Abstract
Chronic food restriction (FR) enhances the rewarding and motor-activating effects of abused drugs, and is accompanied by changes in dopamine (DA) dynamics and increased D-1 DA receptor-mediated cell signaling and transcriptional responses in nucleus accumbens (NAc). However, little is known about effects of FR on DA synthetic activity in the mesoaccumbens and nigrostriatal pathways. In Experiment 1 of the present study, tyrosine hydroxylase (TH) gene expression was measured in ventral tegmental area and substantia nigra, using real time RT-PCR and in situ hybridization; no differences were observed between FR and ad libitum fed (AL) rats. In Experiment 2, TH protein levels, determined by Western blot, were found to be elevated in NAc and caudate-putamen (CPu) of FR relative to AL rats. In the absence of increased transcription, this may reflect a slowing of TH degradation. In Experiments 3 and 4, DA synthetic activity was assessed by Western blot measurement of TH phosphorylation at Ser-40, and HPLC measurement of in vivo tyrosine hydroxylation rate, as reflected by DOPA accumulation following administration of a decarboxylase inhibitor (NSD-1015; 100 mg/kg, i.p.). Basal phospho-Ser(40)-TH levels did not differ between groups but DOPA accumulation was decreased by FR. Decreased DOPA synthesis, despite increased levels of TH protein, may reflect the inhibitory effect of increased DA binding to TH protein or decreased concentrations of cofactor tetrahydrobiopterin. Finally, in response to d-amphetamine (0.5 and 5.0 mg/kg, i.p.), phospho-Ser(40)-TH was selectively decreased in NAc of FR rats. This suggests increased feedback inhibition of DA synthesis - a possible consequence of postsynaptic receptor hypersensitivity, or increased extracellular DA concentration. These results indicate that FR increases TH protein levels, but may decrease the capacity for DA synthesis by decreasing TH activity. According to this scheme, the previously observed upregulation of striatal cell signaling and transcriptional responses to DA receptor agonist administration may include compensatory neuroadaptations.
SECTION: 1. Systems Neuroscience (Regulatory Systems)
Keywords: food restriction, tyrosine hydroxylase, dopamine, nucleus accumbens
1. Introduction
Chronically food-restricted (FR) rats are hyperactive in environments that are novel or associated with food availability (Hart and Turturro, 1998; Hoyenga and Hoyenga, 1974; Timberlake and White, 1990) and are more behaviorally sensitive than ad libitum fed (AL) rats to the rewarding and motor-activating effects of abused drugs and direct dopamine (DA) receptor agonists (Carr, 2002; Carroll and Meisch, 1984). On the other hand, FR rats are less active than controls in their home cage and other familiar environments (Duffy et al., 1990; Hart and Turturro, 1998). Recently, D-1 DA receptor agonist drug challenge was shown to produce greater MAP kinase and CaM kinase II signaling, CREB phosphorylation and transcription of c-fos and neuropeptide genes in nucleus accumbens (NAc) of FR relative to AL rats (Carr et al., 2003; Haberny et al., 2004; Haberny and Carr, 2005a; Haberny and Carr, 2005b). Several findings suggest that the upregulation of NAc postsynaptic cell signaling in response to D-1 DA receptor stimulation could represent compensation for a persistent decrease in basal DA release. For example, to the extent that effects of FR arise from the stressful aspect of FR, it is of interest that other forms of chronic stress (e.g. cold, restraint) are accompanied by decreases in DA cell firing and/or NAc extracellular DA concentrations (Imperato et al., 1993; Moore et al., 1998). Yet, in anesthetized rats that had been subject to a mild FR regimen, VTA DA cell firing was actually higher than in AL controls (Marinelli et al., 2002). A more severe FR regimen was accompanied by decreased basal extracellular DA concentrations in NAc (Pothos et al., 1995), although not all microdialysis studies of striatal extracellular DA in FR subjects reported this decrease (Cadoni et al., 2003; Rouge-Pont et al., 1995). In the extreme case of DA deficiency induced by intra-mesencephalic 6-OHDA injection, enhanced D-1 DA agonist-induced behavioral responses are accompanied by increased striatal Fos-immunostaining and MAP kinase activation, similar to those observed in chronically FR rats (Cai et al., 2000; Gerfen et al., 2002; Kim et al., 2000).
The purpose of the present study was to test the hypothesis that FR is associated with decreased DA synthetic activity in the mesoaccumbens and nigrostriatal pathways. Thus, in Experiment 1, quantitative real time RT-PCR and in situ hybridization were used to evaluate mRNA levels for tyrosine hydroxylase (TH) -the rate-limiting enzyme in DA biosynthesis-in the ventral tegmental area (VTA) and substantia nigra (SN). In Experiment 2, Western blotting was used to evaluate total TH protein concentrations in VTA, SN, caudate putamen (CPu) and NAc. In Experiment 3, Western blotting was used to evaluate TH activation (i.e. phosphorylation at Ser-40) under basal conditions and in response to d-amphetamine challenge. In Experiment 4, HPLC with electrochemical detection was used to measure in vivo tyrosine hydroxylation rate as reflected by DOPA accumulation following inhibition of aromatic L-amino acid decarboxylase.
2. Results
2.1 Experiment 1: Measurements of TH mRNA by quantitative real-time RT-PCR and in situ hybridization
TH mRNA as measured in extracts of microdissected VTA and SN did not differ between AL and FR rats (n=7 per group; t(12)vta =0.0; t(12)sn =1.46, p>0.10; Figure 1). Examination of images obtained from in situ hybridization did not suggest any differences between feeding groups that were localized to particular subdivisions of VTA or SN and densitometric analysis of images corresponding to whole VTA and SN pars compacta confirmed the absence of difference between feeding groups (n= 5 per group; t(8)vta =0.89; t(8)sn =0.86; Figure 2).
Figure 1.
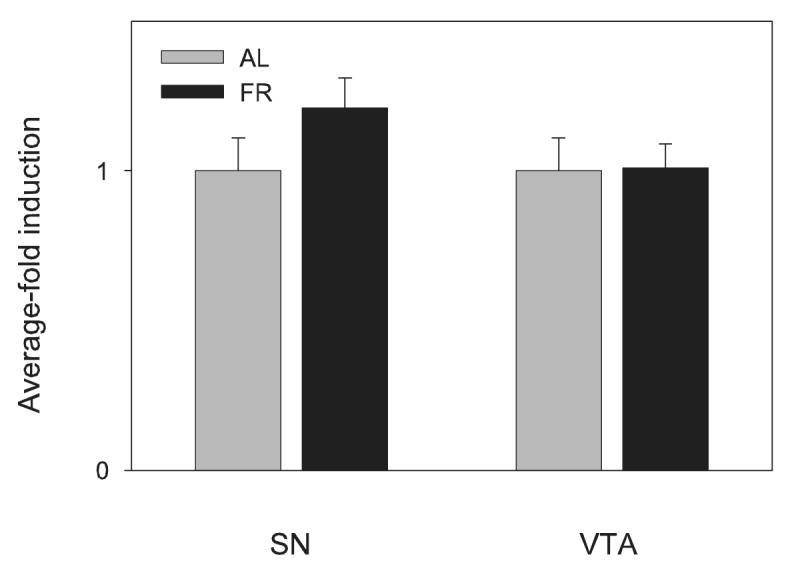
Effects of chronic food restriction (FR) on tyrosine hydroxylase mRNA levels in substantia nigra (SN) and ventral tegmental area (VTA) determined by real-time RT-PCR analysis. All data have been normalized for levels of β-Actin expression within the same sample and represent group means ± SEM (n= 7 per group) of fold-induction over the control ad libitum (AL) fed group.
Figure 2.
Effects of chronic food restriction (FR) on tyrosine hydroxylase mRNA levels in substantia nigra (SN) and ventral tegmental area (VTA) determed by in situ hybridization. Left: mean ± SEM (n=5 per group) optical density (OD) expressed in comparison to the normalized control ad libitum (AL) fed group. Right: Tyrosine hydroxylase mRNA expression in SN and VTA of representative AL and FR rats.
2.2 Experiment 2: Measurement of total TH protein by Western blot
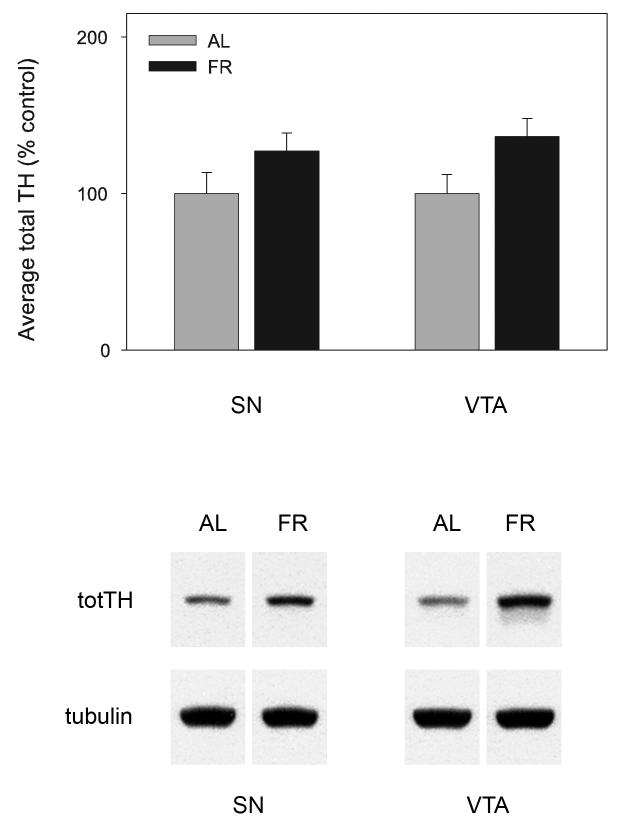
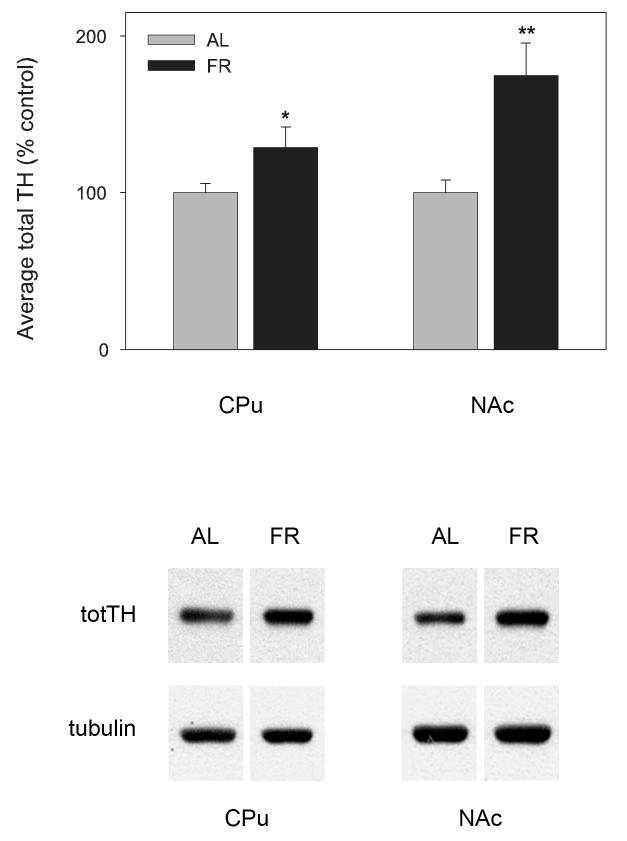
In midbrain, total TH in SN (nAL=6; nFR=5; t(9)=1.4, p>.10) and VTA (t(9)=2.14, p=.06; Figure 3) of FR rats tended to be higher than in AL fed rats but the difference was not significant. In CPu total TH was significantly higher in FR than AL fed rats (n=11 per group; t(20)=2.08, p=.05) and in NAc was markedly higher in FR than AL fed rats (t(20)=3.65, p=.001; Figure 4).
Figure 3.
Mean ± SEM (nAL=6; nFR=5) ratio of total tyrosine hydroxylase protein/tubulin in substantia nigra (SN) and ventral tegmental area (VTA) of food-restricted (FR) rats determined by Western blot and expressed in comparison to the normalized control ad libitum (AL) fed group. Graphed results are displayed with representative immunoblots.
Figure 4.
Mean ± SEM (n = 11 per group) ratio of total tyrosine hydroxylase protein/tubulin in caudate-putamen (CPu) and nucleus accumbens (NAc) of food-restricted (FR) rats determined by Western blot and expressed in comparison to the normalized control ad libitum (AL) fed group. Graphed results are displayed with representative immunoblots. *p=.05; **p=.001
2.3 Experiment 3: Measurement of TH phosphorylation
Basal levels.
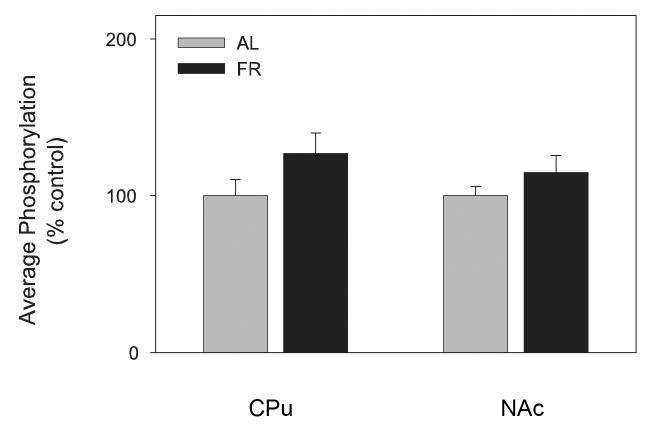
Under basal conditions phospho-(Ser40)-TH did not differ between AL and FR rats in either the CPu (t(20)=1.7, p>.10) or NAc (t(22)=1.3, p>.10; Figure 5)
Figure 5.
Mean ± SEM (nAL=13; nFR = 11) ratio of phospho-(Ser40)-tyrosine hydroxylase/tubulin in caudate-putamen (CPu) and nucleus accumbens (NAc) of food-restricted (FR) rats determined by Western blot and expressed in comparison to the normalized control ad libitum (AL) fed group.
D-Amphetamine.
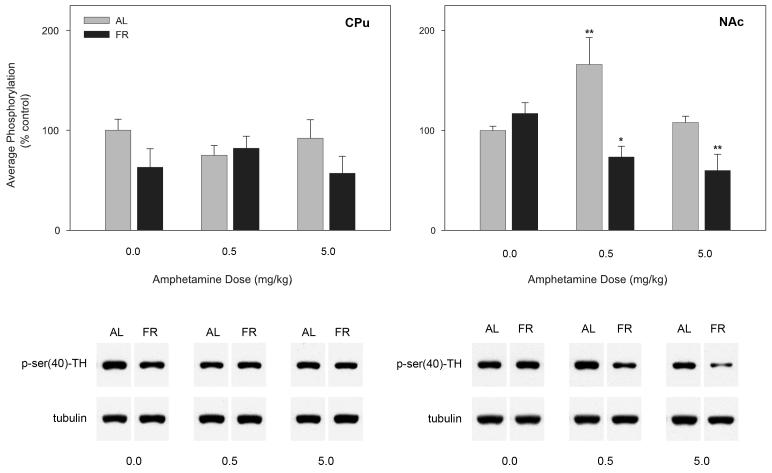
In CPu the ANOVA indicated no effect of feeding condition (n= 5 or 6 per group; F1,28 =3.0, p>.05), no effect of d-amphetamine treatment (F2,28 =0.11), and no interaction between these factors (F2,28 =1.3). In NAc the ANOVA revealed a significant effect of feeding condition (F1,28 =10.9, p<.01), a marginally significant effect of d-amphetamine treatment (F2,28 =3.0, p=.065), and a significant interaction between these factors (F2,28 =6.3, p<.01). Pair-wise Fisher LSD comparisons revealed that the 0.5 mg/kg dose of d-amphetamine increased TH phosphorylation in AL fed rats (p<.01) while both doses of d-amphetamine decreased TH phosphorylation in FR rats (p<.05 for the 0.5 mg/kg dose and p<.01 for the 5.0 mg/kg dose; Figure 6).
Figure 6.
Mean ± SEM (n= 5-6 per group) ratio of phospho-(Ser40)-tyrosine hydroxylase/tubulin in caudate-putamen (CPu) and nucleus accumbens (NAc) of food-restricted (FR) rats injected 20 min prior to sacrifice with 0.0, 0.5 or 5.0 mg/kg d-amphetamine (i.p.) and expressed in comparison to the normalized control ad libitum (AL) fed group injected with saline vehicle. Graphed results are displayed with representative immunoblots. *p<.05; **p<.01
2.4 Experiment 4: Measurement of DOPA accumulation
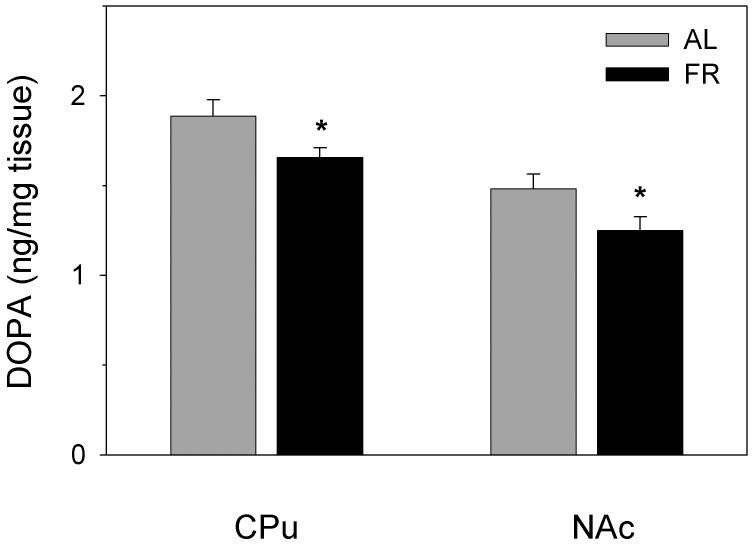
In VTA, mean DOPA concentrations were essentially identical in AL and FR subjects (0.766 vs 0.762 ng/mg tissue) but tended to be lower in the SN of FR relative to AL subjects (0.508 vs 0.665 ng/mg tissue; t(9)=1.96, p=.08). DOPA concentrations were lower in NAc (t(26)=2.09, p<.05) and CPu (t(25)=2.09, p<.05) of FR relative to AL rats (Figure 7).
Figure 7.
Mean ± SEM (n =13-14 per group) DOPA concentration (ng/mg tissue), determined by HPLC, in caudate-putamen (CPu) and nucleus accumbens (NAc) of ad libitum fed (AL) and food-restricted (FR) rats treated with the DOPA decarboxylase inhibitor, NSD-1015 (100 mg/kg, i.p). *p<.05.
3. Discussion
It was previously observed that centrally administered d-amphetamine and the D-1 DA receptor agonist SKF-82958 induce behavioral and striatal cellular responses that are enhanced in chronically FR rats (Cabeza de Vaca and Carr, 1998; Carr and Kutchukhidze, 2000; Carr et al., 2003; Haberny et al., 2004; Haberny and Carr, 2005). The similarity of some of these effects to those observed in DA-deficient animals (Cai et al., 2000; Gerfen et al., 2002; Kim et al., 2000) and the report of decreased basal extracellular DA concentrations in NAc of FR rats (Pothos et al., 1995), suggested that FR may decrease basal DA release, which precedes development of postsynaptic compensatory neuroadaptations. Using quantitative real time RT-PCR to measureTH mRNA, no evidence of decreased VTA or SN TH synthesis was observed in FR relative to AL rats. To pursue this question further, in situ hybridization was used to allow greater neuroanatomical differentiation in the analysis of VTA and SN. Results of this experiment confirmed the RT-PCR result, indicating no difference in TH mRNA levels between FR and AL subjects. These findings indicate that, at least at the time of sacrifice, there was no difference between feeding groups in TH transcription. Contrary to these results, Lindblom and coworkers (2006) recently reported an elevation of TH mRNA in the VTA of FR rats. The different outcomes of these two studies may arise from differences in the ages of subjects (adolescents versus adults), duration of FR (12 versus 21-28 days), or percentage of body weight loss (8 versus 20-25%).
In contrast to measurements of TH mRNA, measurements of total TH protein in Experiment 2 indicated higher concentrations in CPu and NAc of FR relative to AL rats. The increase of total TH in the absence of increase in TH synthesis is suggestive of decreased degradation and turnover. This may represent a mechanism for priming an increase in local DA synthetic capacity without transcriptional change. However, a further important element in developing the overall portrait of DA function in FR subjects is the small but significant decrease in accumulation of striatal DOPA under basal conditions in Experiment 4. Decreased DOPA accumulation and DA synthesis could be the result of decreased phosphorylation of TH at Ser-40 (Dunkley et al., 2004). However, no significant differences in phospho-TH(Ser40) were observed between feeding groups under basal conditions in Experiment 3. The decreased accumulation of DOPA does, nevertheless, indicate decreased tyrosine hydroxylation and, particularly under the circumstance of decreased DA transporter function in these subjects (Zhen et al., 2006), suggests decreased DA utilization. This conclusion is consistent with the microdialysis result that points to decreased basal DA transmission in the NAc of FR rats (Pothos et al., 1995).
Dynamic change in phosphorylation of TH is an established physiological mechanism of TH activation (Dunkley et al., 2004). Phosphorylation at Ser(40), in particular, regulates DA synthesis and is responsive to acute administration of drugs that alter impulse flow and extracellular DA concentrations in DA terminal areas. Stimuli that increase DA neuronal firing produce a short latency increase in phosphorylation of striatal TH (Haycock and Haycock, 1991). The activating effect of haloperidol on TH is pronounced because it increases DA neuronal firing and concurrently blocks DA terminal autoreceptors that would otherwise dampen the activation of terminal TH (Salvatore et al., 2000). Moreover, cessation of impulse flow in DA neurons by treatment with γ-butyroloactone or axotomy also activates TH, presumably due to relief of inhibitory nerve terminal autoreceptor stimulation, since this effect is abolished by DA agonist pretreatment (Lew et al., 1998). Indeed, selective stimulation of terminal D-2 autoreceptors with quinpirole in striatal slices decreases basal phospho-(Ser40)-TH (Lindgren et al., 2001). In vivo, cocaine increases striatal extracellular DA by blocking the DA transporter (DAT) and consequently decreases DA biosynthesis by inhibiting phospho-(Ser40)-TH in a dose-related manner (Jedynak et al., 2002). Amphetamine similarly increases striatal extracellular DA by increasing efflux through the DAT (Kahlig et al., 2005). It is therefore of interest that, in Experiment 3, d-amphetamine decreased phospho-(Ser40)-TH in NAc of FR rats but not AL rats. This result may reflect enhanced DA release by amphetamine in FR subjects and would be consistent with the in vivo microdialysis findings that FR increases cocaine and amphetamine-induced extracellular DA concentrations in NAc (Cadoni et al., 2003; Rouge-Pont et al., 1995). Alternatively, this result may reflect enhanced postsynaptic receptor-mediated feedback inhibition, which would be compatible with the prior findings of upregulated postsynaptic cellular responses to DA receptor agonists (Carr et al., 2003; Haberny et al., 2004). An unexpected result is the increased phospho-(Ser40)-TH in NAc of AL rats injected with the low, 0.5 mg/kg, dose of d-amphetamine. This response is characteristic of increased impulse flow in DA neurons. Interestingly, it has been shown that the typical suppression of DA neuronal impulse flow produced by higher doses of d-amphetamine masks a strong excitatory response mediated via α-2 adrenoreceptors (Shi et al., 2000). It is possible that in AL rats the α-2 adrenoreceptor-mediated excitatory response supersedes the feedback inhibitory effect of released DA in response to the low, 0.5 mg/kg, dose of d-amphetamine.
The findings of the present study are complex and do not fit easily into a simple and coherent picture. On the one hand, the elevated levels of striatal TH, which imply increased DA synthetic capacity, are consistent with findings that salient stimuli, such as food and drugs of abuse, lead to higher extracellular DA concentrations in NAc of FR relative to AL rats (Bassareo and Di Chiara, 1999; Cadoni et al., 2003; Rouge-Pont et al., 1995). Should these events be the prevailing determinants of DA synthesis in FR rats, then increased DA synthetic capacity might be expected. The present observation of decreased phospho-(Ser40)-TH in NAc of FR relative to AL rats challenged with d-amphetamine may be a biochemical correlate of enhanced DA release, reflecting increased feedback inhibition of TH phosphorylation and DA synthesis (Jedynak et al., 2002). However, the lack of difference between feeding groups in basal phospho-(Ser40)-TH and, particularly, the decreased DOPA accumulation in striatal regions of FR rats under basal conditions, suggests that the increased reservoir of TH in FR rats is inactive. This could be a consequence of increased DA binding to TH, or decreased concentrations of cofactor tetrahydrobiopterin (Dunkley et al., 2004; Zigmond et al., 1989). DA conservation under basal conditions during chronic FR would seem to have adaptive value insofar as striatal DA transmission facilitates motor activity, and a downregulation of basal DA transmission may minimize non-essential energy expenditure. Yet, it would be equally adaptive for salient stimuli such as novel environments and food to produce a more robust DA response in NAc of FR relative to AL rats - an adaptive response subject to exploitation by drugs that serve as proxies for natural reinforcers. This effect of FR, documented for psychostimulant drug challenge (Cadoni et al., 2003; Rouge-Pont et al., 1995), cannot be attributed to a greater density or affinity of DA transporters at the plasma membrane for drug interaction (Zhen et al., 2006). A recent study has suggested that the effect may be dependent upon prefrontal-cortical afferents to NAc or VTA that modulate DA release (Ventura and Puglisi-Allegra, 1995). It is also important to note that the enhanced DA response to psychostimulant challenge may emerge only under sustained and relatively severe FR conditions (Stuber et al., 2002), while the enhanced DA responses to food and its anticipation are present after just one day of food deprivation (Wilson et al., 1995).
Chronic FR may induce a host of alterations in DA dynamics, some of which are more durable than others and/or prevalent during circumstances in which there appears to be high versus low behavioral opportunity for drive reduction. Examining a single time point, as in the present study, produces complex data. Examining the time course of changes in these parameters, as well as possible circadian variations, may yield insights which clarify the processes taking place.
4. Experimental Procedures
4.1 Subjects and treatments
All subjects were male Sprague-Dawley rats (375-425 grams) housed individually in plastic cages with free access to food and water except when food restriction conditions applied (see below). Animals were maintained on a 12:12 hour light:dark cycle, with lights on at 0700 h. All experimental procedures were approved by the New York University School of Medicine Institutional Animal Care and Use Committee and were performed in accordance with the “Principles of Laboratory Animal Care” (NIH publication number 85-23, revised 1996). Every measure was taken to minimize the number of animals used and their suffering.
Several days after their arrival in the central animal facility, half of the rats in each experiment were switched to a restricted feeding regimen whereby a single 10 gram meal of Purina rat chow was delivered at approximately 1700 h each day. These rats continued to have ad libitum access to water. Once body weight had declined by 20-25% (approximately 15 days) daily food allotments were titrated to maintain body weight at this value for an additional 7-14 days prior to sacrifice. This feeding regimen and time of analysis are the same as used in the prior studies where behavioral, immediate-early gene (IEG) and striatal cell-signaling responses to d-amphetamine and direct DA receptor agonist challenge were found to be augmented in FR relative to AL rats (e.g., Cabeza de Vaca and Carr, 1998; Carr et al., 2003; Haberny et al., 2004). For all experiments, rats were briefly narcotized with CO2, decapitated, and brains were rapidly removed and immediately frozen in powdered dry ice. As in the prior studies of this series, sacrifice occurred during the light phase 4-5 hours before the FR subjects were to receive their scheduled daily meal. In Experiment 3 some subjects were injected with d-amphetamine (Sigma-Aldrich) at doses of 0.0, 0.5 or 5.0 mg/kg (i.p.). Otherwise, subjects were maintained in a quiet location within their home cages in the period preceding sacrifice. A 20-min interval post-injection to sacrifice was chosen based on reports that systemic cocaine administration produces maximal change in phospho-(Ser40)-TH beginning 15 min post-injection (Jedynak et al., 2002) and systemic haloperidol administration produces a change that is marked when measured 30 min post-injection (Salvatore et al., 2000). Twenty minutes is also the time-point at which increases in D-1 DA agonist-induced striatal cell signaling have been observed in FR relative to AL rats (Haberny et al., 2004; Haberny and Carr, 2005). In Experiment 4, half the subjects were injected with the aromatic L-amino acid decarboxylase inhibitor NSD-1015 (100 mg/kg, i.p.; Astatech, Monmouth Junction, NJ), as in classical studies of in vivo tyrosine hydroxylation (e.g., Carlsson et al., 1977), while half were injected with saline vehicle and returned to home cages for the 30-min interval prior to sacrifice.
4.2 RNA extraction
VTA and SN tissue samples were obtained from two consecutive 500 μm coronal sections (from -6.0 to -5.0 mm in the atlas of Paxinos and Watson (1998) cut from frozen midbrain using an IEC Minotome cryostat. Under an Olympus dissecting microscope VTA and SN (pars compacta and reticulata) were removed bilaterally using a micropunch tool and microknife, respectively. All equipment and surfaces were treated with RNase Zap (Ambion, Austin, TX USA) to minimize RNase contamination. Total RNA was extracted from individual VTA and SN tissue samples using Trizol reagent (Invitrogen, Carlsbad, CA USA) according to the manufacturer’s protocol. Sample RNA concentrations were determined by spectrophotometry.
4.3 Quantitative real-time RT-PCR
Specific mRNA levels in each sample were measured on the Roche Light Cycler Instrument (Roche Diagnostics, Pleasanton, CA USA) in a final volume of 20 μl. Each reaction was performed using reagents from the one-step SYBR Green Quantitative RT-PCR kit (Sigma-Aldrich, St. Louis, MO USA), with 0.3 μM of primer, 20 U of RNasin RNase inhibitor (Promega, Madison, WI USA), and 10 ng total RNA. Amplification consisted of 40 cycles of denaturation at 94°C for 10 seconds, annealing at 50°C - 60°C, depending on the primer pair, for 10 seconds, and extension at 72°C for 40 seconds. Fluorescence signals were monitored sequentially for each sample tube once per cycle at the end of extension. An external standard RNA concentration curve for each primer pair was generated using pooled RNA samples and verified by agarose gel electrophoresis. To evaluate RNA purity, each sample was tested without addition of reverse transcriptase and found to contain no genomic DNA above the threshold detection level. For each experiment, specificity of RT-PCR products was confirmed by analysis of melting curves produced by the Light Cycler Instrument showing the presence of a single species of DNA product per primer pair, and by agarose gel electrophoresis revealing single bands of the predicted molecular weight for each product. Table 1 details the sequence of primer pairs (purchased from GeneLink, Hawthorne, NY USA) and the sources for their design.
TABLE 1.
Sequences of primer pairs used for real-time RT-PCR with the sources for their design.
| β-actin | 5’ GTC GTA CCA CTG GCA TTG TG 3’ |
| 5’ GCC ATC TCT TGC TCG AAG TC 3’ | |
| (Spangler et al., 2004) | |
| Tyrosine | 5’ ATG CCC ACC CCC AGC GCC CC 3’ |
| Hydroxylase | 5’ GAC ACT TTT CTT GGG AAC CA 3’ |
| (Chen et al., 2003) |
Sample mRNA levels for the TH gene were averaged from two separate experiments, each performed in duplicate. To correct for minor variability among samples, each subject’s average TH expression was normalized to its expression level of the housekeeping gene β-actin. Relative expression levels for each subject were subsequently normalized to the corresponding mean of the AL, vehicle-treated (control) group, and -fold changes in induction were calculated and compared, between groups, using t-tests.
4.4 In situ hybridization
Frozen brains were embedded in M-1 embedding matrix (Lipshaw, Pittsburgh, PA). Coronal sections (16 μm) were mounted onto r-Aminopropyltriethoxysilane (Sigma-Aldrich) coated slides and stored at -80° C until used. In situ hybridization was carried out as described by Zheng and Pintar (1995). Briefly, sections were fixed in 4% paraformaldehyde at 4°C for 2 min, followed by several washes in RNAse free water, dehydration and acetylation. A specific [35S] UTP-radiolabeled complementary RNA (cRNA) probe was used. The TH probe consisted of a fragment of 548 bp from nucleotide 946 to 1494 of the TH gene. The fragment was subcloned into pBluescript II plasmid. The construct was a generous gift from Dr. Jeannette Miller (NYU School of Medicine). The single-stranded cRNA probe was synthesized and labeled using the Ambion’s Maxiscript with [35S] UTP (Amersham Biosciences). In situ hybridization of the riboprobe with tissue sections was done at 50° C overnight in a standard hybridization buffer containing 50% formamide. Tissue sections were washed, treated with RNAse A (Sigma-Aldrich, St. Louis MO) at a concentration 0.1 mg/ml, followed by another wash for 2h in 0.2XSSC at 55° C with constant gentle stirring. Finally the sections were dehydrated, air dried and dipped in a 1:1 dilution of Kodak NTB2 emulsion (Eastman Kodak, Rochester, NY) then stored in a light tight box at 4°C. Three weeks later slides were developed, dried and coverslipped with Permount. Images of SN pars compacta and VTA were captured using a Spot InSight QE digital camera (Diagnostic Instruments Inc., Sterling Heights, MI) linked to a Zeiss Axioskop microscope (Carl Zeiss Inc., Thornwood, NY).
The relative optical density in SN and VTA regions were measured using the MCID image analysis system (Imaging Research, St. Catherines, Ontario, Canada). Results shown are averages of bilateral analyses of approximately 16 sections from each brain. Sense control RNA probes gave no specific signal (data not shown). Statistical analyses of the data were carried out using the student’s t-test.
4.5 Tissue sampling for Western blot and HPLC experiments
SN and VTA were obtained from 500 μm frozen sections as described above. NAc and CPu were micropunched, under an Olympus dissecting microscope, from the first five and all six, respectively, of a series of six consecutive 500 μm frozen sections ranging from level +2.7 mm to -0.2 mm in the atlas of Paxinos and Watson (1998).
4.6 Lysate preparation and Western blotting
Tissue samples were homogenized in 10 volumes of 50 mM Tris-HCl, pH 7.5 containing 50 mM NaCl, 5 mM EDTA, 1 mM EGTA, 1mM Na3VO4, 40 mM β-glycerophosphate, 50 mM NaF and 5mM Na4P2O7, 1% Tx-100, 0.5 μM okadaic acid, 0.5 % sodium deoxycholate and 0.1 % SDS, followed by centrifugation and protein determination using BCA reagent kit as described by the manufacturer (Pierce). Supernatants were mixed with 5X SDS-PAGE sample buffer, boiled for 5 min, cooled on ice and kept at -80°C until future use.
Protein (10 μg per lane) was separated by electrophoresis on precast 10% polyacrylamide gels (Cambrex, East Rutherford, NJ, USA). Precision Plus protein standard molecular weight markers (Bio-Rad, Hercules, CA, USA) were also loaded to assure complete electrophoretic transfer and to estimate the size of bands of interest. The gels were transferred to nitrocellulose membrane (Osmonics) for 1 h, with a constant voltage of 100 volts. Membranes were blocked for 1hr at room temperature with blocking buffer, 5% non fat dry milk in 50 mM Tris-HCl, pH 7.5 containing 150 mM NaCl and 0.1 % Tween 20 (TBS-T), then probed overnight at 4°C using primary antibodies for total TH (rat polyclonal, 1:2000-5000; Chemicon, Temecula, CA, USA), or phospho-TH (Ser 40) (rabbit polyclonal, 1:500 dilution, PhosphoSolutions, Aurora, CO). After probing with primary antibodies and washing with TBS-T buffer (3X5 min), membranes were incubated with horseradish peroxidase conjugated anti-rabbit IgG (Cell Signaling). Proteins were visualized using a chemiluminescence ECL kit (Pierce). Densitometric analysis of the bands was performed using the MCID imaging system (St. Catherines, ontario, Canada). Total TH and phospho-(Ser40)-TH values were normalized to tubulin. Results were expressed by comparison to the normalized control, which, when measuring total TH and phospho-(Ser40)-TH in uninjected rats, was the ad libitum fed group; when measuring phospho-(Ser40)-TH in subjects that were injected prior to sacrifice, the ad libitum fed group injected with saline vehicle was the control. Results of the experiment in which phospho-(Ser40)-TH was measured in rats injected with d-amphetamine were analyzed by 2-way ANOVA followed by Fisher’s protected t-test. Otherwise, results were analyzed by student’s t-test.
4.7 HPLC for electrochemical detection of DOPA
Frozen tissues were homogenized in 100-200 volumes of 0.2N perchloric acid containing 5 ng/ml of 3,4-dihydroxybenzylamine (Sigma-Aldrich Chemical Co., St. Louis, MO) as internal standard. After centrifugation (15 min, 30,000xg), supernatants were stored at -80°C until analysis. DOPA levels were measured in 20 μl aliquots of the supernatants injected onto an HPLC using ESA (Chelmsford, MA) instrumentation: an HR-80 column and a model 5100A coulometric detector equipped with a model 5011 dual-electrode high-sensitivity analytical cell. The working electrode potentials were +0.05 and -0.25 V; the mobile phase was ESA CAT-A-PHASE (pH 2.56) supplemented with 3% methanol at a flow rate of 1 ml/min. DOPA was quantitated by reference to the internal standard and results were analyzed using student’s t-test.
Acknowledgments
Acknowledgements: The authors would like to thank Professor Peter Dunkley of the University of Newcastle for valuable comments on portions of this work. This research was supported by T32 DA07254 (Y.P), F31 DA016846 (S.L.H), R01 DA03956 and K02 DA00292 (K.D.C.) from NIDA/NIH.
References
- Bassareo V, Di Chiara G. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur. J. Neurosci. 1999;11:4389–4397. doi: 10.1046/j.1460-9568.1999.00843.x. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J. Neurosci. 1998;18:7502–7510. doi: 10.1523/JNEUROSCI.18-18-07502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C, Solinas M, Valentini V, Di Chiara G. Selective psychostimulant sensitization by food restriction: differential changes in accumbens shell and core dopamine. Eur. J. Neurosci. 2003;18:2326–2334. doi: 10.1046/j.1460-9568.2003.02941.x. [DOI] [PubMed] [Google Scholar]
- Cai G, Zhen X, Uryu K, Friedman E. Activation of extracellular signal-regulated protein kinases is associated with a sensitized locomotor response to D2 dopamine receptor stimulation in unilateral 6-hydroxydopamine-lesioned rats. J. Neurosci. 2000;20:1849–1857. doi: 10.1523/JNEUROSCI.20-05-01849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A, Kehr W, Lindqvist M. Agonist-antagonist interaction on dopamine receptors in brain as reflected in the rates of tyrosine and tryptophan hydroxylation. J. Neural Transm. 1977;40:99–113. doi: 10.1007/BF01250562. [DOI] [PubMed] [Google Scholar]
- Carr KD. Augmentation of drug reward by chronic food restriction: Behavioral evidence and underlying mechanisms. Physiol. Behav. 2002;76:353–364. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- Carr KD, Kutchukhidze N. Chronic food restriction increases Fos-like immunoreactivity (FLI) induced in rat forebrain by intraventricular amphetamine. Brain Res. 2000;861:88–96. doi: 10.1016/s0006-8993(00)02018-7. [DOI] [PubMed] [Google Scholar]
- Carr KD, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neurosci. 2003;119:1157–1167. doi: 10.1016/s0306-4522(03)00227-6. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. Increased drug-reinforced behavior due to food deprivation. Adv. Behav. Pharmacol. 1984;4:47–88. [Google Scholar]
- Chen S, Xianwen C, Dehua X, Zhenguo L, Lingfei X, Smith SW, Zhongcheng Z. Behavioral correction of Parkinsonian rats following the transplantation of immortalized fibroblasts genetically modified with TH and GCH genes. Parkinsonism and Related Disord. 2003;9:591–597. doi: 10.1016/s1353-8020(03)00020-8. [DOI] [PubMed] [Google Scholar]
- Duffy PH, Leakey JE, Pipkin JL, Turturro A, Hart RW. The physiological, neurologic, and behavioral effects of caloric restriction related to aging, disease, and environmental factors. Environ. Res. 1997;73:242–248. doi: 10.1006/enrs.1997.3714. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Borovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW. Tyrosine hydroxylase phosphorylation: regulation and consequences. J. Neurochem. 2004;91:1025–1043. doi: 10.1111/j.1471-4159.2004.02797.x. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Miyachi S, Paletzki R, Brown P. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK 1/2/MAP kinase. J. Neurosci. 2002;22:5042–5054. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberny SL, Berman Y, Meller E, Carr KD. Chronic food restriction increases D-1 dopamine agonist-induced phosphorylation of extracellular signal regulated kinase 1/2 and cyclic AMP response element binding protein in caudate-putamen and nucleus accumbens. Neurosci. 2004;125:289–298. doi: 10.1016/j.neuroscience.2004.01.037. [DOI] [PubMed] [Google Scholar]
- Haberny SL, Carr KD. Food restriction increases NMDA receptor-mediated calcium-calmodulin kinase II and NMDA receptor/extracellular signal-regulated kinase 1/2-mediated cAMP response element-binding protein phosphorylation in nucleus accumbens upon D-1 dopamine receptor stimulation in rats. Neurosci. 2005a;132:1035–1043. doi: 10.1016/j.neuroscience.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Haberny SL, Carr KD. Comparison of basal and D-1 dopamine receptor agonist-stimulated neuropeptide gene expression in caudate-putamen and nucleus accumbens of ad libitum fed and food-restricted rats. Molec. Brain Res. 2005b;141:121–127. doi: 10.1016/j.molbrainres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Hart RW, Turturro A. Evolution and dietary restriction. Exper. Gerontol. 1998;33:53–60. doi: 10.1016/s0531-5565(97)00063-6. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Haycock DA. Tyrosine hydroxylase in rat brain dopaminergic nerve terminals: multiple-site phosphorylation in vivo and in synaptosomes. J. Biol. Chem. 1991;266:5650–5657. [PubMed] [Google Scholar]
- Hoyenga KT, Hoyenga KB. Effects of food deprivation upon cue utilization as measured by novelty incentive. Q. J. Exper. Psychol. 1974;26:206–217. [Google Scholar]
- Imperato A, Cabib S, Puglisi-Allegra S. Repeated stressful experiences differently affect the time dependent responses of the mesolimbic dopamine system to the stressor. Brain Res. 1993;601:333–336. doi: 10.1016/0006-8993(93)91732-8. [DOI] [PubMed] [Google Scholar]
- Jedynak JP, Ali SF, Haycock JW, Hope BT. Acute administration of cocaine regulates the phosphorylation of serine-19, -31 and -40 in tyrosine hydroxylase. J. Neurochem. 2002;82:382–388. doi: 10.1046/j.1471-4159.2002.00982.x. [DOI] [PubMed] [Google Scholar]
- Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, Galli A. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc. Natl. Acad. Sci. 2005;102:3495–3500. doi: 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Szczypka MS, Palmiter RD. Dopamine-deficient mice are hypersensitive to dopamine receptor agonists. J. Neurosci. 2000;20:4405–4413. doi: 10.1523/JNEUROSCI.20-12-04405.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew JY, Garcia-Espana A, Lee KY, Carr KD, Goldstein M, Haycock JW, Meller E. Increased site-specific phosphorylation of tyrosine hydroxylase accompanies stimulation of enzymatic activity induced by cessation of dopamine neuronal activity. Mol. Pharmacol. 1998;55:202–209. doi: 10.1124/mol.55.2.202. [DOI] [PubMed] [Google Scholar]
- Lindblom J, Johansson A, Holmgren A, Grandin E, Nedergard C, Fredriksson R, Schioth HB. Increased mRNA levels of tyrosine hydroxylase and dopamine transporter in the VTA of male rats after chronic food restriction. Eur. J. Neurosci. 2006;23:180–6. doi: 10.1111/j.1460-9568.2005.04531.x. [DOI] [PubMed] [Google Scholar]
- Lindgren N, Xu Z-QD, Herrera-Marschitz M, Haycock J, Hokfelt T, Fisone G. Dopamine D-2 receptors regulate tyrosine hydroxylase activity and phosphorylation at Ser40 in rat striatum. Eur. J. Neurosci. 2001;13:773–780. doi: 10.1046/j.0953-816x.2000.01443.x. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Cooper DC, White FJ. A brief period of reduced food availability increases dopamine neuronal activity and enhances motivation to self-administer cocaine. Behav. Pharmacol. 2002;12(S1):S62. [Google Scholar]
- Moore H, Rose HJ, Grace AA. Chronic cold stress reduces the spontaneous activity of ventral tegmental dopamine neurons. Neuropsychopharmacol. 2001;4:410–419. doi: 10.1016/S0893-133X(00)00188-3. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J. Neurosci. 1995;15:6640–6650. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Sydney: 1998. [Google Scholar]
- Rouge-Pont F, Marinelli M, Le Moal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids. II. Sensitization of the increase in extracellular dopamine induced by cocaine depends on stress-induced corticosterone secretion. J. Neurosci. 1995;15:7189–7195. doi: 10.1523/JNEUROSCI.15-11-07189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Garcia-Espana A, Goldstein M, Deutch AY, Haycock JW. Stoichiometry of tyrosine hydroxylase phosphorylation in the nigrostriatal and mesolimbic systems in vivo: effects of acute haloperidol and related compounds. J. Neurochem. 2000;75:225–232. doi: 10.1046/j.1471-4159.2000.0750225.x. [DOI] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Zhang XX, Jones MD, Bunney BS. Dual effects of d-amphetamine on dopamine neurons mediated by dopamine and nondopamine receptors. J. Neurosci. 2000;20:3504–3511. doi: 10.1523/JNEUROSCI.20-09-03504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Molec. Brain Res. 2004;124:134–42. doi: 10.1016/j.molbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Evans SB, Higgins MS, Pu Y, Figlewicz DP. Food restriction modulates amphetamine-conditioned place preference and nucleus accumbens dopamine release in the rat. Synapse. 2002;46:83–90. doi: 10.1002/syn.10120. [DOI] [PubMed] [Google Scholar]
- Timberlake W, White W. Winning isn’t everything; rats need only food deprivation and not food reward to efficiently transverse a radial arm maze. Learn. Motiv. 1990;21:153–163. [Google Scholar]
- Ventura R, Puglisi-Allegra S. Environment makes amphetamine-induced dopamine release in the nucleus accumbens totally impulse-dependent. Synapse. 2005;58:211–214. doi: 10.1002/syn.20197. [DOI] [PubMed] [Google Scholar]
- Wilson C, Nomikos GG, Collu M, Fibiger HC. Dopaminergic correlates of motivated behavior: importance of drive. J. Neurosci. 1995;15:5169–5178. doi: 10.1523/JNEUROSCI.15-07-05169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen J, Reith MEA, Carr KD. Chronic food restriction and dopamine transporter function in rat striatum. Brain Res. 2006;1082:98–101. doi: 10.1016/j.brainres.2006.01.094. [DOI] [PubMed] [Google Scholar]
- Zheng M, Pintar JE. Analysis of ontogeny of processing enzyme gene expression and regulation. Methods Neurosci. 1995;23:45–64. [Google Scholar]
- Zigmond RE, Schwarzschild MA, Rittenhouse AR. Acute regulation of tyrosine hydroxylase by nerve activity and by neurotransmitters via phosphorylation. Ann. Rev. Neurosci. 1989;12:415–461. doi: 10.1146/annurev.ne.12.030189.002215. [DOI] [PubMed] [Google Scholar]