Abstract
The rate and plane of cell division and anisotropic cell growth are critical for plant development and are regulated by diverse mechanisms involving several hormone signaling pathways. Little is known about peptide signaling in plant growth; however, Arabidopsis thaliana POLARIS (PLS), encoding a 36–amino acid peptide, is required for correct root growth and vascular development. Mutational analysis implicates a role for the peptide in hormone responses, but the basis of PLS action is obscure. Using the Arabidopsis root as a model to study PLS action in plant development, we discovered a link between PLS, ethylene signaling, auxin homeostasis, and microtubule cytoskeleton dynamics. Mutation of PLS results in an enhanced ethylene-response phenotype, defective auxin transport and homeostasis, and altered microtubule sensitivity to inhibitors. These defects, along with the short-root phenotype, are suppressed by genetic and pharmacological inhibition of ethylene action. PLS expression is repressed by ethylene and induced by auxin. Our results suggest a mechanism whereby PLS negatively regulates ethylene responses to modulate cell division and expansion via downstream effects on microtubule cytoskeleton dynamics and auxin signaling, thereby influencing root growth and lateral root development. This mechanism involves a regulatory loop of auxin–ethylene interactions.
INTRODUCTION
Organogenesis in plants is coordinated by complex interactions between diverse signaling systems, leading to changes in the rate and plane of cell division and in cell expansion. The activities of plant hormones, such as the auxins, cytokinins, ethylene, gibberellins, and abscisic acid, depend on cellular context and exhibit interactions that can be either synergistic or antagonistic. For example, auxin can suppress cytokinin biosynthesis (Nordström et al., 2004), auxin and cytokinin can act synergistically to induce ethylene biosynthesis (Vogel et al., 1998), and ethylene can modify auxin responses and meristem function (Morgan and Gausman, 1966; Suttle, 1988; Visser et al., 1996; Haver et al., 2002; Vandenbussche et al., 2003; Souter et al., 2004; Stepanova et al., 2005). Depending on the exposure, ethylene can either inhibit or promote cell division and influence cell fate (Kazama et al., 2004), and in part it acts through interactions with DELLA proteins (Achard et al., 2003). The roles of actin and tubulin components are also receiving much attention, both as being modified by hormones and signaling pathways (Lang et al., 1982; Cyr, 1991; Lloyd et al., 1996; Gardiner et al., 2001; Hussey, 2004) and as themselves being implicated as regulators of hormonal signaling systems (Geldner et al., 2001).
Our understanding of the molecular mechanisms that mediate developmental responses to hormones has improved enormously in recent years through the identification of mutants in Arabidopsis thaliana. These include mutants in hormone biosynthesis, perception, and signal transduction, and many genes have been identified that are transcriptionally upregulated or downregulated in response to hormones. However, despite these very significant advances in understanding signal transduction mechanisms in plants, we still have an incomplete picture of how the different signaling pathways interact to elicit particular developmental responses.
One question we are interested in is how hormones regulate the activity of the root meristem (Casson and Lindsey, 2003). Auxin is transported to the root tip and redistributed there by the polar auxin transport pathway, involving the activity of the AUX1 auxin influx carrier (Bennett et al., 1996; Ljung et al., 2001) and the PIN-FORMED (PIN) proteins, representing components of the efflux carrier system (Gälweiler et al., 1998; Luschnig et al., 1998; Friml et al., 2002, 2003; Blilou et al., 2005; Weijers et al., 2005). Auxin is also a positive regulator of lateral root formation after its redistribution from the root tip, thereby affecting the architecture of the root system (Marchant et al., 2002; Casimiro et al., 2003). Given that the auxin concentration is relatively high in the root tip (Ljung et al., 2001) and cytokinin is synthesized there (Miyawaki et al., 2004), and that both auxin and cytokinin can induce ethylene synthesis, we hypothesize that there must be a negative regulatory system in place to suppress ethylene responses that would otherwise reduce cell division and axial cell elongation and so inhibit root growth. We previously found, for example, that sterol mutants exhibiting enhanced ethylene signaling responses in the root have defective root meristem patterning and growth and that both defects can be rescued by the genetic or pharmacological inhibition of ethylene signaling (Souter et al., 2004). This finding suggests that correct root meristem function requires a tight control over ethylene responses in the root tip.
To better understand the molecular basis of plant cell division and expansion, we have used a genetic approach to identify and characterize mutants showing defective development in the root. One such mutant, identified in a screen of promoter trap transgenic plants, is the polaris (pls) mutant (Topping et al., 1994; Topping and Lindsey 1997). Of interest is the observation that the PLS gene transcribes a short mRNA (∼500 nucleotides in length) encoding a predicted 36–amino acid peptide, translation of which is essential for biological activity (Casson et al., 2002).
Seedlings mutant for the PLS gene show a semidominant phenotype, characterized by relatively short and radially expanded cells in the root with reduced division, leading to short roots; reduced leaf vascularization; and altered responses to exogenous auxins and cytokinins (Casson et al., 2002). pls mutants show a partial rescue of the short-root phenotype in the presence of low (picomolar) concentrations of auxin and reduced growth inhibition by exogenous auxin compared with wild-type plants. Consistent with this reduced inhibition of root growth by auxin, seedlings show reduced expression of the auxin-regulated INDOLE-3-ACETIC ACID1 (IAA1) gene in the absence of exogenous auxin.
To investigate the function of the PLS gene further, we used a combination of biochemical and genetic approaches to characterize the hormonal interactions in the pls mutant. We show that the PLS gene is required for correct control of several ethylene-mediated responses, including growth in the dark, polar auxin transport, auxin homeostasis, and microtubule dynamics. These pathways require PLS for their integration and for two aspects of root development: growth (cell division and elongation) and architecture (lateral root formation).
RESULTS
pls Is Defective in Ethylene Signaling
It is known that enhanced ethylene responses reduce axial growth in light-grown seedlings (Guzman and Ecker, 1990; Abeles et al., 1992; Kieber et al., 1993) and could potentially contribute to the short-root phenotype of the pls mutant. Therefore, we grew pls seedlings in the dark to determine whether they exhibited an abnormal etiolation response, also typical of ethylene effects on seedlings. We found that enhanced ethylene signaling is a key determinant of the short-root phenotype of the pls mutant. pls seedlings were found to exhibit a triple-response phenotype (a phenocopy of the ethylene response in the dark), the phenotype being similar to, but less severe than, that of the ethylene-overproducing eto1 (Figure 1A) or the constitutive triple response mutant ctr1-1 (Figure 1B). To confirm the enhanced ethylene response in pls, we measured the abundance of the ethylene-inducible gene transcript, At GSTF2 (Zhou and Goldsborough, 1993; Smith et al., 2003) by RNA gel blot analysis and found it to be greater in air-grown pls compared with the wild type (Figure 1C). We similarly found that expression of the primary ethylene response gene ERF10, which encodes a transcription factor predicted to bind the core sequence of the ethylene-responsive promoter element (Ohta et al., 2001), is increased in air-grown pls seedlings (Figure 1D).
Figure 1.
The PLS Gene Regulates Ethylene Responses.
(A) Representative seedlings of the wild type (C24 and Col-0 [for Columbia]), pls, eto1-1, PLS transgenic overexpresser (PLSOx), and ein2 grown in the dark in air, showing the triple-response phenotype of eto1-1 and pls and the etiolated phenotypes of the wild-type, ein2, and PLSOx seedlings.
(B) Representative seedlings of the wild type (C24), etr1-1, ctr1, and pls grown in the dark in air, showing the triple-response phenotype of ctr1 and pls and the etiolated phenotypes of the wild-type and etr1-1 seedlings.
(C) Top, RNA gel blot analysis showing increased accumulation of the ethylene-inducible At GSTF2 mRNA in air-grown pls seedlings compared with wild-type seedlings. Bottom, RNA loading control (ethidium bromide–stained 28S rRNA). A total of 10 μg of RNA was loaded per lane.
(D) Semiquantitative RT-PCR of the ethylene-inducible ERF10 transcript in 7-d-old wild-type and pls seedlings. Amplification of ACT2 as an RNA loading control is shown. M, RNA size markers.
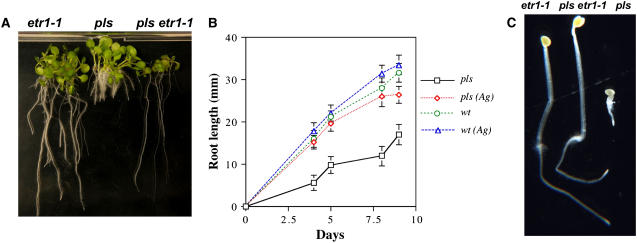
To determine whether the short-root phenotype of pls can be rescued by genetic or pharmacological inhibition of ethylene signaling, pls seedlings were either crossed with the gain-of-function ethylene-insensitive receptor mutant etr1-1 (Figure 2A) or treated with 1 μM silver ions, which inhibit ethylene signaling (Figure 2B). Silver ions may modify the conformation of ETR1 or the signal propagation to the kinase domain of the receptor (Rodriguez et al., 1999). In both cases, the length of the pls primary root was restored to approximately wild-type levels. The etr1-1 mutation was also able to suppress the triple-response phenotype of dark-grown pls seedlings (Figure 2C). These data show that the pls mutant exhibits enhanced ethylene responses and that this determines the short-root phenotype. This finding implicates PLS as a negative regulator of ethylene responses. The failure of the pls mutation to suppress the etr1-1 mutation suggests that PLS may act at or upstream of the ETR1 ethylene receptor.
Figure 2.
Restoration of pls Root Growth by Inhibition of Ethylene Signaling.
(A) Representative light-grown seedlings of etr1-1, pls, and the pls etr1-1 double mutant, showing rescued root growth in the double mutant.
(B) Rescue of primary root growth in pls seedlings treated with 1 μM silver ions [pls (Ag)], which inhibit ethylene signaling by modifying ETR1 conformation or signal propagation to the kinase domain of the receptor (Rodriguez et al., 1999). Error bars represent se; n = 10.
(C) Representative dark-grown seedlings of etr1-1, pls, and the pls etr1-1 double mutant in air, showing suppression of the triple response of pls in the double mutant.
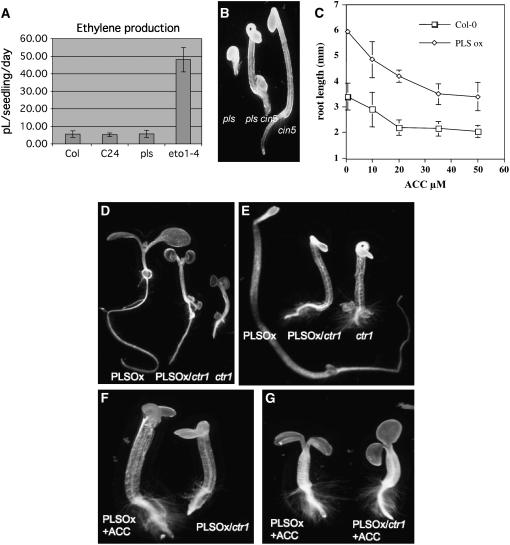
To determine whether the enhanced ethylene responses of pls are attributable to increased ethylene biosynthesis (i.e., upstream of the ethylene receptor), ethylene evolution assays were performed using gas chromatography. These experiments revealed no significant difference in ethylene production between pls and wild-type seedlings (Figure 3A). Levels produced were much lower than for eto1-4 positive controls, which are ethylene overproducers. Moreover, we found that PLS action does not require 1-aminocyclopropane-1-carboxylic acid (ACC) synthase5 (ACS5), an essential enzyme catalyzing the first committed step in cytokinin-mediated ethylene biosynthesis encoded by ACS5/CIN5 (Vogel et al., 1998). cin5 mutants are cytokinin-insensitive and fail to produce ethylene in response to low cytokinin concentrations, and they show no cytokinin-induced triple response in the dark (Figure 3B). However, both the pls mutant and the pls cin5 double mutant showed a strong triple response in the presence of cytokinin, demonstrating that the pls mutation promotes ethylene signaling responses in the absence of ACS5/CIN5 function. Finally, no rescue was achieved by treatment of pls seedlings with the ethylene biosynthesis inhibitor 10 μM aminoethoxyvinylglycine (data not shown). Previously, we showed that pls seedlings did not exhibit increased sensitivity to ethylene (or its precursor, ACC) (Casson et al., 2002).
Figure 3.
pls Is Not an Ethylene Biosynthesis Mutant.
(A) Ethylene evolution by wild-type (Col and C24), pls, and eto1-4 seedlings. Error bars represent sd; n = 6.
(B) Phenotypes of representative seedlings of pls, pls cin5, and cin5 mutants grown in the dark for 3 d in the presence of 0.5 μM kinetin, showing suppression of the cin5 phenotype by pls in the double mutant.
(C) Effect of growth for 7 d on ACC on primary root length of dark-grown seedlings of the wild type (Col-0) and PLS open reading frame-overexpressing line 38. The first data point is 0.5 μM ACC. Error bars represent se; n = 10.
(D) Phenotypes of representative seedlings of light-grown PLSOx (left), ctr1-1 (right), and PLS overexpresser carrying the ctr1-1 mutation (PLSOx/ctr1; middle).
(E) Phenotypes of representative seedlings of dark-grown PLSOx (left), ctr1-1 (right), and PLSOx/ctr1 (middle).
(F) Representative seedlings of dark-grown PLSOx (left) in the presence of 100 μM ACC and PLSOx/ctr1 (right) grown in air.
(G) Representative seedlings of light-grown PLSOx (left) in the presence of 100 μM ACC and PLSOx/ctr1 (right) grown in 100 μM ACC.
Further evidence for a role for PLS in ethylene signaling comes from the analysis of transgenic plants strongly overexpressing the PLS peptide–encoding open reading frame of the PLS cDNA, described previously (Casson et al., 2002). Dark-grown PLS overexpressers failed to show the triple-response phenotype of pls seedlings; instead, they developed a longer root and reduced apical hook compared with wild-type plants, similar to the ethylene-insensitive ein2 (Figure 1A). In addition, although PLS overexpressers produce roots ∼10 to 15% longer than wild-type plants under standard growth conditions (e.g., 19.8 ± 0.4 mm [n = 20] for overexpresser versus 17.9 ± 1.3 mm [n = 20] for the wild type), they maintain ∼60 to 90% longer primary roots than wild-type plants when grown in the presence of the growth-inhibitory ethylene precursor ACC over a wide range of concentrations (Figure 3C). Therefore, the PLS gene acts to suppress the growth-inhibitory effects of ethylene signaling.
To investigate the point of PLS action in the ethylene signaling pathway, we crossed PLS overexpressers with the ctr1-1 mutant. The rationale was that, if PLS functions downstream of CTR1, then PLS overexpression would be predicted to suppress the ctr1 mutant phenotype, whereas if PLS acts upstream of CTR1, at the receptor level, the ctr1 mutation would suppress the phenotype conferred by PLSOx. The results show that PLSOx/ctr1 has an intermediate phenotype (i.e., longer roots in both light- and dark-grown seedlings than ctr1 but much shorter than PLSOx) (Figures 3D and 3E). This finding suggests that there is not complete epistasis: PLS overexpression may be considered to partially suppress ctr1, or ctr1 may partially suppress PLSOx.
If PLS acts downstream of CTR1, it would be expected that the roots and hypocotyls of PLSOx/ctr1 seedlings grown in the dark in air would be longer than those of PLSOx seedlings grown in the presence of ethylene or saturating concentrations of ACC. The results shown in Figure 3F demonstrate that, in fact, the roots and hypocotyls of PLSOx/ctr1 seedlings grown in the dark are ∼50% shorter than those of PLSOx seedlings grown on 100 μM ACC, and the hypocotyl is also shorter, suggesting that CTR1 acts downstream of PLS. By contrast, when grown in the light, air-grown PLSOx/ctr1 seedlings have longer roots and hypocotyls than PLSOx seedlings grown on 100 μM ACC (cf. seedlings in Figures 3D and 3G). When grown in the presence of 100 μM ACC in the light, PLSOx/ctr1 and PLSOx seedlings have identical phenotypes (Figure 3G). Thus, the response of ctr1 to ACC, which leads to some hypocotyl shortening (Resnick et al., 2006), is unaffected by PLS overexpression, and the effect of PLS may be light-dependent.
Together these results suggest that PLS and CTR1 interact to modulate growth in response to ethylene, but they do not act in a single linear pathway.
Enhanced Ethylene Signaling in pls Represses Auxin Transport and Accumulation
It is known that ethylene can inhibit auxin transport (Suttle, 1988), so the enhanced ethylene signaling in pls could cause the reduced auxin responses that we observed previously (Casson et al., 2002). Root architecture is known to be affected by auxin, and the aux1 mutant has defective polar auxin transport, reduced auxin levels in the root tip, and reduced lateral root formation (Bennett et al., 1996; Ljung et al., 2001). Similarly, pls seedlings initiated ∼40% of the number of lateral roots compared with wild-type plants (Figure 4A). To determine whether auxin levels are also affected in pls, the free IAA content of pls was measured and found to be significantly lower than in wild-type plants (Figure 4B). In particular, the peak in root auxin accumulation seen in wild-type seedlings at day 7 was not seen in pls seedlings. Free IAA concentrations were also >70% lower in older pls plants (31 ± 2 ng/100 mg dry weight [n = 5]; growth stage 6.0 to 6.5) (Boyes et al., 2001) than in wild-type plants (114 ± 8 ng/100 mg dry weight [n = 5]). In agreement with a link between PLS and auxin accumulation, transgenic PLS overexpressers showed higher free IAA concentrations than the pls mutant (Figure 4C).
Figure 4.
Auxin Transport and Accumulation Are Defective in pls.
(A) Lateral root numbers in pls, pls etr1-1, and wild-type seedlings at 10 d after germination. Error bars represent se; n = 10.
(B) Free IAA content of pls and wild-type seedlings in aerial and root tissues at 4, 7, and 10 d after germination. Error bars represent sd; n = 5. FW, fresh weight.
(C) Free IAA content of wild-type, pls, pls etr1-1, and PLSOx seedlings at 10 d after germination. Error bars represent sd; n = 3. FW, fresh weight.
(D) Polar transport of auxin in wild-type and pls inflorescence stems. N indicates lack of transport in the basal–to-apical direction. Error bars represent se; n = 8.
(E) Auxin transport in pls and pls etr1-1 mutants. Error bars represent se; n = 7 for pls and pls etr1-1; n = 8 for the wild type.
To investigate further the role of PLS in auxin-regulated root architecture, double mutants were made by crossing the pls mutant with the rooty (rty) mutant, which is characterized by high endogenous levels of auxin and the production of supernumerary lateral roots and adventitious roots on the hypocotyl (King et al., 1995) (Figures 5A and 5B). The rty pls double mutants produced fewer (<10%) and less elongated lateral roots than the rty mutant, indicating that the pls mutation acts to partially suppress the phenotype conferred by rty (Figures 5A and 5B). This finding is consistent with the observation that pls seedlings produce fewer lateral roots per unit length than wild-type plants and exhibit a low-auxin phenotype.
Figure 5.
pls and ACC Suppress the rty Mutant Phenotype.
(A) and (B) Seedlings of the rty single mutant and rty pls double mutant, showing adventitious root formation on the hypocotyl and primary root in rty. In the rty pls double mutant (B), the reduced frequency of lateral roots compared with rty is apparent.
(C) rty seedlings treated with the ethylene precursor ACC (rty + ACC) show a reduced frequency of lateral roots.
(D) Seedlings of ethylene-overproducing eto1-1, rty, and the double mutant eto1-1 rty. The double mutant shows a reduced frequency of lateral roots.
To determine whether there is a link between the enhanced ethylene responses in pls and the effects on the phenotype conferred by rty, rty seedlings were grown in the presence of the ethylene precursor ACC. The results presented in Figure 5C show that treatment of rty seedlings with 100 μM ACC phenocopies the rty pls double mutant, consistent with the notion that the effects of the pls mutation on rty are mediated by ethylene signaling. Similarly, double mutants between rty and the ethylene-overproducing mutant eto1-1 (Chae et al., 2003) also show reduced lateral roots (Figure 5D). These data together show that ethylene inhibits auxin responses (i.e., suppresses the initiation of new lateral roots by auxin) and are consistent with the view that defective ethylene responses in pls cause defects in auxin homeostasis.
To determine whether the low auxin levels in pls are attributable to defective transport from the shoot (a major site of synthesis), the transport of [3H]IAA was measured in isolated inflorescence stem tissue. It was found that the pls mutant has a much reduced ability for auxin transport (∼24% of the level of wild-type plants at 15 h) (Figure 4D). Neither pls nor wild-type stems transported [3H]IAA in the wrong (i.e., basal-to-apical) direction. Therefore, the PLS gene is required for correct auxin transport, accumulation, and root growth.
To investigate further the role of ethylene signaling in auxin transport and accumulation, we measured auxin transport in double mutants between pls and the ethylene-resistant etr1-1. In these double mutants, free IAA levels accumulated to wild-type concentrations (Figure 4C). We also measured [3H]IAA transport in pls etr1-1 double mutants and found it also restored to ∼85% of wild-type levels (Figure 4E). Moreover, lateral root numbers were restored to ∼80% of wild-type levels in the double mutants (Figure 4A).
Together, the data presented demonstrate that the reduced auxin responses and altered root architecture in pls are the result of enhanced ethylene signaling. Given that ethylene is also known to have a dramatic effect on plant cell shape and division, which are processes governed by the cytoskeleton (Abeles et al., 1992; Kieber et al., 1993; Shibaoka, 1994), we investigated whether the ethylene phenotype of pls is responsible for the observed short-root phenotype via effects on the cytoskeleton.
PLS Is Required for Correct Responses to Tubulin Inhibitors
The effect of the pls mutation on the microtubule cytoskeleton was investigated by microscopy and inhibitor and genetic studies. Confocal imaging of root cell interphase microtubules revealed no gross differences in their organization between pls and wild-type plants. However, pls was found to exhibit an abnormal response to certain microtubule inhibitors. Amiprophos-methyl (APM) is an antimicrotubule herbicide that causes severe abnormalities in root architecture as a result of its disruption of the microtubule cytoskeleton (Anthony et al., 1998). pls seedlings grown in the presence of APM did not exhibit the typical root-swelling and growth-inhibition responses of wild-type seedlings (Figures 6A, 6B, and 6G). Furthermore, transgenic plants overexpressing the PLS open reading frame showed a more severe response to APM than did wild-type plants (Figure 6D). Oryzalin, a second microtubule herbicide that is chemically dissimilar to APM, although more potent (Murthy et al., 1994), but which is known to interact with the same target site on tubulin (Ellis et al., 1994), also induced a less severe response in pls compared with wild-type plants (Figure 6E). A third antimicrotubule herbicide, propyzamide, which is of a similar size but has a different mode of action than APM and oryzalin (Anthony et al., 1998), has identical inhibitory effects on root growth of wild-type, pls, and PLS-overexpresser plants (Figures 6F and 6H). As APM and oryzalin (but not propyzamide) inhibit the polymerization of tubulin into microtubules by a similar mechanism, these data indicate that the reduced effect of APM and oryzalin on pls compared with wild-type plants is attributable to altered microtubule dynamics in pls. This may be the result of already defective tubulin dynamics as a consequence of the enhanced ethylene signaling in pls.
Figure 6.
pls Has Reduced Responses to Microtubule Inhibitors.
(A) and (B) Wild-type, pls, etr1-1, and pls etr1-1 seedlings grown for 10 d in the presence ([A] and + in [B]) or absence (− in [B]) of 5 μM APM.
(C) and (D) Wild-type, PLSOx, and ein2 seedlings grown for 10 d in the presence (+) or absence (−) of 5 μM APM.
(E) Wild-type and pls seedlings grown for 10 d in the presence of 5 μM oryzalin.
(F) Effects of propyzamide on the primary root phenotype of wild-type, pls, and PLSOx seedlings.
(G) Kinetics of APM effects on root growth in pls and wild-type seedlings. The 50% inhibitory dose of APM is 1.5 μM for the wild type and 6.0 μM for pls. Each data point represents the mean of six measurements.
(H) Kinetics of propyzamide effects on primary root growth of wild-type, pls, and PLSOx seedlings. Each data point represents the mean of six measurements.
To investigate whether the ethylene signaling defects were responsible for the resistance to APM, pls and etr1-1 single and double mutants and ein2 single mutants were grown in the presence of 5 μM APM. ein2 (Figure 6C) and etr1-1 (Figure 6A) single mutants showed a similar response to wild-type plants in the presence of APM. This finding shows that APM effects are not dependent upon ETR1 and EIN2, respectively, in the wild type. Unlike pls single mutants, pls etr1-1 double mutants exhibited a strong root-swelling response that was identical to that in the wild type, confirming that the enhanced ethylene signaling in pls is responsible for the reduced response to APM (Figure 6A). These observations demonstrate that (1) enhanced ethylene signaling is associated with reduced responses to APM, and (2) PLS is required for correct ethylene signaling, with downstream effects on the microtubule cytoskeleton and root development. Treatment of other ethylene mutants, such as eto1 and ctr1, with APM also indicated a reduced response to APM, but the effect was less pronounced because of the more severe short-root phenotypes of these mutants (data not shown).
PLS Transcription Is Negatively Regulated by Ethylene
To investigate whether the PLS gene is itself regulated by ethylene, both the original promoter trap line At EM101, which contains a gusA gene fused to the PLS gene promoter, and for comparison a transgenic line containing a cloned ∼1.1-kb fragment of the PLS gene promoter fused to a gusA gene in an otherwise wild-type background, PPLS:GUS, were grown in the presence of the ethylene precursor ACC and the ethylene signaling inhibitor silver nitrate. Both promoter trap and PPLS:GUS lines were found previously to respond identically to exogenous auxin (Casson et al., 2002) and were further compared here to establish that the promoter trap activity responds in a similar way to the native promoter in β-glucuronidase (GUS) fusion studies to ethylene signaling. To confirm the GUS expression data, RNA-specific PCR was used to monitor the transcript abundance of the native PLS transcript in wild-type seedlings. For comparative purposes, PPLS promoter activity was also monitored in ethylene-resistant etr1-1 and ethylene-overproducing eto1-1 mutant backgrounds.
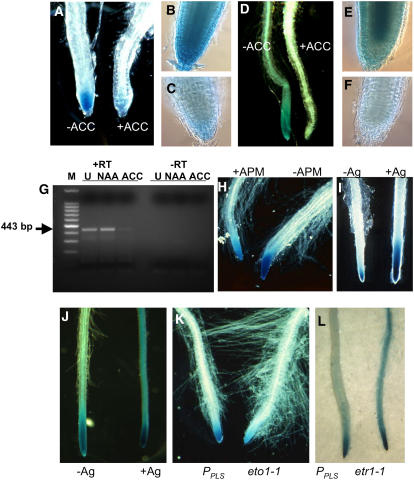
Growing either At EM101 or PPLS:GUS seedlings in the presence of 100 to 150 μM ACC led to the loss of PLS gene promoter activity, as revealed by the lack of GUS activity in seedling root tips (Figures 7A to 7F) or the reduction in the abundance of native PLS transcript (Figure 7G). Interestingly, 1 μM APM had a similar effect to ACC treatment (i.e., was inhibitory) (Figure 7H). By contrast, PLS transcript showed an increase in abundance after treatment with 1-naphthylacetic acid (Figure 7C), as found previously (Casson et al., 2002). The slightly smaller increase than seen previously can be accounted for by the difference in the developmental stage of analysis. Similarly, treatment of seedlings with 5 to 10 μM silver ions led to an increase in the zone of GUS activity in the root, whereby it was no longer restricted to the tip but extended farther back into the older part of the root (Figures 7I and 7J). Analysis of GUS activity in the etr1-1 and eto1-1 mutant backgrounds gave identical results to the pharmacological studies, wherein increased ethylene signaling in the eto1-1 background suppressed PLS promoter activity (Figure 7K) and the suppression of ethylene in etr1-1 led to more extensive GUS activity in the older part of the root (Figure 7L).
Figure 7.
PLS Expression Is Repressed by Ethylene.
(A) to (C) PLS:GUS expression in At EM101 ([A], −ACC, and [B]) is reduced on treatment with 100 μM ACC ([A], +ACC, and [C]).
(D) to (F) GUS activity in wild-type seedlings transformed with PPLS:GUS ([D], −ACC, and [E]) is reduced on treatment with 100 μM ACC ([D], +ACC, and [F]).
(G) RNA-specific RT-PCR of the wild-type PLS transcript (443-bp product) in 6-d-old wild-type seedlings untreated (U), treated for 24 h with 10 μM 1-naphthylacetic acid (NAA), or treated for 24 h with 100 μM ACC. The –RT controls, lacking reverse transcriptase in the reaction, are shown. M, RNA size markers.
(H) Effect of 1 μM APM for 10 d (+APM) on PLS:GUS expression in At EM101.
(I) Effect of 1 μM silver nitrate for 5 d (+Ag) on PLS:GUS expression in At EM101 seedlings, leading to a spread of activity to the older part of the root.
(J) Effect of 1 μM silver nitrate (+Ag) on PPLS:GUS transgenic plants, resulting in a spread of PLS:GUS activity to the older part of the root.
(K) The ethylene-overproducing eto1-1 mutation represses PPLS:GUS expression.
(L) The etr1-1 mutation leads to a spread of PPLS:GUS expression to the older part of the root.
DISCUSSION
The PLS Gene Is Required for Correct Ethylene Signaling
We present evidence that the PLS gene is required for the correct activity of the ethylene signaling pathway. pls seedlings exhibit enhanced ethylene signaling, seen as the triple-response phenotype, and the enhanced expression of both the endogenous At GSTF2, an ethylene-upregulated gene (Zhou and Goldsborough, 1993; Smith et al., 2003), and the primary ethylene response gene ERF10 (Ohta et al., 2001). A key observation is that the defective phenotype of the pls mutant (e.g., the short primary root, reduced polar auxin transport, and low auxin accumulation) is rescued to wild-type status by the pharmacological or genetic inhibition of ethylene signaling. Overexpression of the PLS gene reduces the inhibitory effects of exogenous ACC on primary root growth, further implicating PLS as having a role in the perception or transduction of ethylene signaling.
The suppression by pls of the cin5 mutant's failure to undergo a triple response in the presence of cytokinins suggests that the ethylene response of pls is independent of the ethylene biosynthetic enzyme ACS5, and the high-ethylene-signaling phenotype of pls is unlikely to be attributable to cytokinin-induced ethylene biosynthesis (Vogel et al., 1998). Furthermore, the lack of ethylene overproduction by pls, and the lack of rescue by the ethylene synthesis inhibitor aminoethoxyvinylglycine, confirm a defect in the ethylene signal perception or transduction pathway rather than in the regulation of ethylene biosynthesis. The rescue of the effects of the pls mutation by etr1-1 and the failure of pls to suppress etr1-1 suggest that PLS acts at or close to the ethylene receptor. Similarly, the rte1 mutant cannot suppress the ethylene resistance phenotype of etr1-1 (Resnick et al., 2006). RTE1 (like PLS) acts as a negative regulator of ethylene responses, and it appears to be required for correct ethylene receptor function.
It is likely that PLS acts at more than one level in the ethylene signaling pathway. Although the pls mutation cannot suppress etr1-1, the PLS-overexpression phenotype is incompletely suppressed by ctr1-1 (Figures 3D to 3G), suggesting an additional role for PLS downstream of CTR1. There also appears to be a light-mediated effect on the interaction between pls and ctr1 mutants: the light-grown PLSOx/ctr1 has relatively longer roots than those grown in the dark (Figures 3D and 3F), perhaps suggesting a light dependence of PLS action.
At present, we do not know the precise mode of action of the PLS peptide. The semidominance of the pls mutation suggests a dose-dependent effect of the peptide in suppressing ethylene responses. It is possible, for example, that the peptide interacts with an ethylene receptor upstream of CTR1 to inhibit ethylene binding and so suppress ethylene signaling in a dose-dependent manner; or it may inhibit receptor–CTR1 interactions to suppress ethylene responses. The notion that PLS works at more than one point in the ethylene signaling pathway opens the possibility that it may represent a point of crosstalk between ethylene and other pathways of root development.
Ethylene Signaling Modifies Auxin Transport and Accumulation
It has been recognized that ethylene can reduce auxin responses and transport in other systems (Morgan and Gausman, 1966; Suttle, 1988; Haver et al., 2002), but the molecular mechanism of this effect remains obscure. The observation that the low-auxin phenotype and reduced polar auxin transport and reduced numbers of lateral roots of pls can be restored to approximately wild-type levels in double mutants with the ethylene-resistant etr1-1 demonstrates that the enhanced ethylene-signaling phenotype of pls is most likely responsible for the repression of auxin synthesis and transport. This shows both that ethylene can have an inhibitory effect on auxin synthesis, transport, and biological function and that PLS is a new molecular component of this signaling interaction. We can speculate that a reduced export of auxin from the sites of synthesis might repress auxin biosynthesis in pls.
The pls mutant shows a reduced rate of [3H]IAA transport (Figure 4). The reduced auxin in pls seedlings and older plants is consistent with the observed reduced levels of expression of the auxin-regulated IAA1 gene, reduced leaf vasculature, and reduced growth inhibition in response to low concentrations of exogenous auxin (Casson et al., 2002). Also in agreement is the reduced frequency of lateral root initiation, a process that is regulated at least in part by auxin (Figure 4).
Although the mechanism of the relationship between ethylene signaling and auxin transport is unclear, a number of mutants have been identified in which both signaling pathways are affected. For example, the ethylene-insensitive mutant eir1-1 is defective in the auxin efflux carrier component PIN2 (Luschnig et al., 1998), linking auxin transport and ethylene responses. The mutant alh1 shows a constitutive ethylene triple response and also altered responses to auxin signaling and transport, but not accumulation (Vandenbussche et al., 2003). The hookless mutant similarly shows defective auxin and ethylene interactions that regulate apical hook formation (Lehman et al., 1996). Recently, Stepanova et al. (2005) found that anthranilate synthases, enzymes induced by ethylene and catalyzing the synthesis of Trp and auxin, account in part at least for ethylene-mediated growth inhibition in roots. The relationship between auxin transport and ethylene is intriguing, and the pls mutant offers new opportunities to investigate it.
Ethylene and APM May Affect the Same Process
Of interest is the link between signaling and the cytoskeleton revealed by the pls mutant. pls seedlings show reduced responses to the microtubule inhibitors APM and oryzalin. These inhibitors are members of different chemical classes (phosphorothioamidates and dinitroanilines, respectively), but they have the same predicted mode of action, in the binding of tubulin and the subsequent inhibition of tubulin polymerization (Murthy et al., 1994; Anthony and Hussey, 1999a). This affects microtubule dynamics (by destabilizing them), leading to an inhibition of cell division and axial cell expansion. This is phenotypically similar to the treatment of roots with ethylene, with short roots, radial cell expansion, and swollen lateral root tips in particular.
Wild-type seedlings treated with either APM or oryzalin had similar phenotypes, notably radially swollen root tips. The etr1-1 and ein2 mutants showed a wild-type response to APM, suggesting that ETR1 and EIN2 act upstream of the point of action of APM on microtubules. This finding is in agreement with the evidence that APM binds tubulin directly (Anthony and Hussey, 1999a). The wild-type response to APM and oryzalin is reduced dramatically in pls seedlings (Figure 6), and the physiological importance of ethylene signaling in this apparent resistance is clearly demonstrated by the observation that pls etr1-1 double mutant seedlings showed a response to APM that is restored to wild-type levels. A central role for the PLS gene in this response is demonstrated by the enhanced response to APM in PLS-overexpressing seedlings.
A possible interpretation of these observations is that the reduced response of the pls mutant to tubulin inhibitors may be the result of already defective tubulin dynamics (e.g., a stabilization of the microtubules) in the mutant, as a consequence of the observed enhanced ethylene signaling. The effects of APM and oryzalin are correspondingly reduced. PLS/ethylene signaling and APM/oryzalin both affect microtubule dynamics, but by different mechanisms, with the herbicides acting downstream of ethylene signaling.
The mechanism by which ethylene affects the cytoskeleton and growth is unclear. The role of tubulin in the control of cell division and expansion in roots of Arabidopsis has been shown, for example, in transgenic studies in which reduced levels of α-tubulin led to an inhibition of root elongation, among other effects (Bao et al., 2001), and in mutants defective in a range of microtubule-associated proteins, including botero (Bichet et al., 2001), pleiade (Muller et al., 2004), mor1/gem1 (Whittington et al., 2001; Twell et al., 2002), lefty (Abe et al., 2004), and spiral (Nakajima et al., 2004; Sedbrook et al., 2004). The dwarfed fass/tonneau is also defective in the organization of cell division and expansion and is mutant in a protein phosphatase 2A subunit required for correct cortical microtubule regulation (Camilleri et al., 2002). Microtubule-associated proteins may play important roles in linking hormonal and other signals to the microtubule cytoskeleton (Lloyd et al., 1996; Gardiner et al., 2001). Ethylene, like mutations that affect herbicide responses (Anthony et al., 1998; Anthony and Hussey, 1999b), may affect tubulin conformation so that either herbicide binding is less efficient and/or by stabilizing tubulin heterodimer interactions in the microtubule against the destabilizing effect of the herbicides (Anthony et al., 1999). This view is consistent with the rescued response to inhibitors in the pls etr1-1 double mutants.
Therefore, we propose that a downstream effect of PLS is the suppression of ethylene signaling to the microtubule cytoskeleton, to control cell shape and division. Polar auxin transport requires a functional actin cytoskeleton for correct vesicle trafficking (Geldner et al., 2001), and it is possible that interactions between actin and defective microtubule components of the cytoskeleton account for the reduced polar auxin transport in pls. This possibility is supported by the evidence that auxin-regulated PLS expression is inhibited by both ethylene and APM, each of which acts on the microtubule cytoskeleton. Although we cannot exclude the possibility that the enhanced ethylene-signaling phenotype in pls suppresses polar auxin transport by an independent mechanism from APM, it is tempting to suggest that each represses auxin transport and auxin-regulated PLS expression via effects on the cytoskeleton—and, by implication, that ethylene regulates auxin transport/homeostasis/responses in part, at least, by this mechanism. This could account for defects in pls, such as in vascular patterning, lateral root formation, and root meristem maintenance, that depend on correct auxin transport (Mattsson et al., 1999; Casimiro et al., 2003; Blilou et al., 2005).
Ethylene and Auxin Interact to Establish a Regulatory Loop for the Patterning of PLS Gene Expression in the Root
The ethylene precursor ACC has a suppressive effect on PLS gene expression. This has been shown by RNA-specific PCR analysis of the native gene transcript and by both promoter-GUS analysis in the original promoter trap line At EM101, in which the PLS gene promoter is tagged by a promoterless gusA gene, and in transgenic plants containing gusA cloned downstream of the PLS gene promoter sequence (Figure 7). Similarly, the ethylene-overproducing eto1-1 mutation has an identical suppressive effect on PLS promoter activity. Consistent with this, the inhibition of ethylene signaling in both classes of transgenic plants, either by silver ions or by the etr1-1 mutation, leads to an extended zone of PLS promoter activity back into the proximal region of the root.
We previously showed in detailed studies using quantitative competitor PCR, RNA gel blot analysis, and promoter-GUS fusion transgenic plants that the PLS gene is upregulated approximately threefold within 30 min after the application of auxin (Casson et al., 2002). Furthermore, we have also shown that correct patterning of PLS-GUS expression is dependent on GNOM activity (Topping and Lindsey, 1997), which in turn is required for correct PIN protein localization and auxin distribution via the actin cytoskeleton (Steinmann et al., 1999; Geldner et al., 2003). Therefore, we propose that the patterning and level of PLS expression in the root is itself regulated by an antagonistic interaction between auxin and ethylene signaling, in which auxin localization to the root tip promotes PLS transcriptional activation while ethylene signaling, in a more proximal position, represses expression. As ethylene signaling is suppressed, the zone of PLS expression is extended (Figures 7I, 7J, and 7L). This observed interaction between auxin and ethylene signaling in the root provides one possible mechanism for regulating gene expression domains, at least in a crude way.
A simple prediction from our data would be that, in a pls mutant background, which exhibits enhanced ethylene signaling in the root, PLS transcription itself should be reduced compared with its expression in a wild-type background. The more extensive expression in the proximal region of the roots of transgenic plants containing the PPLS:GUS gene construct in a wild-type background, compared with the tighter PPLS:GUS promoter trap expression in the pls mutant background (Figures 7A and 7I versus Figures 7D and 7J), supports this view and suggests that ethylene suppresses PLS expression farther back in the root to allow ethylene signaling to take place, as is required, for example, for root hair formation at that position (Tanimoto et al., 1995).
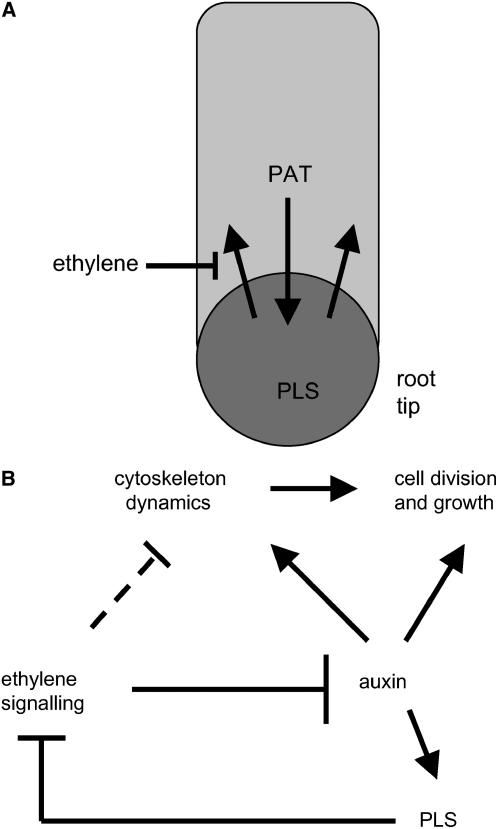
A Model for PLS Function in Root Development
PLS is required for two aspects of root development: elongation (growth) and lateral root formation. We propose a model (Figure 8) in which PLS transcription is activated at the root tip by the relatively high auxin concentration that accumulates and is required for correct cell division at that position (Sabatini et al., 1999; Ljung et al., 2001; Friml et al., 2002; Blilou et al., 2005). Here, PLS acts as a negative regulator of ethylene signaling, which is inhibitory to cell division and expansion, and therefore root growth (Souter et al., 2004). This model could account for the suppression of the inductive effects of auxin and cytokinin on ethylene biosynthesis at the root tip (cytokinin is synthesized there) (Vogel et al., 1998).
Figure 8.
Model for PLS Function in the Root.
(A) Auxin moves into the root tip via polar auxin transport (PAT) and is also transported by this mechanism back up the root, triggering lateral root initiation. Ethylene suppresses polar auxin transport, which may prevent auxin both entering and leaving the tip.
(B) Auxin is a positive regulator of PLS expression and also plays a role in cytoskeleton function, cell division, and cell expansion. Ethylene suppresses these effects, and PLS suppresses the effects of ethylene. According to this model, ethylene treatment suppresses auxin entry and exit at the root tip, and turnover of auxin would lead to a decline in the concentration of active auxin in the root tip in ethylene-treated seedlings. This in turn would lead to a reduction in PLS transcription, allowing ethylene to alter root architecture (e.g., leading to the production of thicker roots and fewer lateral roots). Auxin signaling from the shoot would antagonize this effect via PLS, which the model suggests acts as a modulator of the auxin–ethylene interaction. It is proposed that this interaction modifies root cell division, shape, and ultimately growth through effects, at least in part, on the cytoskeleton. The inhibitory effects of ethylene on auxin transport and cytoskeleton dynamics may be independent or interdependent, but they are currently unclear mechanistically.
We can speculate on potential ecological implications for this mechanism. Mechanical stresses in the soil that can induce relatively high levels of ethylene synthesis/signaling could produce thicker, and potentially mechanically stronger, roots to allow better soil penetration. PLS expression would also suppress the ethylene-mediated inhibition of auxin transport in the root tip, again ensuring correct auxin signaling for cell division and patterning. This model suggests a number of experiments for the further elucidation of the roles of auxin and ethylene interactions at the root tip. PLS is also required for correct lateral root initiation, presumably via ethylene-mediated control of auxin transport to the pericycle.
In conclusion, we have identified PLS as an essential component in the regulation of auxin homeostasis and root growth by restricting ethylene signaling. We show that one downstream component of the cellular machinery that transduces these hormonal signals in the modulation of cell division and expansion at the root tip is the microtubule cytoskeleton. Given the paucity of identified small polypeptides that are known to be biologically functional in plants (Lindsey et al., 2002; Fiers et al., 2005) and the current interest in small RNAs in gene regulation (Kidner and Martienssen, 2005), these data add to the view that many new signaling components, unrecognized by most computational gene identification tools because of their size or genomic organization, remain to be discovered.
METHODS
Plant Materials and Growth Conditions
The transgenic line At EM101 (Arabidopsis thaliana ecotype C24) contains the pls gene mutated by the promoter trap pΔgusBin19 (Topping et al., 1991; Casson et al., 2002). PPLS:GUS transgenic plants have been described previously (Casson et al., 2002). PLS-overexpressing transgenic plants contained a partial PLS cDNA encoding the 36–amino acid open reading frame cloned behind the cauliflower mosaic virus 35S promoter (Casson et al., 2002). For in vitro growth studies, seeds were vernalized, surface-sterilized, and plated on growth medium (half-strength Murashige and Skoog medium; Sigma-Aldrich), 1% sucrose, and 2.5% Phytagel (Sigma-Aldrich) at 22 ± 2°C at a photon flux density of ∼150 μmol·m−2·s−1, as described previously (Casson et al., 2002). etr1-1, eto1-1, ctr1, and cin5 mutants were obtained from the Nottingham Arabidopsis Stock Centre. For hormone/inhibitor application experiments, seeds were germinated aseptically on growth medium containing various concentrations of hormones. 2,4-D, 1-naphthylacetic acid, benzyladenine, and ACC were obtained from Sigma-Aldrich. Oryzalin, APM, and propyzamide were obtained from Fluka.
Gene Expression Analysis
Tissue localization of GUS enzyme activity was performed as described (Casson et al., 2002). For transcript analysis, RNA was extracted using the RNeasy plant RNA extraction kit (Qiagen) and the PolyATract mRNA isolation system (Promega). RNA was blotted, hybridized, and probed as described (Casson et al., 2002). RNA gel blot analysis was performed using 50 μg of total RNA isolated from 7-d-old wild-type and pls seedlings. RNA markers were Promega G319. RNA-specific PCR was used to monitor PLS transcriptional changes, and ACT2 was used as a control, using primers and conditions as described (Casson et al., 2002). Primers for RT-PCR of the ERF10 transcript were 5′-GGACTTGCGTTGAGGTCA-3′ and 5′-GCCAGAGCCTACGACTC-3′.
Hormone Analysis
For each assay, ∼50 seedlings were grown in air on half-strength Murashige and Skoog medium containing 1% sucrose in the dark for 72 h in 22-mL vials for ethylene measurement by gas chromatography as described previously (Wang et al., 2004). Each assay represents an average number from six samples/vials of the same lines and was duplicated. The ethylene production of each line was normalized accordingly, with a unit represented as picoliters per seedling per day. Auxins were assayed in in vitro–grown seedlings up to 10 d after germination, or hydroponically as described (Ljung et al., 2005), or in plants grown in controlled-environment chambers (16-h day, 23°C, 75% humidity; 8-h night, 18°C, 80% humidity; harvested at floral transition, growth stage 6.0 to 6.5) (Boyes et al., 2001). Analysis was by HPLC/gas chromatography–mass spectrometry (Micromass GCT or Quattro Ultima triple quadrupole), essentially as described (Ljung et al., 2005). The polar transport of [3H]IAA (GE Healthcare) was measured in inflorescence stem segments essentially as described (Okada et al., 1991). The apical or (as a control) basal end of each 2.5-cm stem segment was immersed in an Eppendorf tube containing 0.08 μCi/mL 1.5 μM [3H]IAA, and radioactivity accumulating in the other 0.5 cm was measured over a time course by scintillation counting (Tri-Carb 1600 TR; Packard Instruments).
Microscopy
Light micrographs were taken using a CoolSNAPcf digital camera (Photometrics; Roper Scientific) with Openlab 3.1.1 software (Improvision) on Leica MZ125 (Leica Microsystems UK), Olympus SZH10 (Olympus UK), or Zeiss Axioskop (Carl Zeiss) microscopes. Images were processed in Adobe Photoshop 5.0.
Accession Numbers
GenBank/EMBL accession numbers or Arabidopsis Genome Initiative locus identifiers for the genes mentioned in this article are as follows: POLARIS, AF285768; At GSTF2, At4g02520; ERF10, At1g03800.
Acknowledgments
K.L. gratefully acknowledges funding from the Biotechnology and Biological Sciences Research Council, the Gatsby Charitable Foundation, and the County Durham Subregional Partnership, which has supported this work.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Keith Lindsey (keith.lindsey@durham.ac.uk).
Open Access articles can be viewed online without a subscription.
References
- Abe, T., Thitamadee, S., and Hashimoto, T. (2004). Microtubule defects and cell morphogenesis in the lefty1lefty2 tubulin mutant of Arabidopsis thaliana. Plant Cell Physiol. 45 211–220. [DOI] [PubMed] [Google Scholar]
- Abeles, F., Morgan, P., and Saltveit, M. (1992). Ethylene in Plant Biology. San Diego, CA: Academic Press.
- Achard, P., Vriezen, W.H., Van der Straeten, D., and Harberd, N.P. (2003). Ethylene regulates Arabidopsis development via the modulation of DELLA protein growth repressor function. Plant Cell 15 2816–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony, R.G., and Hussey, P.J. (1999. a). Dinitroaniline herbicide resistance and the microtubule cytoskeleton. Trends Plant Sci. 4 112–116. [DOI] [PubMed] [Google Scholar]
- Anthony, R.G., and Hussey, P.J. (1999. b). Double mutation in Eleusine indica alpha-tubulin increases the resistance of transgenic maize calli to dinitroaniline and phosphorothioamidate herbicides. Plant J. 18 669–674. [DOI] [PubMed] [Google Scholar]
- Anthony, R.G., Reichelt, S., and Hussey, P.J. (1999). Dinitroaniline herbicide-resistant transgenic tobacco plants generated by co-overexpression of a mutant alpha-tubulin and a beta-tubulin. Nat. Biotechnol. 17 712–716. [DOI] [PubMed] [Google Scholar]
- Anthony, R.G., Waldin, T.R., Ray, J.A., Bright, S.W.J., and Hussey, P.J. (1998). Herbicide resistance caused by spontaneous mutation of the cytoskeletal protein tubulin. Nature 393 260–263. [DOI] [PubMed] [Google Scholar]
- Bao, Y., Kost, B., and Chua, N.-H. (2001). Reduced expression of α-tubulin genes in Arabidopsis thaliana specifically affects root growth and morphology, root hair development and root gravitropism. Plant J. 28 145–157. [DOI] [PubMed] [Google Scholar]
- Bennett, M.J., Marchant, A., Green, H.G., May, S.T., Ward, S.P., Millner, P.A., Walker, A.R., Schulz, B., and Feldmann, K.A. (1996). Arabidopsis AUX1 gene—A permease-like regulator of root gravitropism. Science 273 948–950. [DOI] [PubMed] [Google Scholar]
- Bichet, A., Desnos, T., Turner, S., Grandjean, O., and Hofte, H. (2001). BOTERO1 is required for normal orientation of cortical microtubules and anisotropic cell expansion in Arabidopsis. Plant J. 25 137–148. [DOI] [PubMed] [Google Scholar]
- Blilou, I., Xu, J., Wildwater, M., Willemsen, V., Papanov, I., Friml, J., Heidstra, R., Aida, M., Palme, K., and Scheres, B. (2005). The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433 39–44. [DOI] [PubMed] [Google Scholar]
- Boyes, D.C., Zayed, A.M., Ascenzi, R., McCaskill, A.J., Hoffman, N.E., Davis, K.R., and Gorlach, J. (2001). Growth stage-based phenotypic analysis of Arabidopsis: A model for high throughput functional genomics in plants. Plant Cell 13 1499–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri, C., Azimzadeh, J., Pastuglia, M., Bellini, C., Grandjean, O., and Bouchez, D. (2002). The Arabidopsis TONNEAU2 gene encodes a putative novel protein phosphatase 2A regulatory subunit essential for the control of the cortical cytoskeleton. Plant Cell 14 833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro, I., Beeckman, T., Graham, N., Bhalerao, R., Zhang, H., Casero, P.J., Sandberg, G., and Bennett, M.J. (2003). Dissecting Arabidopsis lateral root development. Trends Plant Sci. 8 165–171. [DOI] [PubMed] [Google Scholar]
- Casson, S.A., Chilley, P.M., Topping, J.F., Evans, I.M., Souter, M.A., and Lindsey, K. (2002). The POLARIS gene of Arabidopsis encodes a predicted peptide required for correct root growth and leaf vascular patterning. Plant Cell 14 1705–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson, S.A., and Lindsey, K. (2003). Genes and signalling in root development. New Phytol. 158 11–38. [Google Scholar]
- Chae, H.S., Faure, F., and Kieber, J.J. (2003). The eto1, eto2 and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of the ACS protein. Plant Cell 15 545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr, R.J. (1991). Calcium calmodulin affects microtubule stability in lysed protoplasts. J. Cell Sci. 100 311–317. [Google Scholar]
- Ellis, J.R., Taylor, R., and Hussey, P.J. (1994). Molecular modeling indicates that two chemically distinct classes of anti-mitotic herbicide bind to the same receptor site(s). Plant Physiol. 105 15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers, M., Golemiec, E., Xu, J., van der Geest, L., Heidstra, R., Stiekema, W., and Liu, C.-M. (2005). The 14-amino acid CLV3, CLE19, and CLE40 peptides trigger consumption of the root meristem in Arabidopsis through a CLAVATA2-dependent pathway. Plant Cell 17 2542–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml, J., Benkova, E., Blilou, I., Wisniewska, J., Hamann, T., Ljung, K., Woody, S., Sandberg, G., Scheres, B., Jürgens, G., and Palme, K. (2002). AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 108 661–673. [DOI] [PubMed] [Google Scholar]
- Friml, J., Vieten, A., Sauer, M., Weijers, D., Schwarz, H., Hamann, T., Offringa, R., and Jurgens, G. (2003). Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426 147–153. [DOI] [PubMed] [Google Scholar]
- Gälweiler, L., Guan, C., Muller, A., Wisman, E., Mendgen, K., Yephremov, A., and Palme, K. (1998). Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282 2226–2230. [DOI] [PubMed] [Google Scholar]
- Gardiner, J.C., Harper, J.D.I., Weerakoon, N.D., Collins, D.A., Ritchie, S., Gilroy, S., Cyr, R.J., and Marc, J. (2001). A 90 kD phospholipase D from tobacco binds microtubules and the plasma membrane. Plant Cell 13 2143–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner, N., Anders, N., Wolters, H., Keicher, J., Kornberger, W., Muller, P., Delbarre, A., Ueda, T., Nakano, A., and Jürgens, G. (2003). The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112 219–230. [DOI] [PubMed] [Google Scholar]
- Geldner, N., Friml, J., Stierhof, Y.D., Jürgens, G., and Palme, K. (2001). Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413 425–428. [DOI] [PubMed] [Google Scholar]
- Guzman, P., and Ecker, J.R. (1990). Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haver, D.L., Schuch, U.K., and Lovatt, C.J. (2002). Exposure of petunia seedlings to ethylene decreased apical dominance by reducing the ratio of auxin to cytokinin. J. Plant Growth Regul. 21 459–468. [Google Scholar]
- Hussey, P.J., ed (2004). The Plant Cytoskeleton in Cell Differentiation and Development. Oxford, UK: Blackwell Publishers.
- Kazama, H., Dan, H., Imaseki, H., and Wasteneys, G.O. (2004). Transient exposure to ethylene stimulates cell division and alters the fate and polarity of hypocotyl epidermal cells. Plant Physiol. 134 1614–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidner, C.A., and Martienssen, R.A. (2005). The developmental role of microRNA in plants. Curr. Opin. Plant Biol. 8 38–44. [DOI] [PubMed] [Google Scholar]
- Kieber, J.J., Rohenberg, M., Roman, G., Feldmann, K.A., and Ecker, J.R. (1993). CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72 427–441. [DOI] [PubMed] [Google Scholar]
- King, J.J., Stimart, D.P., Fisher, R.H., and Bleecker, A.B. (1995). A mutation altering auxin homeostasis and plant morphology in Arabidopsis. Plant Cell 7 2023–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, J.M., Hedden, P., and Graebe, J.E. (1982). Effects of ethylene on the orientation of microtubules and cellulose microfibrils of pea epicotyl cells with polylamellate cell walls. Protoplasma 110 5–14. [Google Scholar]
- Lehman, A., Black, R., and Ecker, J.R. (1996). HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell 85 183–194. [DOI] [PubMed] [Google Scholar]
- Lindsey, K., Casson, S., and Chilley, C. (2002). Peptides: New signalling molecules in plants. Trends Plant Sci. 7 78–83. [DOI] [PubMed] [Google Scholar]
- Ljung, K., Bhalerao, R.P., and Sandberg, G. (2001). Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative development. Plant J. 28 465–474. [DOI] [PubMed] [Google Scholar]
- Ljung, K., Hull, A.K., Celenza, J., Yamada, M., Estelle, M., Normanly, J., and Sandberg, G. (2005). Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell 17 1090–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd, C., Shaw, P.J., Warn, R.M., and Yuan, M. (1996). Gibberellic acid-induced reorientation of cortical microtubules in living plant cells. J. Microsc. (Oxf.) 181 140–144. [Google Scholar]
- Luschnig, C., Gaxiola, R.A., Grisafi, P., and Fink, G.R. (1998). EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 12 2175–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant, A., Bhalerao, R., Casimiro, I., Eklof, J., Casero, P.J., Bennett, M., and Sandberg, G. (2002). AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson, J., Sung, Z.R., and Berleth, T. (1999). Responses of plant vascular systems to auxin transport inhibition. Development 126 2979–2991. [DOI] [PubMed] [Google Scholar]
- Miyawaki, K., Matsumoto-Kitano, M., and Kakimoto, T. (2004). Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: Tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J. 37 128–138. [DOI] [PubMed] [Google Scholar]
- Morgan, P.W., and Gausman, H.W. (1966). Effects of ethylene on auxin transport. Plant Physiol. 41 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, S., Smertenko, A., Wagner, V., Heinrich, M., Hussey, P.J., and Hauser, M.-T. (2004). The plant microtubule-associated protein AtMAP65–3/PLE is essential for cytokinetic phragmoplast function. Curr. Biol. 14 412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy, J.V., Kim, H.-H., Hanesworth, V.R., Hugdahl, J.D., and Morejohn, L.C. (1994). Competitive inhibition of high-affinity oryzalin binding to plant tubulin by the phosphoric amide herbicide amiprophos-methyl. Plant Physiol. 105 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima, K., Furutani, I., Tachimoto, H., Matsubara, H., and Hashimoto, T. (2004). SPIRAL1 encodes a plant-specific microtubule-localized protein required for directional control of rapidly expanding Arabidopsis cells. Plant Cell 16 1178–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström, A., Tarkowski, P., Tarkowska, D., Norbaek, R., Astot, C., Dolezal, K., and Sandberg, G. (2004). Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: A factor of potential importance for auxin-cytokinin-regulated development. Proc. Natl. Acad. Sci. USA 101 8039–8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta, M., Matsui, K., Hiratsu, K., Shinshi, H., and Ohme-Takagi, M. (2001). Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell 13 1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada, K., Ueda, J., Komaki, M.K., Bell, C.J., and Shimura, Y. (1991). Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick, J.S., Wen, C.-K., Shockey, J.A., and Chang, C. (2006). REVERSION-TO-ETHYLENE SENSITIVITY1, a conserved gene that regulates ethylene receptor function in Arabidopsis. Proc. Natl. Acad. Sci. USA 103 7917–7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, F.I., Esch, J.J., Hall, A.E., Binder, B.M., Schaller, G.E., and Bleeker, A.B. (1999). A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science 283 996–998. [DOI] [PubMed] [Google Scholar]
- Sabatini, S., Beis, D., Wolkenfelt, H., Murfett, J., Guilfoyle, T., Malamy, J., Benfey, P., Leyser, O., Bechtold, N., Weisbeek, P., and Scheres, B. (1999). An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99 463–472. [DOI] [PubMed] [Google Scholar]
- Sedbrook, J.C., Ehrhardt, D.W., Fisher, S.E., Scheible, W.R., and Somerville, C.R. (2004). The Arabidopsis SKU6/SPIRAL1 gene encodes a plus end-localized microtubule-interacting protein involved in directional cell expansion. Plant Cell 16 1506–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibaoka, H. (1994). Plant hormone-induced changes in the orientation of cortical microtubules: Alterations in the cross-linking between microtubules and the plasma membrane. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45 527–544. [Google Scholar]
- Smith, A.P., Nourizadeh, S.D., Peer, W.A., Xu, J., Bandyopadhyay, A., Murphy, A.S., and Goldsborough, P.B. (2003). Arabidopsis AtGSTF2 is regulated by ethylene and auxin, and encodes a glutathione S-transferase that interacts with flavonoids. Plant J. 36 433–442. [DOI] [PubMed] [Google Scholar]
- Souter, M.A., Pullen, M., Topping, J.F., Zhang, X., and Lindsey, K. (2004). Rescue of defective auxin-mediated gene expression and root meristem function by inhibition of ethylene signalling in sterol biosynthesis mutants of Arabidopsis. Planta 219 773–783. [DOI] [PubMed] [Google Scholar]
- Steinmann, T., Geldner, N., Grebe, M., Mangold, S., Jackson, C.L., Paris, S., Gälweiler, L., Palme, K., and Jürgens, G. (1999). Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286 316–318. [DOI] [PubMed] [Google Scholar]
- Stepanova, A.N., Hoyt, J.M., Hamilton, A.A., and Alonso, J.M. (2005). A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell 17 2230–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle, J.C. (1988). Effect of ethylene treatment on polar IAA transport, net IAA uptake and specific binding of N-1-naphthylthalamic acid in tissues and microsomes isolated from etiolated pea epicotyls. Plant Physiol. 88 795–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto, M., Roberts, K., and Dolan, L. (1995). Ethylene is a positive regulator of root hair development in Arabidopsis thaliana. Plant J. 8 943–948. [DOI] [PubMed] [Google Scholar]
- Topping, J.F., Agyeman, F., Henricot, B., and Lindsey, K. (1994). Identification of molecular markers of embryogenesis in Arabidopsis thaliana by promoter trapping. Plant J. 5 895–903. [DOI] [PubMed] [Google Scholar]
- Topping, J.F., and Lindsey, K. (1997). Promoter trap markers differentiate structural and positional components of polar development in Arabidopsis. Plant Cell 9 1713–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topping, J.F., Wei, W., and Lindsey, K. (1991). Functional tagging of regulatory elements in the plant genome. Development 112 1009–1019. [DOI] [PubMed] [Google Scholar]
- Twell, D., Park, S.K., Hawkins, T.J., Schubert, D., Schmidt, R., Smertenko, A., and Hussey, P.J. (2002). MOR1/GEM1 has an essential role in the plant-specific cytokinetic phragmoplast. Nat. Cell Biol. 4 711–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche, F., et al. (2003). The Arabidopsis mutant alh1 illustrates a cross talk between ethylene and auxin. Plant Physiol. 131 1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser, E.J.W., Cohen, J.D., Barendse, G.W.M., Blom, C.W.P.M., and Voesenek, L.A.C.J. (1996). An ethylene-mediated increase in sensitivity to auxin induces adventitious root formation in flooded Rumex palustris Sm. Plant Physiol. 112 1687–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, J.P., Woeste, K.E., Theologis, A., and Kieber, J.J. (1998). Recessive and dominant mutations in the ethylene biosynthetic gene ACS5 of Arabidopsis confer cytokinin insensitivity and ethylene overproduction, respectively. Proc. Natl. Acad. Sci. USA 95 4766–4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, K.L.-C., Yoshida, H., Lurin, C., and Ecker, J.R. (2004). Regulation of ethylene gas biosynthesis by the Arabidopsis ETO1 protein. Nature 428 945–950. [DOI] [PubMed] [Google Scholar]
- Weijers, D., Sauer, M., Meurette, O., Friml, J., Ljung, K., Sandberg, G., Hooykaas, P., and Offringa, R. (2005). Maintenance of embryonic auxin distribution for apical-basal patterning by PIN-FORMED-dependent auxin transport in Arabidopsis. Plant Cell 17 2517–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington, A.T., Vugrek, O., Wei, K.J., Hasenbein, N.G., Sugimoto, K., Rashbrooke, M.C., and Wasteneys, G.O. (2001). MOR1 is essential for organizing cortical microtubules in plants. Nature 411 610–613. [DOI] [PubMed] [Google Scholar]
- Zhou, J., and Goldsborough, P.B. (1993). An Arabidopsis gene with homology to glutathione S-transferases is regulated by ethylene. Plant Mol. Biol. 22 517–523. [DOI] [PubMed] [Google Scholar]