Abstract
Type 2 diabetes mellitus (T2DM) is strongly associated with obesity in most, but not all, ethnic groups, suggesting important ethnic differences in disease susceptibility. Although it is clear that insulin resistance plays a major role in the pathogenesis of T2DM and that insulin resistance is strongly associated with increases in hepatic (HTG) and/or intramyocellular lipid content, little is known about the prevalence of insulin resistance and potential differences in intracellular lipid distribution among healthy, young, lean individuals of different ethnic groups. To examine this question, 482 young, lean, healthy, sedentary, nonsmoking Eastern Asians (n = 49), Asian-Indians (n = 59), Blacks (n = 48), Caucasians (n = 292), and Hispanics (n = 34) underwent an oral glucose tolerance test to assess whole-body insulin sensitivity by an insulin sensitivity index. In addition, intramyocellular lipid and HTG contents were measured by using proton magnetic resonance spectroscopy. The prevalence of insulin resistance, defined as the lower quartile of insulin sensitivity index, was ≈2- to 3-fold higher in the Asian-Indians compared with all other ethnic groups, and this could entirely be attributed to a 3- to 4-fold increased prevalence of insulin resistance in Asian-Indian men. This increased prevalence of insulin resistance in the Asian-Indian men was associated with an ≈2-fold increase in HTG content and plasma IL-6 concentrations compared with Caucasian men. These data demonstrate important ethnic and gender differences in the pathogenesis of insulin resistance in Asian-Indian men and have important therapeutic implications for treatment of T2DM and for the development of steatosis-related liver disease in this ethnic group.
Keywords: gender and ethnic differences, hepatic steatosis, insulin resistance, risk of type 2 diabetes
The worldwide prevalence of type 2 diabetes mellitus (T2DM) is expected to double within the next two decades, with the greatest increase occurring in Asia and the Indian subcontinent, where it will affect >130 million individuals (1). In contrast to other ethnic groups, Eastern-Asians and Asian-Indians tend to develop T2DM without the same degree of generalized adiposity (2) and with a greater tendency to develop central obesity (3). In addition, the prevalence of diabetes has been found to be much higher in Hispanics and Blacks compared with Caucasians (4). These data suggest important ethnic differences in T2DM susceptibility (5–8). Although the primary cause of T2DM is unknown, insulin resistance plays a major role in the pathogenesis of this disease and is strongly associated with increases in intracellular fat content in liver and skeletal muscle (9–13).
To examine whether there are differences in the prevalence of insulin resistance between different ethnic groups, we examined insulin sensitivity by oral glucose tolerance testing (OGTT) in young, lean, healthy, sedentary Eastern-Asian, Asian-Indian, Black, Hispanic, and Caucasian volunteers. The advantage of studying this cohort of young, lean, healthy individuals is that they have none of the confounding factors that might contribute to insulin resistance and the pathogenesis of insulin resistance, and T2DM can be examined at its earliest steps.
Because increases in intracellular lipid metabolism have been implicated in the pathogenesis of insulin resistance in liver and muscle, these studies were complemented by proton magnetic resonance spectroscopy (MRS) measurements of hepatic triglyceride (HTG) and intramyocellular lipid (IMCL) content.
Results
Prevalence of Insulin Resistance.
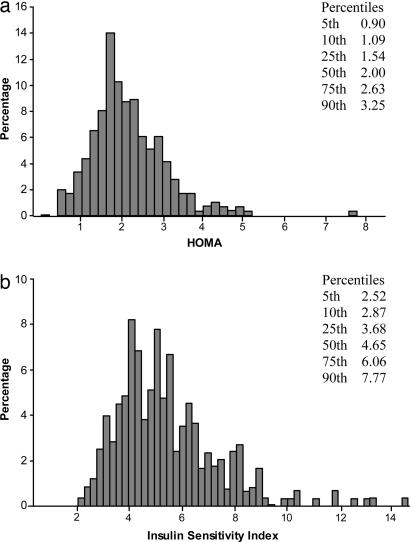
All subjects were young, lean, healthy, and similar in age, weight, and BMI among the different ethnic groups, although the Eastern-Asian subjects tended to be on average slightly smaller and leaner than the other ethnic groups (Table 1). Because smoking and physical activity can affect insulin sensitivity, only subjects who were nonsmoking and sedentary were selected to participate in these studies. Physical activity questionnaires demonstrated that all groups were indeed sedentary and had similar daily activity levels (Table 1). Furthermore, all subjects were weight-stable, and there were no significant differences in dietary composition among the ethnic groups as assessed by dietary questionnaire (data not shown). The distribution of the homeostasis model assessment (HOMA) for the entire study population is shown in Fig. 1a. The 25th, 50th, and 75th percentiles for HOMA were 1.54, 2.00, and 2.63, respectively.
Table 1.
Metabolic characteristics of the different ethnic groups
| Gender (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects | n | Male | Female | Age, years | Weight, kg | Height, m | BMI, kg/m2 | Activity index | Fasting glucose, mg/dl* | Fasting insulin,*† microunits/ml | HOMA*† | ISI,*† 10–4 dl/min per microunit/ml |
| Eastern-Asian | 59 | 20 (33.9) | 39 (66.1) | 26.3 ± 6.8 | 59.6 ± 9.7 | 1.66 ± 0.08 | 21.4 ± 2.2 | 2.7 ± 0.1 | 90.4 (88.4, 92.4) | 7.9 (7.0, 8.9) | 1.74 (1.53, 1.97) | 4.41 (3.94, 4.94) |
| Asian-Indian | 49 | 31 (63.3) | 18 (36.7) | 28.7 ± 8.3 | 63.4 ± 9.3 | 1.68 ± 0.08 | 22.4 ± 2.3 | 2.1 ± 0.1 | 92.2 (90.1, 94.3)B2 | 12.1 (10.7, 13.8)A2,C2,B1,H2 | 2.75 (2.40, 3.15)A2,C2,B2,H2 | 3.02 (2.67, 3.41)A2,C2,B2,H2 |
| Caucasian | 292 | 124 (42.5) | 168 (57.5) | 26.0 ± 7.0 | 65.4 ± 9.6 | 1.71 ± 0.09 | 22.2 ± 2.1 | 2.3 ± 0.1 | 89.2 (88.3, 90.0) | 8.7 (8.3, .2) | 1.95 (1.84, 2.05) | 4.75 (4.53, 4.99) |
| Black | 48 | 14 (29.2) | 34 (70.8) | 23.8 ± 7.0 | 65.8 ± 8.7 | 1.70 ± 0.07 | 22.6 ± 1.8 | 2.3 ± 0.1 | 86.5 (84.2, 88.8) | 8.9 (7.7, 10.2) | 1.90 (1.64, 2.20) | 4.46 (3.91, 5.08) |
| Hispanic | 34 | 15 (44.1) | 19 (55.9) | 26.5 ± 6.4 | 65.7 ± 10.2 | 1.69 ± 0.09 | 22.9 ± 2.1 | 2.3 ± 0.2 | 88.6 (86.1, 91.1) | 7.5 (6.4, 8.7) | 1.65 (1.41, 1.94) | 5.21 (4.52, 6.00) |
Superscript letters and numbers: A2, significantly different from the Eastern-Asian group (Tukey's corrected P < 0.005); C2, significantly different from the Caucasian group (Tukey's corrected P < 0.005); B1, significantly different from the Black group (Tukey's corrected P < 0.05); B2, significantly different from the Black group (Tukey's corrected P < 0.005); H2, significantly different from the Hispanic group (Tukey's corrected P < 0.005).
*Data were adjusted for age and BMI.
†Data are presented as geometric means.
Fig. 1.
Shown are HOMA (a) and ISI (b) distribution in the entire cohort.
Despite being matched for dietary and activity history as well as for BMI fasting plasma glucose and insulin concentrations were significantly increased in the Asian-Indians compared with the other ethnic groups (Table 1). Mean HOMA was also significantly increased in the Asian-Indians compared with all other ethnic groups, suggesting that on average young, lean, healthy Asian-Indians are more insulin-resistant than other tested ethnic groups.
The distribution of the insulin sensitivity index (ISI) for the entire population is shown in Fig. 1b. The 25th, 50th, and 75th percentiles for ISI were 3.68, 4.65, and 6.06, respectively. Similar to the higher HOMA index reflecting more insulin resistance in the Asian-Indians the ISI, which operates in the reverse direction, was significantly lower in the Asian-Indian group compared with the other ethnic groups. Furthermore, the prevalence of insulin resistance, as defined by the lower ISI quartile, was 2- to 3-fold higher in the Asian-Indian group (59%) compared with other ethnic groups [Caucasian (20%), Eastern-Asian (30%), Black (33%), and Hispanic (18%)].
When gender was taken into account the difference in prevalence of insulin resistance in the Asian-Indian population could entirely be accounted for by an increased prevalence (81%) of insulin resistance in the Asian-Indian men (Table 2). There were no differences in either ISI or HOMA among the other ethnic or gender groups (Tables 1 and 3).
Table 2.
Metabolic characteristics of the men in the different ethnic groups
| Subjects | n | Age, years | Weight, kg | Height, m | BMI, kg/m2 | Fasting glucose,* mg/dl | Fasting insulin,*† microunits/ml | HOMA*† | ISI,*†, 10–4 dl/min per microunits/ml |
|---|---|---|---|---|---|---|---|---|---|
| Eastern-Asian | 20 | 27.2 ± 7.2 | 67.6 ± 8.5 | 1.73 ± 0.07 | 22.5 ± 2.1 | 91.3 (88.1, 94.5) | 7.7 (6.4, 9.4) | 1.69 (1.38, 2.07) | 4.49 (3.74, 5.39) |
| Asian-Indian | 31 | 30.0 ± 8.7 | 67.8 ± 7.3 | 1.72 ± 0.06 | 22.8 ± 2.2 | 97.2 (94.6, 99.8)C,B2 | 14.0 (12.0, 16.4)A,C,B1,H | 3.29 (2.78, 3.89)A,C,B2,H | 2.38 (2.05, 2.76)A,C,B2,H |
| Caucasian | 124 | 26.2 ± 7.2 | 72.1 ± 8.5 | 1.79 ± 0.07 | 22.5 ± 2.2 | 91.0 (89.7, 92.3) | 8.6 (7.9, 9.2) | 1.94 (1.79, 2.11) | 4.76 (4.43, 5.13) |
| Black | 14 | 23.9 ± 7.0 | 72.8 ± 6.1 | 1.76 ± 0.05 | 23.5 ± 1.5 | 87.8 (83.9, 91.7) | 8.5 (6.7, 10.7) | 1.83 (1.43, 2.33) | 4.86 (3.90, 6.05) |
| Hispanic | 15 | 27.6 ± 7.9 | 71.9 ± 6.7 | 1.75 ± 0.05 | 23.4 ± 1.9 | 91.1 (87.3, 94.8) | 7.1 (5.7, 8.9) | 1.61 (1.27, 2.03) | 5.45 (4.41, 6.74) |
Superscript letters and numbers: A, significantly different from the Eastern-Asian group (Tukey's corrected P < 0.005); C, significantly different from the Caucasian group (Tukey's corrected P < 0.005); B1, significantly different from the Black group (Tukey's corrected P < 0.05); B2, significantly different from the Black group (Tukey's corrected P < 0.005); H, significantly different from the Hispanic group (Tukey's corrected P < 0.005).
*Data were adjusted for age and BMI.
†Data are presented as geometric means.
Table 3.
Metabolic characteristics of the women in the different ethnic groups
| Subjects | n | Age, years | Weight, kg | Height, m | BMI, kg/m2 | Fasting glucose,* mg/dl | Fasting insulin,*† microunits/ml | HOMA*† | ISI,*†, 10–4 dl/min per microunits/ml |
|---|---|---|---|---|---|---|---|---|---|
| Eastern-Asian | 39 | 25.8 ± 6.7 | 55.5 ± 7.5 | 1.63 ± 0.07 | 20.9 ± 2.1 | 89.4 (87.1, 91.8) | 8.0 (6.9, 9.2) | 1.79 (1.54, 2.08) | 4.33 (3.79, 4.95) |
| Asian-Indian | 18 | 26.4 ± 7.1 | 55.7 ± 7.3 | 1.60 ± 0.05 | 21.7 ± 2.4 | 87.2 (83.8, 90.6) | 10.5 (8.6, 12.9) | 2.30 (1.85, 2.86) | 3.84 (3.17, 4.66) |
| Caucasian | 168 | 25.8 ± 6.8 | 60.6 ± 7.2 | 1.66 ± 0.06 | 22.0 ± 2.0 | 87.3 (86.2, 88.4) | 8.9 (8.4, 9.6) | 1.95 (1.82, 2.09) | 4.74 (4.45, 5.05) |
| Black | 34 | 23.7 ± 7.6 | 62.9 ± 8.0 | 1.68 ± 0.07 | 22.3 ± 1.9 | 85.2 (82.7, 87.6) | 9.3 (8.0, 10.8) | 1.97 (1.69, 2.31) | 4.09 (3.56, 4.71) |
| Hispanic | 19 | 25.7 ± 5.0 | 60.8 ± 9.9 | 1.64 ± 0.08 | 22.5 ± 2.2 | 86.1 (82.8, 89.4) | 7.8 (6.4, 9.6) | 1.70 (1.38, 2.10) | 4.97 (4.12, 6.00) |
*Data were adjusted for age and BMI.
†Data are presented as geometric means.
Liver and Intramyocellular Triglyceride Content.
To examine the mechanism for the increased prevalence of insulin resistance in the Asian-Indian men we measured intracellular lipid content in muscle and liver in a subgroup of these individuals using MRS and compared them to a subgroup of Caucasian men. Both groups of individuals who underwent MRS were similar to the Asian-Indian and Caucasian men, who did not undergo MRS with respect to age and BMI. However, the MRS subgroup differed slightly with respect to fasting plasma glucose concentrations 94.2 mg/dl vs. 90.1 mg/dl in the subjects who did not undergo MRS. This resulted in corresponding differences in ISI (MRS group median of 3.5 vs. without-MRS median of 4.8) and HOMA (MRS group median of 2.64 vs. without-MRS group median of 1.93). Nevertheless, the proportion of Asian-Indian subjects who underwent MRS was higher than the proportion of the Caucasian men who underwent MRS (74% vs. 59%), suggesting that our estimates of differences between Asian-Indian and Caucasian men may be conservative.
Both intrahepatic triglyceride and IMCL content were increased in the Asian-Indian men compared with the Caucasian men (Table 4). However, when adjusted for by the ISI, the Asian-Indian men had a >2-fold increase in HTG content compared with the Caucasian men whereas the differences in the amount of IMCL between the two groups did not persist (Table 4). The 90th and 95th percentiles for HTG concentration were 1.75% and 3.00%, respectively, for the entire Caucasian cohort (73 men/97 women) who underwent1H MRS measurements of liver triglyceride content. These upper limits of HTG content are somewhat lower than those previously reported by Szczepaniak et al. (14) (90th and 95th percentiles of 4.3% and 5.6%, respectively). The reason for their higher upper limits is unclear but may be attributed to the fact that, in contrast to our studies, these workers did not gate for respiration, which may lead to overestimation of liver triglyceride content because of contamination from fat in the gall bladder moving into the field of observation during respiration. Furthermore, these workers do not provide any specific information about the mean or median BMI of these subjects other than stating that they all had a BMI of <25, so it is possible that our subjects were on average leaner than their subjects.
Table 4.
Plasma fatty acid, adipocytokine concentrations, and tissue lipid content in Caucasian and Asian-Indian men
| Plasma metabolite concentrations and tissue lipid content | n | Asian-Indian | Caucasian | P value | Adjusted Asian-Indian | Adjusted Caucasian | Adjusted P value |
|---|---|---|---|---|---|---|---|
| Fatty acids, mmol/liter | 15/87 | 0.43 (0.33, 0.56) | 0.39 (0.36, 0.42) | 0.41 | 0.39 (0.31, 0.48) | 0.39 (0.36, 0.42) | 0.96 |
| Adiponectin, μg/ml | 27/120 | 6.2 (5.0, 7.6) | 8.0 (7.3, 8.9) | 0.02 | 6.9 (5.5, 8.7) | 7.9 (7.2, 8.7) | 0.28 |
| IL-6, pg/ml | 22/107 | 1.60 (1.16, 2.21) | 0.78 (0.67, 0.91) | <0.001 | 1.48 (1.05, 2.10) | 0.79 (0.68, 0.91) | 0.001 |
| TNF-α, pg/ml | 15/82 | 1.29 (1.02, 1.62) | 1.13 (1.02, 1.25) | 0.32 | 1.28 (1.00, 1.64) | 1.13 (1.02, 1.25) | 0.35 |
| Leptin, ng/ml | 21/119 | 4.7 (3.6, 6.1) | 2.4 (2.2, 2.7) | <0.001 | 3.6 (2.8, 4.7) | 2.5 (2.2, 2.8) | 0.01 |
| Liver triglyceride, % | 23/73 | 1.94 (1.31, 2.89) | 0.75 (0.60, 0.93) | <0.001 | 1.54 (1.00, 2.38) | 0.77 (0.62, 0.96) | 0.007 |
| IMCL, % | 24/99 | 1.03 (0.86, 1.24) | 0.74 (0.68, 0.81) | 0.001 | 0.90 (0.74, 1.09) | 0.76 (0.69, 0.83) | 0.12 |
All comparisons were made with adjustment for BMI and age. Adjusted values show comparisons further adjusted for ISI. IMCL, intramyocellular lipid.
Plasma Adipocytokines.
Plasma concentrations of IL-6 and leptin were both increased in Asian-Indian men compared with Caucasian men both adjusted and unadjusted for by the ISI (Table 4). However, there were no differences in plasma adiponectin or TNF-α concentrations between these two groups after adjusting for the ISI (Table 4).
β Cell Responsiveness.
β cell responsiveness was examined in a subgroup of Asian-Indian (n = 21) and Caucasian (n = 71) men during the OGTT as previously described (15). Asian-Indian men had increased basal β cell responsivity (Phib) compared with the Caucasian men (7.98 ± 2.1 for Asian-Indian men vs. 6.21 ± 2.32 for Caucasian men; P < 0.04); however, this was inadequate for their degree of insulin resistance as reflected by a lower disposition index in the Asian-Indian men compared with the Caucasian men (994 ± 1,012 for Asian-Indian men vs. 2,517 ± 2,518 for Caucasian men; P < 0.01).
Discussion
These studies demonstrate that the prevalence of insulin resistance in healthy, young, lean Asian-Indian men is 3- to 4-fold greater than lean men of other ethnic groups. Furthermore, when adjusted for the ISI, this increased prevalence of insulin resistance in Asian-Indian men was associated with an ≈2-fold increase in HTG content and plasma IL-6 concentrations compared with Caucasian men. In contrast, there were no differences in the prevalence of insulin resistance among Asian-Indian women compared with the other ethnic groups, suggesting a potentially important protective role of estrogen in this process (16, 17).
There is increasing evidence for a causal relationship between hepatic steatosis and hepatic insulin resistance both in rodent models with hepatic steatosis and in patients with nonalcoholic fatty liver disease (4, 18–25). Short-term high-fat feeding in rats has been shown to result in hepatic steatosis and hepatic insulin resistance, which were both reversed after treatment with the mitochondrial uncoupling agent 2,4-dinitrophenol (21). Comparable results were observed in patients with severe lipodystrophy where chronic leptin replacement therapy was shown to reverse their hepatic steatosis and hepatic insulin resistance (18). In addition, rosiglitazone treatment in patients with T2DM has been shown to lower HTG content and ameliorate hepatic insulin resistance (26, 27). Finally, recent studies have demonstrated that a relatively small weight loss (≈8 kg) in patients with poorly controlled T2DM reversed their hepatic steatosis and normalized fasting plasma glucose concentrations, rates of hepatic glucose production, and hepatic insulin responsiveness (19).
The molecular mechanism of fat-induced hepatic insulin resistance is unclear; however, recent studies in rodents suggest that intracellular increases in diacylglycerol activates protein kinase Cε, which activates a serine kinase cascade that in turn blocks insulin stimulation of IRS-2 tyrosine phosphorylation, an early step in insulin signaling (21, 22, 25).
Recent studies have also suggested that visceral adiposity may play an important role in causing hepatic insulin resistance through its release of fatty acids and adipocytokines to the liver via the portal vein (28–31). However, we found no differences in the circulating concentrations of adiponectin or TNF-α between the Asian-Indian men and Caucasian men, suggesting that these adipocytokines are not likely playing a major role in causing the increased prevalence of insulin resistance in the Asian-Indian men. In contrast, we did observe an ≈2-fold increase in plasma IL-6 concentrations in the Asian-Indian men that persisted even after adjustment for the ISI. Recent studies in rodents have demonstrated that acute infusions of IL-6 caused insulin resistance in both liver and skeletal muscle (32). Whether this increase in plasma IL-6 concentration is playing a causative role for the increased prevalence of insulin resistance in the Asian-Indian men or is secondary to the hepatic steatosis and insulin resistance remains to be determined.
We also assessed pancreatic β cell function in a subgroup of Asian-Indian men and Caucasian men and found that there was an ≈30% increase in basal β cell responsivity (Phib) in the Asian-Indian men compared with the Caucasian men. However, this compensatory increase in pancreatic β cell function was inadequate for their degree of insulin resistance as reflected by a 60% reduction in the disposition index in the Asian-Indian men.
In summary, the results of this study indicate that the prevalence of insulin resistance is 3- to 4-fold higher in young, lean, healthy Asian-Indian men compared with men in other ethnic groups. This increase prevalence in insulin resistance in the Asian-Indian men was associated with increased HTG content and plasma IL-6 concentrations. These data suggest that Asian-Indian men may be genetically predisposed to develop hepatic steatosis and hepatic insulin resistance at a lower BMI than other ethnic groups. Furthermore, increased prevalence of non-alcoholic fatty liver disease in the Asian-Indian men has important implications for future health risks in these individuals because this condition is associated with steatohepatitis, which may progress to cirrhosis and end-stage liver disease. Finally, these data suggest that there are significant ethnic differences in the pathogenesis of insulin resistance between Asian-Indian men and Caucasian men, which may have important therapeutic implications for prevention and treatment of T2DM in this ethnic group and possibly explain the lower efficacy of lifestyle intervention in the Indian diabetes prevention study compared with the U.S. and Finnish diabetes prevention studies (33–35).
Experimental Procedures
The protocol was approved by the Yale University Human Investigation Committee, and written consent was obtained from each subject after the purpose, nature, and potential complications of the studies were explained. The studies were conducted according to the principles expressed in the Declaration of Helsinki.
Subjects.
Young, healthy, lean, nonsmoking, sedentary volunteers were sequentially recruited from the New Haven community by local advertisement from 1998 to 2006. From this recruitment, we sequentially studied 482 subjects: Caucasian (n = 292), Asian (Chinese and Japanese) (n = 59), Asian-Indian (n = 49), Black (non-Hispanic) (n = 48), and Hispanic (n = 34) individuals (Table 1A).
Dietary Records.
Each participant answered a questionnaire about his or her usual daily intake of food and snacks, any changes with seasons, and changes in body weight and eating habits over the past 12 months. The participants were also asked to describe the food and snacks consumed the day before the visit. All subjects had low (≤30 g/day in men and ≤20 g/day in women) or no alcohol consumption. Each dietary questionnaire was evaluated by the dietician, who calculated intake of total amount of calories and the composition of the diet of each participant.
HOMA and ISI.
Whole-body insulin sensitivity was assessed in all subjects with a 2-h, 75-g OGTT in combination with the HOMA and the ISI. Thirty minutes after insertion of an antecubital i.v. line, fasting blood samples were collected for determination of plasma glucose, insulin, fatty acid, leptin, adiponectin, IL-6, and TNF-α concentrations. The dextrose drink (Glucola; Curtin Matheson Scientific, Houston, TX) was administered, and blood samples were collected at 10, 20, 30, 60, 90, and 120 min for determination of plasma glucose and insulin concentrations. ISI (10–4 dl/min per microunit/ml) was estimated from plasma glucose and insulin concentrations measured during the OGTT by using the oral glucose minimal model (15, 36). This ISI measures overall effects of insulin to stimulate glucose disposal and inhibit glucose production.
β Cell Responsiveness.
An index of β cell responsiveness [Phib (10−9 min−1)] was estimated from plasma glucose and C-peptide concentrations measured during the OGTT by using the oral C-peptide minimal model (37). To determine whether β cell function was appropriate for the degree of insulin resistance, the overall β cell responsiveness was expressed in relation to insulin sensitivity through the disposition index (15).
Proton MRS of Intramyocellular Triglyceride and HTG Content.
To examine whether alterations in the amount of intramyocellular and hepatic lipid content might be responsible for the increased prevalence of insulin resistance in the Asian-Indian men we assessed these parameters in a subgroup of Asian-Indian and Caucasian subjects by proton MRS. Localized proton MRS spectra of the soleus muscle were acquired on a 2.1 Biospec system by using a STEAM sequence or on a 4 T Biospec system by using a PRESS sequence (both from Bruker Instruments, Billerica, MA), in conjunction with a 1H-quadrature probe with twin 13-cm coils as described (11).
Localized proton MRS spectra of the liver at 2.1 T were obtained by using a 12- × 14-cm butterfly proton observation coil placed rigidly over the lateral aspect of the abdomen or on the 4 T system by using a STEAM sequence with respiratory gating and outer volume suppression as described (11).
Physical Activity Monitoring.
Physical activity (during work, leisure activities, and exercise) was assessed by a questionnaire defining a sedentary lifestyle by an exercise index of <2.8 (38).
Analytical Methods.
Plasma glucose concentrations were measured by using an YSI STAT 2700 Analyzer (Yellow Springs Instruments, Yellow Springs, CA). Plasma concentrations of insulin, leptin, and adiponectin were measured by using double-antibody RIA kits in samples obtained after an overnight fast (Linco, St. Louis, MO). The RIA for leptin did not cross-react with human proinsulin, insulin, or glucagon. Plasma concentrations of IL-6 and TNF-α were measured by Quantine High Sensitivity kits (R & D Systems, Minneapolis, MN). Plasma fatty acid and triglyceride concentrations were determined by using a microfluorimetric method (39).
Calculations.
Insulin sensitivity was calculated by the HOMA proposed by Matthews et al. (40). HOMA is based on the assumption that normal-weight healthy subjects aged <35 years have an IR of 1 and β cell function of 100%. HOMA calculates IR and β cell function from fasting glucose (mmol/liter) and insulin (microunits/ml) concentrations: HOMA, IR = (FPG × FPI)/(22.5 × 18), where IR is the calculated IR, FPG is fasting plasma glucose concentration, and FPI is fasting plasma insulin concentration. The higher the value, the more resistant an individual is to insulin.
The ISI was calculated from the plasma glucose and insulin concentrations before and during the OGTT by using the following formula: 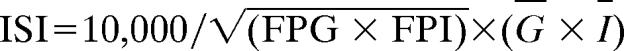 , where FPG is the fasting plasma glucose concentration, FPI the fasting plasma insulin concentration, Ḡ is the average plasma glucose concentration during OGTT (t = 10–120 min), and Ī is the average plasma insulin concentration during OGTT (t = 10–120 min) (11, 41). The ISI represents the composite whole-body insulin sensitivity, reflecting both hepatic and peripheral tissue insulin sensitivity. The higher the ISI, the more sensitive an individual is to insulin. IR was defined by an ISI in the lowest tertile of the entire population (all ethnic groups combined).
, where FPG is the fasting plasma glucose concentration, FPI the fasting plasma insulin concentration, Ḡ is the average plasma glucose concentration during OGTT (t = 10–120 min), and Ī is the average plasma insulin concentration during OGTT (t = 10–120 min) (11, 41). The ISI represents the composite whole-body insulin sensitivity, reflecting both hepatic and peripheral tissue insulin sensitivity. The higher the ISI, the more sensitive an individual is to insulin. IR was defined by an ISI in the lowest tertile of the entire population (all ethnic groups combined).
Statistical Analysis.
Statistical analyses were performed by using SAS 9.1 (SAS, Cary, NC). The distributions of HOMA and ISI were projected by weighting our sample data by the U.S. population proportions of our racial/ethnic groups: Caucasian = 0.691, Asian = 0.011, Black = 0.121, Hispanic = 0.060, Asian-Indian = 0.006 (U.S. Census Bureau 2000, www.census.gov).
Where appropriate, positively skewed variables were log-transformed (fasting insulin concentrations, ISI, HOMA, fatty acid, adiponectin, IL-6, TNF-α, leptin, liver triglyceride, and IMCL content). Differences across racial groups were evaluated by analysis of covariance with adjustment for BMI and age with pairwise comparisons corrected by Tukey's HSD test. In these models, BMI and age were included as continuous covariates. After we observed that gender significantly modified the effect of race on insulin sensitivity (P < 0.001), we chose to stratify our analysis by gender. For some variables (adiponectin, IL-6, fatty acid, TNF-α, leptin, liver triglyceride, and IMCL content), results are restricted to Caucasian and Asian-Indian groups because data were available only for subsets of these groups. To evaluate whether the differences observed between the Caucasian and Asian-Indian groups were reflected by differences in insulin sensitivity, we further adjusted analyses for ISI. Unless otherwise stated, data are expressed as means or geometric means with 95% confidence intervals.
Acknowledgments
We thank Sandra Alfano, Andrea Belous, Carolyn Canonica, Donna Casseria, Donna D'Eugenio, Robin DeGraaf, Aida Groszmann, Douglas Rothman, Hedy Sarafino, Christine Simpson, Irina Smolgovsky, Mikhail Smolgovsky, Gina Solomon, and the staff of the Yale/New Haven Hospital General Clinical Research Center for expert technical assistance with the studies and the volunteers for participating in this study. This work was supported by Public Health Service Grants R01 AG-23686, R01 DK-49230, P01 DK-068229, P30 DK-45735, and M01 RR-00125; the Yamanouchi USA Foundation; and a Distinguished Clinical Scientist Award from the American Diabetes Association (to G.I.S.).
Abbreviations
- MRS
magnetic resonance spectroscopy
- T2DM
type 2 diabetes mellitus
- OGTT
oral glucose tolerance test
- HOMA
homeostasis model assessment
- IMCL
intramyocellular lipid
- ISI
insulin sensitivity index
- HTG
hepatic triglyceride.
Footnotes
The authors declare no conflict of interest.
References
- 1.Zimmet P, Alberti KG, Shaw J. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.WHO Expert Consultation. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 3.McKeigue PM, Shah B, Marmot MG. Lancet. 1991;337:382–386. doi: 10.1016/0140-6736(91)91164-p. [DOI] [PubMed] [Google Scholar]
- 4.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 5.King H, Rewers M. Diabetes Care. 1993;16:157–177. doi: 10.2337/diacare.16.1.157. [DOI] [PubMed] [Google Scholar]
- 6.Raji A, Gerhard-Herman MD, Warren M, Silverman SG, Raptopoulos V, Mantzoros CS, Simonson DC. J Clin Endocrinol Metab. 2004;89:3965–3972. doi: 10.1210/jc.2004-0087. [DOI] [PubMed] [Google Scholar]
- 7.Chiu KC, Cohan P, Lee NP, Chuang LM. Diabetes Care. 2000;23:1353–1358. doi: 10.2337/diacare.23.9.1353. [DOI] [PubMed] [Google Scholar]
- 8.Valsamakis G, Chetty R, Anwar A, Banerjee AK, Barnett A, Kumar S. Diabet Med. 2004;21:1339–1345. doi: 10.1111/j.1464-5491.2004.01361.x. [DOI] [PubMed] [Google Scholar]
- 9.Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 10.Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L. Diabetes. 1999;48:1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 11.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGarry JD. Science. 1992;258:766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- 13.Shulman GI. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Am J Physiol. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 15.Dalla Man C, Campioni M, Polonsky KS, Basu R, Rizza RA, Toffolo G, Cobelli C. Diabetes. 2005;54:3265–3273. doi: 10.2337/diabetes.54.11.3265. [DOI] [PubMed] [Google Scholar]
- 16.Galipeau D, Verma S, McNeill JH. Am J Physiol. 2002;283:H2478–H2484. doi: 10.1152/ajpheart.00243.2002. [DOI] [PubMed] [Google Scholar]
- 17.Vasudevan H, Xiang H, McNeill JH. Am J Physiol. 2005;289:H1335–H1342. doi: 10.1152/ajpheart.00399.2005. [DOI] [PubMed] [Google Scholar]
- 18.Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI. J Clin Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A, Halavaara J, Yki-Jarvinen H. J Clin Endocrinol Metab. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 21.Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 22.Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, Cline GW, Pongratz RL, Zhang XM, Choi CS, et al. Cell Metab. 2005;2:55–65. doi: 10.1016/j.cmet.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, Pypaert M, Lutz EP, Kako Y, Velez-Carrasco W, Goldberg IJ, et al. Proc Natl Acad Sci USA. 2001;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JK, Gavrilova O, Chen Y, Reitman ML, Shulman GI. J Biol Chem. 2000;275:8456–8460. doi: 10.1074/jbc.275.12.8456. [DOI] [PubMed] [Google Scholar]
- 25.Savage DB, Choi CS, Samuel VT, Liu ZX, Zhang D, Wang A, Zhang XM, Cline GW, Yu XX, Geisler JG, et al. J Clin Invest. 2006;116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayerson AB, Hundal RS, Dufour S, Lebon V, Befroy D, Cline GW, Enocksson S, Inzucchi SE, Shulman GI, Petersen KF. Diabetes. 2002;51:797–802. doi: 10.2337/diabetes.51.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bajaj M, Suraamornkul S, Pratipanawatr T, Hardies LJ, Pratipanawatr W, Glass L, Cersosimo E, Miyazaki Y, DeFronzo RA. Diabetes. 2003;52:1364–1370. doi: 10.2337/diabetes.52.6.1364. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein BJ. Am J Cardiol. 2002;90:3G–10G. doi: 10.1016/s0002-9149(02)02553-5. [DOI] [PubMed] [Google Scholar]
- 29.Wellen KE, Hotamisligil GS. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjorntorp P. Ann Med. 1992;24:465–468. doi: 10.3109/07853899209166997. [DOI] [PubMed] [Google Scholar]
- 31.Kabir M, Catalano KJ, Ananthnarayan S, Kim SP, Van Citters GW, Dea MK, Bergman RN. Am J Physiol. 2005;288:E454–E461. doi: 10.1152/ajpendo.00203.2004. [DOI] [PubMed] [Google Scholar]
- 32.Kim HJ, Higashimori T, Park SY, Choi H, Dong J, Kim YJ, Noh HL, Cho YR, Cline G, Kim YB, Kim JK. Diabetes. 2004;53:1060–1067. doi: 10.2337/diabetes.53.4.1060. [DOI] [PubMed] [Google Scholar]
- 33.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, et al. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 34.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. Diabetologia. 2006;49:289–297. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 36.Breda E, Toffolo G, Polonsky KS, Cobelli C. Diabetes. 2002;51(Suppl 1):S227–S233. doi: 10.2337/diabetes.51.2007.s227. [DOI] [PubMed] [Google Scholar]
- 37.Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Diabetes. 2001;50:150–158. doi: 10.2337/diabetes.50.1.150. [DOI] [PubMed] [Google Scholar]
- 38.Baecke JA, Burema J, Frijters JE. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 39.Miles J, Glasscock R, Aikens J, Gerich J, Haymond M. J Lipid Res. 1983;24:96–99. [PubMed] [Google Scholar]
- 40.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 41.Matsuda M, DeFronzo RA. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]