specific tracer.
Depletion of Releasable Vesicles After Stimulation in Dynasore.
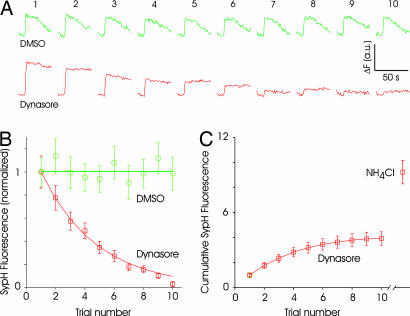
If dynasore blocks all compensatory synaptic vesicle endocytosis, synapses should run out of releasable vesicles when stimulated repeatedly. To confirm this hypothesis, we subjected synapses expressing sypH to multiple rounds of a brief stimulus (cycles of 20 APs at 20 Hz, spaced 2 min apart). We chose this method rather than continuous stimulation to avoid the possibility of inactivating calcium channels or the release machinery and to separate exocytosis and endocytosis temporally. Surface appearance of sypH remained undiminished for many cycles of stimulation in the absence of dynasore (Fig. 4A). By contrast, the surface appearance of sypH diminished to virtually zero during the course of consecutive cycles of stimulation in the presence of dynasore (Fig. 4A). Average data from several experiments indicated that responses diminished to 35 ± 5% after four trials, and no response could be discerned by the 10th trial, indicating depletion of releasable vesicles (Fig. 4B). Although evoked responses were abolished, an additional pool of vesicles could be revealed by alkalinizing the synapse with NH4Cl (Fig. 4C), suggesting that not all vesicles at a synapse are recruited even when endocytosis is blocked (32, 34, 39). The additional fluorescence change induced by NH4Cl was equivalent to 5.3 ± 0.94 times the response to the first trial in dynasore.
Fig. 4.
Rundown of synaptic responses in the presence of dynasore. (A) Example of responses of sypH-expressing synapses to repeated stimulation (20 APs at 20 Hz) delivered every minute. In control conditions (DMSO), responses remain stable. But responses diminish with repeated stimulation in the presence of dynasore. Note the lack of endocytosis in the presence of dynasore. Each set of traces is an average of 20 synapses from one experiment. Numbers on top refer to the trial number. (B) Average responses normalized to the first response clearly shows the decay of responses in the presence of dynasore. DMSO data were fitted with a straight line and dynasore data with a single exponential (n = 2 and 3 experiments, 40 and 60 boutons, respectively, for DMSO and dynasore). (C) Integrated response over the 10 trials reaches an asymptotic value of ≈4 (normalized to the first response) for dynasore-treated synapses. The addition of NH4Cl after the 10th trial reveals an additional pool of vesicles that is similar in size to the total released vesicles.
Endocytic Intermediates Appear After Stimulation in the Presence of Dynasore.
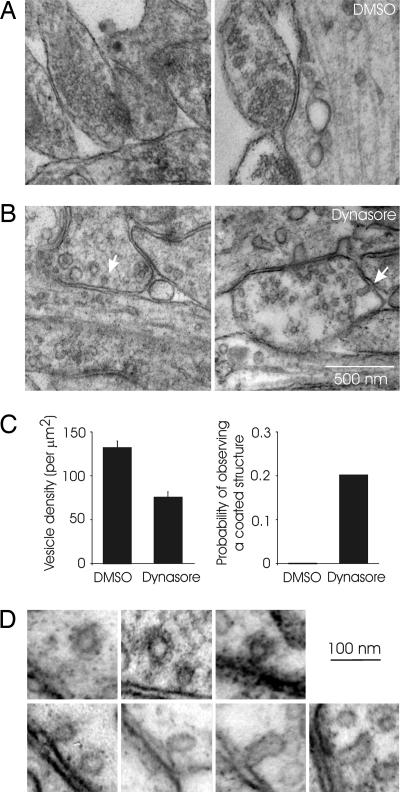
To look for endocytic intermediates, we visualized synapses by using electron microscopy after stimulation at low frequency (300 APs at 1 Hz) in the presence of dynasore or DMSO. Control cultures fixed after stimulation in the presence of DMSO had readily identifiable synapses with tight clusters of vesicles and intact active zones (Fig. 5A). By contrast, synapses in dynasore-treated cultures were depleted of vesicles (Fig. 5B), with their density approximately half that of synapses from vehicle-treated cultures (Fig. 5C; DMSO, 132 ± 7 vesicles per μm2; dynasore, 76 ± 6 vesicles per μm2; n = 45, P < 0.001). The plasma membrane near the active zone often appeared ruffled, and we could identify coated pits and Ω-shaped structures resembling those observed in nonneuronal cells (28) only in dynasore-treated cultures (Fig. 5D). Whether or not these Ω-shaped structures were coated could not be ascertained because of their low abundance. In no case did we observe an Ω-shaped structure with elongated and ringed necks such as those seen in synapses treated with GTPγS (40). Coated structures in presynaptic terminals were observed in 9 of 45 synapses examined in dynasore-treated cultures, and they were not seen in >50 synapses examined in DMSO-treated cultures. In rare instances, we could also identify tubular invaginations from plasma membrane (Fig. 5D). Interestingly, larger coated pits were often present in the postsynaptic dendritic regions in dynasore-treated samples, possibly representing endocytic intermediates in postsynaptic receptor traffic as described in ref. 41.
Fig. 5.
Stimulation in the presence of dynasore reveals endocytic intermediates. (A) Examples of synapses from a culture that was stimulated with 300 APs at 1 Hz in the presence of DMSO. Synapses with densely packed vesicles could be easily identified. (B) Synapses were sparsely populated with vesicles in samples prepared from cultures stimulated (300 APs, 1 Hz) in the presence of dynasore. Arrows point to putative endocytic intermediates such as coated pits (Left) and invaginations (Right). (C) The density of synaptic vesicles in identified presynaptic boutons (Left) and the probability of observing coated structures at synapses (Right) from cultures stimulated in DMSO or dynasore. (D) A gallery of endocytic structures such as coated pits, Ω-shaped structures, and tubular invaginations.
Discussion
The occurrence of rapid endocytosis after brief stimulation (time constant of ≈1 s) has prompted investigators to invoke a clathrin-independent endocytic pathway at neuronal synapses (22, 35, 42, 43). In parallel, other studies have suggested a fast mode of recycling termed kiss-and-run, in which a synaptic vesicle undergoing exocytosis never quite collapses onto the plasma membrane, and it is retrieved intact (7, 23, 44). The molecular mechanisms in kiss-and-run recycling remain unspecified. In this work, we present the an experimental manipulation in which endocytosis is blocked completely at a small central synapse, and we suggest that dynamin is essential for all forms of compensatory synaptic vesicle endocytosis.
Our finding of endocytic block after strong stimulation is best explained by perturbations in the clathrin- and dynamin-mediated pathway. Stimuli of the sort used here are known to cause redistribution of many synaptic vesicle proteins (45–47) as well as clathrin (48), suggesting full collapse of the vesicle onto the plasma membrane and subsequent retrieval by the classical pathway. Ultrastructurally, there is evidence for the formation of a variety of endocytic compartments, including coated vesicles and larger cisternae (6, 39) after strong stimulation. Our data indicate that endocytosis after strong stimulation, whether it is by clathrin-coated vesicles or by bulk invagination of large pieces of membrane, depends on dynamin.
Kiss-and-run recycling, where synaptic vesicles are retrieved without fully collapsing onto the plasma membrane, is thought to occur more frequently (as a proportion of the total events) during low-frequency stimulation or during brief stimulation (23). Two recent studies found evidence for the existence of a pool of synaptic vesicle proteins on the plasma membrane that is selectively and readily retrieved after brief or mild stimuli (49, 50). Partially preassembled endocytic structures waiting to be recovered after a stimulus could, in principle, account for the rapid endocytosis elicited under some conditions. We found that endocytosis is blocked by dynasore during low-frequency stimulation as well as after brief stimulation. Ultrastructurally, the presence of coated pits after low-frequency stimulation argues for a role of clathrin-dependent endocytosis even under these conditions. We did not find Ω-shaped or coated structures at the active zone, but this result could be caused by undersampling. The relative paucity of coated pits at synapses is not surprising because their density is expected to be rather low. Assuming that coated vesicles can arise anywhere within a synaptic bouton and that ≈50 vesicles are released by the stimuli used here, the frequency of coated pits would be ≈0.1 per section (section thickness, 70 nm).
We note that dynasore had no effect on exocytosis, suggesting that acute and rapid block of dynamin function does not have an immediate effect on synaptic vesicle exocytosis. This observation also rules out any effect of dynasore on action potential propagation, calcium channel function, or exocytic machinery. Extensive in vitro experiments by Macia et al. (28) have demonstrated the specificity of dynasore for the dynamin family proteins, and our data are entirely consistent with highly specific block of dynamin function. A block of endocytosis with negligible effect on exocytosis should lead to a depletion of synaptic vesicles during sustained activity. We confirmed this prediction by using ultrastructural analysis, which suggested that the density of synaptic vesicles was reduced (Fig. 5C). The reduction in vesicle density was ≈40%, but an accurate count of the total number of vesicles can only be obtained by careful serial reconstruction of synapses. We were able to find clathrin-coated structures after endocytic block during low-frequency stimulation, which clearly indicates that this canonical pathway mediates endocytosis even under conditions in which rapid recycling of vesicles occurs (23).
Repeated stimulation of synapses in the presence of dynasore led to a progressive loss of fusion events, indicating the importance of local recycling of vesicles for sustained release. In the absence of compensatory endocytosis, releasable vesicles cannot be replenished. Interestingly, responses approached zero even though there was still a population of vesicles at synapses that are revealed by alkalinization of the internal pool of vesicles (Fig. 4C). This observation is compatible with previous proposals of the existence of a resting population of vesicles that is resistant to stimulus-evoked release (32, 34, 39). The morphological or molecular basis of the heterogeneity of vesicles is unknown, and we expect that the use of dynasore might facilitate future investigation.
The identity of the specific isoforms or splice variants of dynamin involved in endocytosis triggered by different stimuli cannot be resolved here because dynasore is equally effective at perturbing dynamin 1 and 2 (28). From experiments in neuroendocrine cells, dynamin 1 has been proposed to be involved in rapid endocytosis that is clathrin-independent and dynamin 2 to be involved in endocytosis that follows extended stimulation (43). Our results offer strong evidence for an essential role for dynamin in all forms of synaptic vesicle endocytosis. By blocking both dynamin 1 and dynamin 2 acutely and rapidly with dynasore, we prevented recovery of synaptic vesicles after exocytosis. In this blocked state, we found coated pits and Ω-shaped structures, similar to those found in nonneuronal cells treated with dynasore.
In summary, we found that dynasore, a small molecule inhibitor of dynamin GTPase, blocks synaptic vesicle endocytosis completely, without an immediate effect on exocytosis. Dynasore was equally effective in blocking endocytosis triggered by high- as well as low-frequency stimulation and brief as well as sustained stimulation, which suggests that dynamin may be essential for all forms of compensatory synaptic vesicle endocytosis.
Materials and Methods
Cultures and Transfection.
Hippocampal neurons were dissociated from 1- to 2-day-old rats by using methods described in ref. 46. All animal experiments were approved by the Harvard University standing committee on the use of animals in research and training. The following fluorescent fusion proteins were introduced into neurons between days 6 and 8 by using the calcium phosphate transfection method: spH, sypH, and synaptotagmin–pHluorin. Experiments were performed at 14–28 days in vitro. All experiments were done at room temperature (20–22°C).
Stimulation and Imaging.
Coverslips were mounted on a custom-built chamber equipped with a pair of parallel platinum electrodes. The normal external medium contained 136 mM NaCl, 2.5 mM KCl, 10 mM Hepes, 10 mM d-glucose, 2 mM CaCl2, 1.3 mM MgCl2, and the glutamate receptor blockers 50 μm 2-amino-5-phosphonovaleric acid and 10 μm mM 6-cyano-7-nitroquinoxaline-2,3-dione. The pH was adjusted to 7.2–7.3. For the ammonium chloride-containing solution, 50 mM NaCl was replaced with NH4Cl, and the pH was adjusted to be the same as that of the normal external medium. The acidic solution contained 2-[N-morpholino]ethanesulfonic acid in lieu of Hepes, and it was brought to a final pH of 5.5. Bafilomycin A (Calbiochem, San Diego, CA) was stored frozen and diluted to a final concentration of 1 μM (0.2% DMSO). Dynasore was stored frozen at a concentration of 20 mM in 20-μl aliquots and diluted to a final concentration of 20–80 μM (0.1–0.4% DMSO) in medium that contained no serum or albumin.
Electrical pulses (1-ms, 70–90 V, bipolar) were controlled by software SLIDEBOOK (Intelligent Imaging Innovations, Santa Monica, CA) and administered through a SD9 stimulator (Grass Instruments, Quincy, MA). pHluorin and FM4-64 were excited with a xenon lamp, with the filter set no. 51008 from Chroma (Rockingham, VT). For pHluorin, the excitation filter was 465–487 nm, and the emission filter was 500–570 nm. For FM4-64, the excitation filter was 605–648 nm, and emitted light collected through a long-pass filter above 670 nm. A cooled charge-coupled device camera (PCO Sensicam, Cooke Corporation, Romulus, MI) on an Olympus inverted microscope (IX 70; Olympus, Melville, NY) with an oil lens (×60, 1.25 NA, Olympus) was used to acquire images.
Unless otherwise noted, a test stimulus (different pattern depending on the experiment) was administered to spH-expressing neurons in normal external solution. After a 5 min poststimulus recovery period, the extracellular media was replaced with external solution supplemented with either DMSO or dynasore for 5–10 min. Solutions containing bafilomycin (with DMSO or dynasore where required) were added a minute before onset of the next stimulus. For reversibility experiments, the media was washed out and substituted with normal external solution immediately after the dynasore image acquisition. The postwash stimulus was applied after a 25-min period. Where indicated, ammonium chloride solution (and DMSO or dynasore) was added immediately after image acquisition.
Relative surface fluorescence before and after stimulation in the presence of dynasore was determined as follows. A short series of images (four images taken every second) were taken of synapses expressing spH in the presence of various extracellular solutions, all containing 80 μM dynasore. Images were first acquired in normal medium, then after replacement with acidic solution to quench the surface fluorescence, and again with normal medium. After a resting period of 5 min, a stimulus of 300 APs at 10 Hz was delivered, and images were acquired. Finally, additional images were obtained after the medium was again exchanged with acidic solution.
FM 4-64 Labeling.
Neurons in normal external medium containing 10 μM FM 4-64 (Molecular Probes, Eugene, OR) were stimulated with 300 APs at 10 Hz to load the dye into the synaptic vesicles. After leaving the FM dye for an additional 30 s, the coverslip was perfused with normal extracellular solution for 10 min to remove excess dye. The perfusion was stopped, and medium containing either 0.4% DMSO or 80 μM dynasore was added. After 5–10 min, a destaining stimulus of 1,200 APs at 10 Hz was given to monitor vesicle exocytosis. Immediately after image acquisition, neurons were washed with 5 ml of normal medium containing 0.4% DMSO for 25 min, and a final stimulus of 1,200 APs at 10 Hz was delivered. Images taken after the final stimulus were used to determine baseline values for relative releasable fluorescence.
Electron Microscopy.
Hippocampal cultures expressing spH were first imaged optically as described above to confirm the block of endocytosis by dynasore. A stimulus of 300 APs at 1 Hz was used. Cells were then fixed in 2.5% glutaraldehyde/2% paraformaldehyde in 0.1 M sodium cacoldylate buffer at pH 7.4. Cells were then processed for thin-section electron microscopy after epon resin embedding and osmium contrast enhancement. Seventy-nanometer sections were cut horizontal to the coverslip on an Reichert Ultracut S (Leica Microsystems, Bannockburn, IL). The micrographs were acquired digitally with a Tecnai Spirit microscope (FEI Company, Hillsboro, OR) at a direct magnification of ×6,800 or ×23,000.
Data Analysis.
Images were analyzed with custom routines in MATLAB (The MathWorks, Inc., Natick, MA) as described in ref. 46. Fluorescence intensities reported are the average of the fluorescence values for all pixels contained in a region of interest drawn around a synaptic bouton. Electron micrographs were analyzed by using ImageJ (National Institutes of Health, Bethesda, MD). Synapses were visually identified, and a perimeter was drawn around vesicle clusters. Vesicle densities were then estimated by dividing the number of vesicles within this region by the area. Data are presented as mean ± SEM, unless otherwise stated. Statistical comparisons were made by using Student's t test.
Supplementary Material
Acknowledgments
We thank Charles F. Stevens and Yongling Zhu (The Salk Institute, La Jolla, CA) for the sypH construct and Juergen Klingauf (MPI, Göttingen, Germany) for synaptotagmin-pHluorin. We also thank Henry Pelish for resynthesis and chemical and biological characterization of dynasore and Maria Ericksson for preparing the samples for electron microscopy. This work was supported by National Institutes of Health Grant NS39059 and grants from the National Science Foundation (IBN-0133742) and the Klingenstein Fund (all to V.N.M.) and National Institutes of Health Grant GM075252 (to T.K.).
Abbreviations
- AP
action potential
- GTPγS
guanosine 5′-[γ-thio]triphosphate
- spH
synaptopHluorin
- sypH
synaptophysin and pHluorin.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Murthy VN, De Camilli P. Annu Rev Neurosci. 2003;26:701–728. doi: 10.1146/annurev.neuro.26.041002.131445. [DOI] [PubMed] [Google Scholar]
- 2.Royle SJ, Lagnado L. J Physiol. 2003;553:345–355. doi: 10.1113/jphysiol.2003.049221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sudhof TC. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 4.Schweizer FE, Ryan TA. Curr Opin Neurobiol. 2006;16:298–304. doi: 10.1016/j.conb.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Holt M, Cooke A, Wu MM, Lagnado L. J Neurosci. 2003;23:1329–1339. doi: 10.1523/JNEUROSCI.23-04-01329.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takei K, Mundigl O, Daniell L, De Camilli P. J Cell Biol. 1996;133:1237–1250. doi: 10.1083/jcb.133.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fesce R, Grohovaz F, Valtorta F, Meldolesi J. Trends Cell Biol. 1994;4:1–4. doi: 10.1016/0962-8924(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 8.Aravanis AM, Pyle JL, Tsien RW. Nature. 2003;423:643–647. doi: 10.1038/nature01686. [DOI] [PubMed] [Google Scholar]
- 9.Stevens CF, Williams JH. Proc Natl Acad Sci USA. 2000;97:12828–12833. doi: 10.1073/pnas.230438697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klyachko VA, Jackson MB. Nature. 2002;418:89–92. doi: 10.1038/nature00852. [DOI] [PubMed] [Google Scholar]
- 11.Jackson MB, Chapman ER. Annu Rev Biophys Biomol Struct. 2006;35:135–160. doi: 10.1146/annurev.biophys.35.040405.101958. [DOI] [PubMed] [Google Scholar]
- 12.Gandhi SP, Stevens CF. Nature. 2003;423:607–613. doi: 10.1038/nature01677. [DOI] [PubMed] [Google Scholar]
- 13.Hinshaw JE. Annu Rev Cell Dev Biol. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Praefcke GJK, McMahon HT. Nat Rev Mol Cell Biol. 2004;5:133–147. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- 15.Koenig JH, Ikeda K. J Neurobiol. 1983;14:411–419. doi: 10.1002/neu.480140602. [DOI] [PubMed] [Google Scholar]
- 16.Koenig JH, Ikeda K. J Neurosci. 1989;9:3844–3860. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Artalejo CR, Henley JR, McNiven MA, Palfrey HC. Proc Natl Acad Sci USA. 1995;92:8328–8332. doi: 10.1073/pnas.92.18.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham ME, O’Callaghan DW, McMahon HT, Burgoyne RD. Proc Natl Acad Sci USA. 2002;99:7124–7129. doi: 10.1073/pnas.102645099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan TA. Proc Natl Acad Sci USA. 2003;100:2171–2173. doi: 10.1073/pnas.0530260100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita T, Hige T, Takahashi T. Science. 2005;307:124–125. doi: 10.1126/science.1103631. [DOI] [PubMed] [Google Scholar]
- 21.Holroyd P, Lang T, Wenzel D, De Camilli P, Jahn R. Proc Natl Acad Sci USA. 2002;99:16806–16811. doi: 10.1073/pnas.222677399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jockusch WJ, Praefcke GJ, McMahon HT, Lagnado L. Neuron. 2005;46:869–878. doi: 10.1016/j.neuron.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Harata NC, Choi S, Pyle JL, Aravanis AM, Tsien RW. Neuron. 2006;49:243–256. doi: 10.1016/j.neuron.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 24.Richards DA, Bai J, Chapman ER. J Cell Biol. 2005;168:929–939. doi: 10.1083/jcb.200407148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shupliakov O, Low P, Grabs D, Gad H, Chen O, David C, Takei K, De Camilli P, Brodin L. Science. 1997;276:259–263. doi: 10.1126/science.276.5310.259. [DOI] [PubMed] [Google Scholar]
- 26.Mozhayeva MG, Matos MF, Liu X, Kavalali ET. J Neurosci. 2004;24:1680–1688. doi: 10.1523/JNEUROSCI.3801-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sara Y, Mozhayeva MG, Liu X, Kavalali ET. J Neurosci. 2002;22:1608–1617. doi: 10.1523/JNEUROSCI.22-05-01608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Miesenbock G, De Angelis DA, Rothman JE. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 30.Betz WJ, Bewick GS. Science. 1992;255:200–203. doi: 10.1126/science.1553547. [DOI] [PubMed] [Google Scholar]
- 31.Ryan TA, Smith SJ. Neuron. 1995;14:983–989. doi: 10.1016/0896-6273(95)90336-4. [DOI] [PubMed] [Google Scholar]
- 32.Murthy VN, Sejnowski TJ, Stevens CF. Neuron. 1997;18:599–612. doi: 10.1016/s0896-6273(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 33.Sankaranarayanan S, Ryan TA. Nat Neurosci. 2001;4:129–136. doi: 10.1038/83949. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Burrone J, Tyler WJ, Hartman KN, Albeanu DF, Murthy VN. Proc Natl Acad Sci USA. 2005;102:6131–6136. doi: 10.1073/pnas.0501145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neves G, Gomis A, Lagnado L. Proc Natl Acad Sci USA. 2001;98:15282–15287. doi: 10.1073/pnas.261311698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun JY, Wu XS, Wu LG. Nature. 2002;417:555–559. doi: 10.1038/417555a. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Alfonso T, Ryan TA. Neuron. 2004;41:943–953. doi: 10.1016/s0896-6273(04)00113-8. [DOI] [PubMed] [Google Scholar]
- 38.Pennuto M, Bonanomi D, Benfenati F, Valtorta F. Mol Biol Cell. 2003;14:4909–4919. doi: 10.1091/mbc.E03-06-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harata N, Pyle JL, Aravanis AM, Mozhayeva M, Kavalali ET, Tsien RW. Trends Neurosci. 2001;24:637–643. doi: 10.1016/s0166-2236(00)02030-0. [DOI] [PubMed] [Google Scholar]
- 40.Takei K, McPherson PS, Schmid SL, De Camilli P. Nature. 1995;374:186–190. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]
- 41.Bredt DS, Nicoll RA. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 42.Klingauf J, Kavalali ET, Tsien RW. Nature. 1998;394:581–585. doi: 10.1038/29079. [DOI] [PubMed] [Google Scholar]
- 43.Artalejo CR, Elhamdani A, Palfrey HC. Proc Natl Acad Sci USA. 2002;99:6358–6363. doi: 10.1073/pnas.082658499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palfrey HC, Artalejo CR. Curr Biol. 2003;13:R397–9. doi: 10.1016/s0960-9822(03)00320-8. [DOI] [PubMed] [Google Scholar]
- 45.Sankaranarayanan S, Ryan TA. Nat Cell Biol. 2000;2:197–204. doi: 10.1038/35008615. [DOI] [PubMed] [Google Scholar]
- 46.Li Z, Murthy VN. Neuron. 2001;31:593–605. doi: 10.1016/s0896-6273(01)00398-1. [DOI] [PubMed] [Google Scholar]
- 47.Star EN, Newton AJ, Murthy VN. J Physiol. 2005;569:103–117. doi: 10.1113/jphysiol.2005.092528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mueller VJ, Wienisch M, Nehring RB, Klingauf J. J Neurosci. 2004;24:2004–2012. doi: 10.1523/JNEUROSCI.4080-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wienisch M, Klingauf J. Nat Neurosci. 2006;9:1019–1027. doi: 10.1038/nn1739. [DOI] [PubMed] [Google Scholar]
- 50.Fernandez-Alfonso T, Kwan R, Ryan TA. Neuron. 2006;51:179–186. doi: 10.1016/j.neuron.2006.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.