Abstract
In some individuals, fearful experiences (e.g., combat) yield persistent and debilitating psychological disturbances, including posttraumatic stress disorder (PTSD). Early intervention (e.g., debriefing) after psychological trauma is widely practiced and argued to be an effective strategy for limiting subsequent psychopathology, although there has been considerable debate on this point. Here we show in an animal model of traumatic fear that early intervention shortly after an aversive experience yields poor long-term fear reduction. Extinction trials administered minutes after aversive fear conditioning in rats suppressed fear acutely, but fear suppression was not maintained the next day. In contrast, delivering extinction trials 1 day after fear conditioning produced an enduring suppression of fear memory. We further show that the recent experience of an aversive event, not the timing of the extinction intervention per se, inhibits the development of long-term fear extinction. These results reveal that the level of fear present at the time of intervention is a critical factor in the efficacy of extinction. Importantly, our work suggests that early intervention may not yield optimal outcomes in reducing posttraumatic stress, particularly after severe trauma.
Keywords: aversive learning, context, fear conditioning, memory retrieval, stress
Traumatic events such as military combat, motor vehicle accidents, or sexual assault can lead to debilitating psychological disturbances, including posttraumatic stress disorder (PTSD) (1). Although PTSD is estimated to develop in <10% of individuals experiencing trauma in the general population (2), it presents at significantly higher rates in individuals exposed to extremely traumatic events (such as combat). For example, rates of PTSD as high as 17% have been reported in military personnel 3–4 months after returning from combat (3). It is not surprising then that traumatic events exact an incredible toll on mental health, affecting millions of people worldwide (1–3).
Because of the staggering costs and consequences of PTSD and other anxiety disorders, clinical interventions to reduce the long-term consequences of psychological trauma are essential. As a first line of defense against the development of mental illness in the aftermath of a traumatic event, it has been argued that early interventions (such as psychological debriefing) are critical to manage the stress response to trauma (4, 5). In a typical debriefing session, victims of a traumatic event are encouraged to talk about their experience in a supportive group setting, which presumably facilitates psychological recovery from the trauma. Although early intervention is intuitively reasonable, considerable work has challenged the efficacy of debriefing in curbing the development of PTSD after trauma (for review, see ref. 6). Moreover, little work has systematically examined whether early interventions, whatever form they take, are more effective than delayed interventions in reducing the incidence of psychopathology after trauma (7). Indeed, intervening too early, particularly when the intense and acute stress of the experience has not waned, might even exacerbate relapse of fear (6–9). Nonetheless, recent work in rats suggests that an early intervention may more effectively suppress fear than a delayed intervention would (10).
In the present study, we sought to compare the efficacy of early and delayed interventions in reducing fear associated with a traumatic event. To address this question, we used an animal model of traumatic fear, Pavlovian fear conditioning in rats (11–14). In this form of associative learning, innocuous stimuli (i.e., conditioned stimuli, CSs) that predict aversive events (i.e., unconditioned stimuli, USs) come to yield fear responses themselves. This type of learning may be involved in the development of pathological fear in patients with a variety of anxiety disorders, including PTSD and panic disorder (15–17). Extinction training, in which CSs are presented without the US, suppresses conditioned responses learned during fear conditioning. Considerable evidence indicates that fear conditioning and extinction yield excitatory and inhibitory memories, respectively, and that these memories compete with each other for expression in behavior. In most cases, extinction does not erase fear memory. Nonetheless, extinction is an important component of exposure therapy in humans and is emerging as a powerful model for understanding the mechanisms of fear suppression relevant to the treatment of anxiety disorders (18–22). Hence, this behavioral paradigm affords many advantages, because it allows us to precisely control both the nature of the traumatic event (i.e., conditioning) and the timing of the intervention (i.e., extinction) in a clinically relevant model of traumatic fear.
Results
Experiment 1: Immediate or Delayed Extinction After Fear Conditioning.
The first experiment aimed to compare the efficacy of extinction training at two different times after fear conditioning. We were particularly interested in whether an early intervention delivered minutes after fear conditioning would produce superior extinction relative to a standard delayed intervention (24 h). Rats were submitted to a standard fear conditioning procedure in which an auditory CS was paired with a noxious footshock US in a novel chamber. After either a short (15 min) or long (24 h) delay, half of the animals received 45 extinction trials in which the CS was presented alone; the other half of the animals remained in the chambers without the presentation of either the CS or US (these animals served as a no-extinction control group). Forty-eight hours after conditioning, rats were tested for their fear to the CS by assessing freezing behavior, which is manifested as somatomotor immobility (except for breathing). For this retention test, rats were once again returned to the conditioning chambers and presented with five auditory CSs.
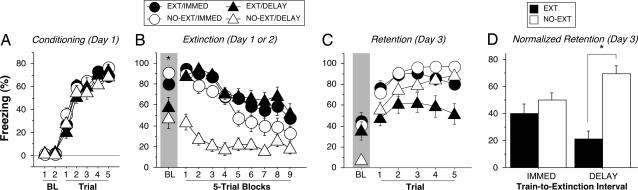
Freezing behavior during the conditioning session is shown in Fig. 1A. There were very low levels of freezing behavior before the first conditioning trial; freezing behavior emerged only after the first conditioning trial and steadily increased in frequency thereafter. During the extinction session (Fig. 1B), which was conducted in the conditioning context either 15 min (immediate) or 24 h (delayed) after fear conditioning, group differences emerged. All animals exhibited high levels of fear before the onset of extinction trials, a consequence of fear conditioned to the testing context. However, recently conditioned rats exhibited significantly higher levels of freezing behavior before the first extinction trial compared with rats in the delayed extinction groups [F(1, 60) = 20.7, P < 0.0001]. Once extinction training commenced, CS presentations yielded robust freezing behavior in both the immediate and delayed extinction groups, and there was an equivalent decline in freezing in both groups across the session [extinction × interval × block, F(8, 480) = 1.9]. Shock-induced sensitization of fear contributed to the elevation of fear in the immediate groups, which potentiated fear above that generated by context fear alone in the delay groups. Rats that were placed in the boxes 15 min after conditioning, but not exposed to the CS, exhibited a similar pattern of freezing behavior to animals in both of the extinction groups [interval × extinction interaction, F(1, 60) = 16.0, P < 0.0005].
Fig. 1.
Immediate or delayed extinction after fear conditioning. (A) Freezing behavior on the conditioning day. Data are 1-min averages for the period before (baseline, BL) and after each of five tone–shock conditioning trials. (B) Freezing behavior during the extinction session, which occurred either 15 min (IMMED) or 24 h (DELAY) after conditioning. Control rats did not receive CS presentations during extinction (NO-EXT). (C) Freezing behavior during the retention test 48 h after conditioning. (D) Baseline freezing data were averaged and subtracted from the average freezing across test trials to yield normalized freezing for the retention test data shown in C. All data are means ± SEM. ∗, P < 0.05.
Despite similar levels of fear reduction during the extinction session, rats that had received immediate or delayed extinction training differed with respect to their retention of the extinction memory (Fig. 1C). Forty-eight hours after conditioning, only rats that had received the delayed extinction procedure exhibited a significant reduction in freezing relative to nonextinguished controls when presented with the CS [interval × extinction, F(1, 60) = 10.6, P < 0.002; Fig. 1D]. Hence, fear memories exhibited substantial spontaneous recovery (i.e., a return in conditional responding with the passage of time after extinction) after an early intervention, but remained inhibited in rats with a 24-h delay between conditioning and the extinction intervention.
Experiment 2: Retention Testing with a Common Extinction Test Interval.
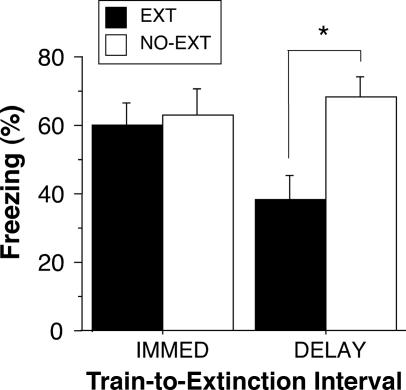
The different levels of extinction in the immediate and delayed groups cannot be explained by the time elapsed between fear conditioning and retention testing; this interval was constant in both groups. However, the design of Experiment 1 confounded the interval between extinction training and the retention test. That is, animals in the immediate extinction group were tested 48 h after extinction, whereas those in the delayed group were tested only 24 h after extinction. It is possible that the longer test interval in the immediate group allowed for more spontaneous recovery of fear than the shorter test interval in the delay group. In Experiment 2, we examined this possibility by equating the test interval in the immediate and delayed extinction groups. The experiment was identical to Experiment 1, except that rats in both the immediate and delayed groups were tested 48 h after extinction training.
Behavior during the conditioning and extinction sessions was similar to that reported in Experiment 1 (data not shown). As shown in Fig. 2, rats in the immediate extinction condition exhibited significantly weaker extinction than those animals in the delayed extinction condition. Planned comparisons indicated that only rats in the delayed extinction condition exhibited significant extinction relative to their no-extinction controls (P < 0.05). Thus, early extinction trials failed to yield long-term extinction, even when the test interval was equated among the immediate and delayed groups.
Fig. 2.
Retention testing with a common extinction test interval. Shown is freezing behavior during the retention test 48 h after extinction. The extinction test interval was equated in rats that were extinguished either 15 min (IMMED) or 24 h (DELAY) after conditioning. Control rats did not receive CS presentations during extinction (NO-EXT). Data were normalized as in Fig. 1D. All data are means ± SEM. ∗, P < 0.05.
Experiment 3: Massed or Distributed Extinction Trials Immediately After Fear Conditioning.
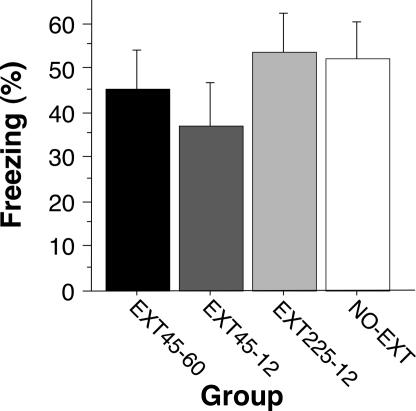
Recent work suggests that massed extinction training [delivering CS-alone trials with a short interstimulus interval (ISI)] produces more robust long-term extinction than extinction training with distributed trials (23). In Experiment 3, we examined the possibility that delivering many massed extinction trials might enable extinction in the immediate groups. To this end, we replicated the immediate condition in Experiment 1 (45 trials with 1-min ISIs), and also examined groups receiving either 45 or 225 massed extinction trials (12-sec ISI); the time all animals spent in the conditioning context was equated across the groups (i.e., animals in the short ISI groups were left in the boxes after their extinction trials). Animals in all groups exhibited similar decrements in freezing behavior during the extinction training session (data not shown). However, as shown in Fig. 3, neither massing the extinction trials (45 trials, 12-sec ISI) nor increasing the number of extinction trials (225 trials, 12-sec ISI) yielded long-term retention of extinction relative to the no-extinction controls. These data reveal that neither massed nor distributed (Experiments 1 and 2) extinction trials yield long-term fear suppression when delivered shortly after training.
Fig. 3.
Massed or distributed extinction trials immediately after fear conditioning. Rats received 45 or 225 extinction trials 15 min after fear conditioning. For two groups of rats (EXT45–12 and EXT225–12), the extinction trials were massed (12-sec ISI). The EXT45–60 group was treated identically to that in Experiment 1 (45 trials; 60-sec ISI). Total time in the extinction context was equated in all of the groups. The graph displays freezing behavior during the retention test 24 h after extinction. Data were normalized as in Fig. 1D. All data are means ± SEM.
Experiment 4: Reducing Fear Before Immediate Extinction.
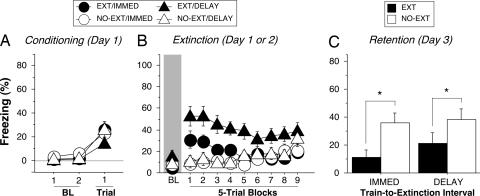
In Experiment 1, we observed much higher levels of fear before the onset of extinction training among rats in the immediate condition compared with those in the delayed condition (see Fig. 1B, baseline). This result is likely due to the sensitization of fear produced by recent shock summating with fear conditioned to the context. It has recently been reported in both rats and humans that the arousal of fear before extinction training can interfere with the development of long-term extinction (24, 25). We therefore investigated whether the different levels of fear at the outset of extinction training contributed to the different levels of long-term extinction in the immediate and delayed groups. In Experiment 4, rats were submitted to the identical behavioral procedures described in Experiment 1, except that they received only a single conditioning trial (Fig. 4A), and extinction training and testing were conducted outside of the conditioning context. The goal of these manipulations was to reduce the level of fear before the onset of extinction training.
Fig. 4.
Reducing fear before immediate extinction. (A) Freezing behavior on the conditioning day. Data are 1-min averages for the periods before (baseline, BL) and after a single tone–shock conditioning trial. (B) Freezing behavior during the extinction session in a novel context, which occurred either 15 min (IMMED) or 24 h (DELAY) after conditioning. Control rats were exposed to the context but did not receive CS presentations during extinction (NO-EXT). (C) Freezing behavior during the retention test 48 h after conditioning; data from the retention test were normalized as in Fig. 1D. All data are means ± SEM. ∗, P < 0.005.
As shown in Fig. 4B (see baseline), reducing the number of conditioning trials and shifting the context between conditioning and extinction greatly reduced freezing behavior at the outset of extinction training in both immediate and delay groups. Importantly, reducing fear before the onset of extinction training yielded robust extinction, even in the immediate extinction group [extinction, F(1, 60) = 8.9, P < 0.005; extinction × interval, F(1, 60) = 0.6; Fig. 4C]. Therefore, early extinction is effective in producing long-term fear suppression when fear is relatively low at the onset of extinction training. In fact, extinction obtained under these conditions did not exhibit spontaneous recovery in a retention test conducted 1 week after the first retention test (data not shown). This finding is consistent with a recent study showing that early extinction training produces a lasting fear suppression that does not show either spontaneous recovery or renewal upon a change in context (10).
Experiment 5: Arousing Fear Before Delayed Extinction.
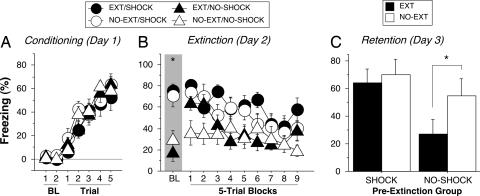
If the level of fear at the outset of extinction training influences the long-term retention of extinction, then arousing fear before a delayed extinction intervention should impair extinction memory. To test this hypothesis, we examined whether arousing fear before a delayed extinction intervention compromises long-term extinction. Rats were submitted to the same procedures as the delayed groups in Experiment 1, except that they were exposed to additional unsignaled footshocks in a novel context 15 min before the extinction session.
Conditioning proceeded normally in all of the rats (Fig. 5A). As shown in Fig. 5B, exposing rats to footshock 15 min before extinction training elevated their levels of fear before the onset of the extinction trials [F(1, 28) = 32.6, P < 0.0001]. Rats that received extinction trials 15 min after unsignaled shock decreased their fear over the course of the extinction session and reached levels of freezing similar to those of rats that received unsignaled shock but did not receive extinction trials. Nonetheless, rats in the extinction (EXT/SHOCK) condition showed substantial recovery when tested 24 h later. As shown in Fig. 5C, only rats that were not shocked before extinction training exhibited a normal reduction in fear during the retention test (planned comparisons, P < 0.05). Collectively, these results indicate that the level of acute fear at the time of the extinction intervention determines both the nature and extent of extinction memory. Moreover, these experiments indicate that the conditioning–extinction interval per se is not the critical factor regulating the efficacy of extinction, but that recent fear appears to mitigate long-term extinction memory.
Fig. 5.
Arousing fear before delayed extinction. (A) Freezing behavior on the conditioning day. Data are 1-min averages for the periods before (baseline, BL) and after each of five tone–shock conditioning trials. (B) Freezing behavior during the extinction session. Extinction training was conducted in the context in which the rats had been conditioned a day earlier. Fifteen minutes before the extinction session, rats received either five unsignaled footshocks (SHOCK) in a novel context or exposure without shock (NO-SHOCK) in that context. Control rats did not receive CS presentations during extinction (NO-EXT). (C) Freezing behavior during the retention test 48 h after conditioning. All data are means ± SEM. ∗, P < 0.05.
Discussion
The major finding of the present work is that long-term extinction is minimal when extinction training is conducted shortly after fear conditioning in rats. This deficit in long-term extinction appears to be related to the level of fear present at the outset of extinction training, rather than the interval between conditioning and extinction per se. These results indicate that attempts to extinguish fear shortly after a traumatic experience may not be effective, particularly if the trauma is extreme.
Interestingly, recent work by Davis and colleagues (10) in another fear-conditioning paradigm in rats, fear-potentiated acoustic startle, has revealed that the properties of extinction also depend on the interval in between conditioning and extinction. In this study, short intervals between conditioning and extinction yielded a form of extinction that was both enduring (i.e., it did not spontaneously recover with the passage of time) and insensitive to context shifts that normally attenuate extinction. Although there was a trend for weaker extinction with early intervention, our observations would appear to be at odds with the relatively robust extinction observed by these investigators with early extinction. But this disparity can be explained when one considers that Davis and colleagues used relatively weak footshocks during conditioning, which is typical in the fear-potentiated startle paradigm. Although these investigators did not measure fear during the extinction session, it is reasonable to assume that their conditioning procedure limited fear before the extinction session. And, as we observed in Experiment 4, an early intervention does yield extinction if the conditioning procedure does not arouse fear before the extinction session; early interventions appear to fail only when there are high levels of fear at the outset of extinction training. It is also possible that the greater number of conditioning trials used by Davis and colleagues influenced subsequent extinction. Together, these reports reveal that both the nature and magnitude of long-term extinction depend on an interaction between the timing of extinction relative to conditioning and the level of fear present when extinction trials are delivered (9). This interaction has clinical relevance, because it suggests that, although an early intervention may be optimal after mild trauma, a delayed intervention may be more suitable after a severe trauma.
A key question from a theoretical point of view is whether the arousal of fear before extinction training interferes with extinction learning (i.e., learning an inhibitory CS–US association), the consolidation of the extinction memory, or the later retrieval of the extinction memory. Because rats do reduce their fear response to the CS during extinction training (independently of when extinction trials are administered relative to training), it is unlikely that they simply fail to encode inhibitory associations. Therefore, the decrement in long-term extinction is either a failure to consolidate the extinction memory or a generalization decrement from extinction to testing that interferes with the retrieval of the extinction memory. The latter possibility is particularly compelling, insofar as there is substantial evidence that the inhibitory associations acquired during extinction are modulated by both time and context (16, 26, 27). Indeed, some theoretical accounts of spontaneous recovery after extinction predict that there will be greater spontaneous recovery of conditional responding when the interval between conditioning and extinction is short (28, 29). Consistent with this view, Rescorla (30) has recently reported that there is greater spontaneous recovery in responding to a CS that is extinguished 1 day versus 8 days after conditioning in an appetitive conditioning paradigm. Although these results are consistent with what we have observed in the present experiments, it is unlikely that the interval between conditioning and extinction alone accounts for our results. As we have shown, a critical variable, at least in our hands, is the level of fear present before the delivery of extinction trials. Nonetheless, it is reasonable to suggest that the deficit in long-term extinction in both cases is related to a failure to retrieve the extinction memory during the retention test. This possibility awaits further examination.
It is important to note that other models of associative learning predict that either short intervals between conditioning and extinction or high levels of background fear (that has been aroused by another excitatory CS or a fearful context, for example) will enhance extinction (31, 32). In Wagner's SOP (Standard Operating Procedures) model, for example, inhibitory associations between the CS and US are more likely to occur if CS-alone trials occur shortly after exposure to the US. And in the Rescorla–Wagner model, high levels of fear before the onset of extinction should strongly predict footshock when the CS is presented, resulting in especially large decrements in associative strength to the CS when it is presented in the absence of the US. There is some evidence for the latter effect in appetitive conditioning procedures (33), and it has recently been shown that compound presentation of excitatory CSs during extinction yields greater extinction than extinction of either element alone (34). Therefore, it will be important to use other measures (e.g., summation, retardation) to determine whether CSs that undergo extinction shortly after conditioning under high levels of fear gain any inhibitory value, and if so, the retrieval processes that work against the expression of that inhibition during retention testing.
From a neurobiological perspective, it is surprising that our early extinction manipulation did not produce more effective extinction. Indeed, it is well known that memories (including fear memories) are most susceptible to disruption within an hour of encoding (35, 36). Neurobiological studies (37, 38) have recently shown that extinction training reverses some of the biochemical changes that develop during conditioning in brain structures such as the amygdala that are essential for fear conditioning (11–14). And, as already noted, delivering CS-alone trials shortly after fear conditioning can produce a form of extinction that appears to be more an erasure of fear memory than an acquisition of inhibition (10). Thus, the influence of CS-alone trials on fear memory may be determined by the degree to which those trials either disrupt cellular consolidation of the conditioning memory or engage new inhibitory learning that permits extinction. By this view, the present experiments suggest that high levels of fear prevent CS-alone trials that are delivered shortly after conditioning from disrupting cellular consolidation, and it remains to be seen how these conditions influence the inhibitory associations learned during extinction training.
Psychological interventions are not always effective when administered shortly after a traumatic event (6–9). The present work indicates that recent fear, interfering with either the consolidation or retrieval of long-term extinction memories, may be the cause of this result. Of course, we have not examined the longevity of fear suppression obtained with delayed extinction training (we assessed behavior up to 48 h after extinction), and understanding the factors that contribute to a suppression of fear lasting weeks to months is important when developing clinical interventions. Likewise, in our experiments, interposing a 24-h delay in the delivery of the intervention was sufficient to enable fear suppression (lasting at least 2 days). Whether a 24-h delay is always optimal is not clear, and our data suggest that this interval will critically depend on the duration and extent of acute stress associated with trauma. Indeed, for people who experience severe trauma, this interval may extend beyond days or even weeks (39). Clearly, the modulation of extinction by concurrent levels of fear and stress has important implications for optimizing clinical interventions for psychological trauma in humans.
Methods
Subjects.
The subjects were 240 male Long–Evans rats (Harlan–Sprague–Dawley, Indianapolis, IN) weighing 250–330 g. They were housed in individual cages on a 14-h light/10-h dark cycle (lights on at 7:00 a.m.), and allowed food and water ad libitum. During the first 5 days, they were handled for 10 sec to habituate them to the experimenter.
Apparatus.
Eight identical observation chambers (30 × 24 × 21 cm; Med Associates, St. Albans, VT) were used in all experiments. The chambers were constructed of aluminum and Plexiglas and were situated in sound-attenuating cabinets located in a brightly lit and isolated room. The floor of each chamber consisted of 19 stainless steel rods (4 mm in diameter) spaced 1.5 cm apart (center to center). Rods were wired to a shock source and solid-state grid scrambler for the delivery of footshock US (0.5 sec, 1 mA). A speaker mounted outside a grating in one wall of the chamber was used for the delivery of acoustic CS (2 sec, 80 dB, 2 kHz). Illumination, odor, and ambient noise were manipulated to create two distinct contexts for some of the experiments.
Each conditioning chamber rested on a load-cell platform that was used to record chamber displacement in response to each rat's motor activity, therefore allowing our detection of freezing behavior. Freezing was determined during each 1-min interval after the CS offset during conditioning, extinction, and the retention test and during the minutes preceding the first CS presentation during extinction training.
Behavioral Procedures.
Rats were submitted to three phases of training: fear conditioning, extinction, and an extinction retention test. All of these phases were conducted in the same context in Experiments 1–3 and Experiment 5; in Experiment 4, fear conditioning was conducted in a different context than extinction and retention testing. For fear conditioning, rats received one (Experiment 4) or five (Experiments 1–3 and 5) tone–footshock trials (62-sec intertrial interval) beginning 3 min after being placed in the chambers. For extinction (EXT), rats received 45 tone-alone presentations (60-sec ISI) either 15 min (IMMED) or 24 h (DELAY) after conditioning (again with a 3-min baseline preceding the extinction trials). Rats that received immediate extinction trials were transported home 2 min after the last footshock and returned to the conditioning context (Experiment 1–3 and 5) or a novel context (Experiment 4) 15 min later for extinction. Rats in the delay condition received extinction training 24 h after conditioning in the conditioning context (Experiments 1–3 and 5) or a novel context (Experiment 4). In Experiment 3, rats received either 45 (60-sec or 12-sec ISI) or 225 (12-sec ISI) extinction trials 15 min after conditioning; time in the extinction context was equated among the groups. In Experiment 5, the rats were placed in a novel context and were either shocked (SHOCK) or not shocked (NO-SHOCK) 15 min before being returned to the conditioning chamber for delayed extinction trials. Under the no-extinction condition (NO-EXT), rats were placed in the chamber for the same amount of time as the EXT rats but were not exposed to the tone CS. Two days after conditioning, all rats were returned to the extinction context and exposed to five CS-alone presentations 3 min after placement in the chambers. Retention test freezing was averaged across the five CS trials and subtracted from the 3-min baseline. All behavioral data are expressed as means ± SEM.
Acknowledgments
We thank Drs. Michael Davis, Robert A. Rescorla, Terry E. Robinson, Martin Sarter, and R. Fred Westbrook for critical commentary. This work was supported by National Institutes of Health Grant R01 MH065961 (to S.M.).
Abbreviations
- PTSD
posttraumatic stress disorder
- CS
conditioned stimulus
- US
unconditioned stimulus
- ISI
interstimulus interval.
Footnotes
The authors declare no conflict of interest.
References
- 1.McNally RJ. Annu Rev Psychol. 2003;54:229–252. doi: 10.1146/annurev.psych.54.101601.145112. [DOI] [PubMed] [Google Scholar]
- 2.Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Adreski P. Arch Gen Psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- 3.Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. N Engl J Med. 2004;351:13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- 4.Campfield KM, Hills AM. J Trauma Stress. 2001;14:327–340. doi: 10.1023/A:1011117018705. [DOI] [PubMed] [Google Scholar]
- 5.Everly GS, Jr, Mitchell JT. Critical Incident Stress Management (CISM): A New Era and Standard of Care in Crisis Intervention. 2nd Ed. Ellicott City, MD: Chevron; 1999. [Google Scholar]
- 6.McNally RJ, Bryant RA, Ehlers A. Psychol Sci Publ Inter. 2003;4:45–79. doi: 10.1111/1529-1006.01421. [DOI] [PubMed] [Google Scholar]
- 7.Gray MJ, Litz BT. Behav Modif. 2005;29:189–215. doi: 10.1177/0145445504270884. [DOI] [PubMed] [Google Scholar]
- 8.Bisson JI, Jenkins PL, Alexander J, Bannister C. Br J Psychiatry. 1997;171:78–81. doi: 10.1192/bjp.171.1.78. [DOI] [PubMed] [Google Scholar]
- 9.Rothbaum BO, Davis M. Ann NY Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- 10.Myers KM, Ressler KJ, Davis M. Learn Mem. 2006;13:216–223. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maren S. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 12.Fanselow MS, Poulos AM. Annu Rev Neurosci. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- 13.LeDoux JE. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 14.Davis M. J Clin Neurophysiol. 1998;15:378–387. doi: 10.1097/00004691-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Grillon C, Southwick SM, Charney DS. Mol Psychiatry. 1996;1:278–297. [PubMed] [Google Scholar]
- 16.Bouton ME, Mineka S, Barlow DH. Psychol Rev. 2001;108:4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- 17.Rau V, DeCola JP, Fanselow MS. Neurosci Biobehav Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Bouton ME. Behav Res Ther. 1988;26:137–149. doi: 10.1016/0005-7967(88)90113-1. [DOI] [PubMed] [Google Scholar]
- 19.Rothbaum BO, Schwartz AC. Am J Psychother. 2002;56:59–75. doi: 10.1176/appi.psychotherapy.2002.56.1.59. [DOI] [PubMed] [Google Scholar]
- 20.Myers KM, Davis M. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- 21.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Barad M. Curr Opin Neurobiol. 2005;15:710–715. doi: 10.1016/j.conb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Cain CK, Blouin AM, Barad M. J Exp Psychol Anim Behav Process. 2003;29:323–333. doi: 10.1037/0097-7403.29.4.323. [DOI] [PubMed] [Google Scholar]
- 24.Morris RW, Furlong TM, Westbrook RF. J Exp Psychol Anim Behav Process. 2005;31:40–55. doi: 10.1037/0097-7403.31.1.40. [DOI] [PubMed] [Google Scholar]
- 25.Lovibond PF, Davis NR, O'Flaherty AS. Behav Res Ther. 2000;38:967–983. doi: 10.1016/s0005-7967(99)00121-7. [DOI] [PubMed] [Google Scholar]
- 26.Bouton ME. Psychol Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- 27.Maren S, Quirk GJ. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 28.Devenport LD. Anim Learn Behav. 1998;26:172–181. [Google Scholar]
- 29.Spear NE. In: Animal Memory, Honig WK, James PHR, editors. New York: Academic; 1971. pp. 45–109. [Google Scholar]
- 30.Rescorla RA. Learn Behav. 2004;32:401–408. doi: 10.3758/bf03196037. [DOI] [PubMed] [Google Scholar]
- 31.Rescorla RA, Wagner AR. In: Classical Conditioning II: Current Research and Theory, Black AH, Prokasy WF, editors. New York: Appleton Century Crofts; 1972. pp. 64–99. [Google Scholar]
- 32.Wagner AR. In: Information Processing in Animals: Memory Mechanisms, Spear NE, Miller RR, editors. Hillsdale, NJ: Erlbaum; 1981. pp. 5–47. [Google Scholar]
- 33.Rescorla RA. J Exp Psychol Anim Behav Process. 2000;26:251–260. doi: 10.1037//0097-7403.26.3.251. [DOI] [PubMed] [Google Scholar]
- 34.Rescorla RA. J Exp Psychol Anim Behav Process. 2006;32:135–144. doi: 10.1037/0097-7403.32.2.135. [DOI] [PubMed] [Google Scholar]
- 35.McGaugh JL. Science. 2000;14:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 36.Schafe GE, Nader K, Blair HT, LeDoux JE. Trends Neurosci. 2001;24:540–546. doi: 10.1016/s0166-2236(00)01969-x. [DOI] [PubMed] [Google Scholar]
- 37.Lin CH, Yeh SH, Leu TH, Chang WC, Wang ST, Gean PW. J Neurosci. 2003;23:1574–1579. doi: 10.1523/JNEUROSCI.23-05-01574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin CH, Lee CC, Gean PW. Mol Pharmacol. 2003;63:44–52. doi: 10.1124/mol.63.1.44. [DOI] [PubMed] [Google Scholar]
- 39.Pennebaker JW. Int J Emerg Ment Health. 1999;1:9–18. [PubMed] [Google Scholar]