Abstract
Low-energy surface acoustic waves generated from electrically activated piezo elements are shown to effectively prevent microbial biofilm formation on indwelling medical devices. The development of biofilms by four different bacteria and Candida species is prevented when such elastic waves with amplitudes in the nanometer range are applied. Acoustic-wave-activated Foley catheters have all their surfaces vibrating with longitudinal and transversal dispersion vectors homogeneously surrounding the catheter surfaces. The acoustic waves at the surface are repulsive to bacteria and interfere with the docking and attachment of planktonic microorganisms to solid surfaces that constitute the initial phases of microbial biofilm development. FimH-mediated adhesion of uropathogenic Escherichia coli to guinea pig erythrocytes was prevented at power densities below thresholds that activate bacterial force sensor mechanisms. Elevated power densities dramatically enhanced red blood cell aggregation. We inserted Foley urinary catheters attached with elastic-wave-generating actuators into the urinary tracts of male rabbits. The treatment with the elastic acoustic waves maintained urine sterility for up to 9 days compared to 2 days in control catheterized animals. Scanning electron microscopy and bioburden analyses revealed diminished biofilm development on these catheters. The ability to prevent biofilm formation on indwelling devices and catheters can benefit the implanted medical device industry.
Indwelling device-related infections constitute a major cause of morbidity and mortality in hospitalized patients, adding considerably to medical costs. Microbial biofilms readily develop on all types of devices, urinary, endotracheal, intravenous, and other types of catheters and implants inserted into more than 25% of patients during hospitalization. The incidence of bacterial infections in patients with urinary catheters is approximately 5 to 10% per day, with virtually all patients who undergo long-term catheterization (≥28 days) becoming infected (13, 14, 17).
The first stage in biofilm formation from planktonic microorganisms is attachment to solid surfaces (6). Attachment stimulates microbial aggregation and proliferation to form microcolonies. The colonies excrete an encasing exopolysaccharide “slime,” which consolidates the attachment to surfaces, and the microaggregates differentiate into characteristic biofilms (20). Quorum-sensing molecules that generate concentration gradient-dependent signals that control and alter expression of a large number of genes also aid biofilm differentiation (15, 25). Encasing the extracellular polysaccharide matrix of biofilms regulates exchange of ions and nutrients with the surrounding environment. This regulation contributes to increases of up to 1,000-fold in biofilm resistance to antibiotics compared to planktonic bacteria (9, 11) and protects the biofilms from biocides, surfactants, and predators. Microbial biofilms also present serious challenges to the immune system because expression of bacterial antigens within the encasing polysaccharide matrix is suppressed and the colonies are highly resistant to phagocytosis by polymorphonuclear cells (12). Altogether these properties render biofilms exceedingly difficult to eradicate and explain the severity, persistence, and high levels of morbidity associated with the infections that they produce.
The harsh and potentially fatal consequences of microbial biofilm infections generated efforts to prevent their formation, particularly on indwelling medical devices using chemical and mechanical approaches. Catheters coated with hydrogel, silver salts, and antimicrobials have been evaluated; however, they provide minimal reduction in infection incidence (21). Mechanical approaches to preventing biofilm formation have utilized ultrasonic energy, yet the focus has thus far been on increasing biofilm sensitivity to antibiotics (18). The combination of ultrasound with antibiotics was found effective only in reducing the burden of Escherichia coli biofilms in animal models, falling short of providing a comprehensive solution to the biofilm problem (3).
We devised an innovative approach in which we generate low-energy elastic acoustic waves of practically nonthermal range from electrically activated piezo ceramic elements. The vibration energy is transmitted directly to indwelling medical devices in an integrated unit. Our aim was to achieve dispersion of the acoustic energy on entire surfaces of indwelling medical devices with different consistencies and structures. We analyzed the physical and power requirements for harnessing these waves to prevent microbial attachment and biofilm formation. The findings were consolidated into piezo actuators generating low-power acoustic waves at frequencies ranging from 100 to 300 kHz. The results of studies evaluating the efficacy of these actuators in preventing biofilm formation on indwelling medical devices from several microorganisms, in vitro and in animal models, are presented.
MATERIALS AND METHODS
Generation and dispersion of acoustic vibration energy on the surfaces of catheters.
A device generating surface acoustic waves (SAW) and capable of transmitting the vibration energy directly onto indwelling catheters has been constructed. A battery-powered electronic driver delivers periodical rectangular electrical pulses to an actuator harboring a thin piezo ceramic plate. Piezoelectric vibrations are generated in the actuator at frequencies of 100 to 300 kHz with an acoustic intensity of 200 mW/cm2 and amplitudes of 300 to 800 nm.
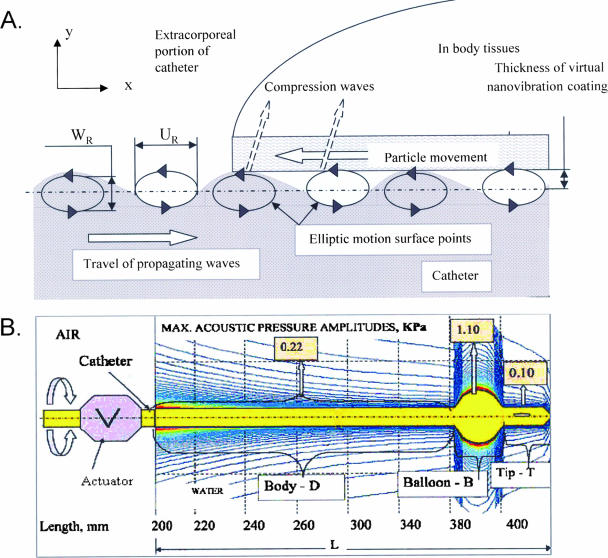
Low-energy SAW spread from an actuator to catheters, covering all surfaces with waves at amplitudes between 0.2 and 2 nm. These waves acquire two vectors as shown in Fig. 1A. A longitudinal vector spreads parallel to the wave propagation x axis along the catheter surface, triggering horizontal particle displacement. Another transversal compression wave component develops on the y axis in the direction of surrounding tissues or fluid. Consequently, all catheters are covered with a virtual vibrating coat (24).
FIG. 1.
(A) Schematic illustration of the modes of dispersion of surface acoustic waves on solid surfaces. Horizontal particle displacement (UR) and another transversal compression wave component (WR) are indicated. (B) Schematic illustration of acoustic pressure amplitude distribution of the coating nanowaves among the different parts of a urinary catheter (body, balloon, and tip). Max., maximum; L, length.
The acoustic pressure amplitudes of the waves vary on different parts of urinary catheters (body, balloon, and tip) as shown in a simulation of their measurements (Fig. 1B). The largest transversal vector directed perpendicular to the catheter surface is detected around the balloon with maximal power intensities of ≤1.1 mW/cm2. These noncavitational power intensities are 3 orders of magnitude lower than the thresholds beyond which cavitation is produced (frequency f = 100 kHz at acoustic intensities of 0.5 × 103 to 2 × 103 mW/cm2) (5, 8).
Evaluation of biofilm prevention on urinary catheters by SAW in vitro.
Sections of 16Fr Foley catheters 6 cm long (siliconized latex; Unomedical, Denmark) were attached to piezo actuators, sterilized with isopropyl alcohol, and placed in 25-ml tissue culture flasks (Corning) through an opening created at the top of the flask. Several commercial microbial strains (E. coli ATCC 25922, Enterococcus faecalis ATCC 19433, Candida albicans ATCC 10231, and Proteus mirabilis ATCC 4630 supplied by Hylabs, Rehovot, Israel) were cultured overnight in Bacto tryptic soy broth (TSB) (Difco). The log-phase cultures were brought to a concentration of 109 CFU/ml determined by optical density at 640 nm and confirmed by plate counts. The selected bacteria were brought to a concentration of 103 CFU/ml in a mixture of (i) 50% of a solution containing 8 g of TSB and 8 ml fetal calf serum (Gibco) in 1 liter of phosphate-buffered saline (PBS) (Gibco) and (ii) 50% heat-sterilized human urine from a healthy donor and placed in a chemostat to which the flasks were connected and sealed with plastic covers. The media were passed over the catheters in the flasks continuously for the 3-day duration of each experiment. Flow was achieved via a peristaltic pump at a rate of 0.5 ml/min under a temperature of ∼30°C with the input medium replaced daily (batch system). Signals for surface acoustic nanowaves were monitored twice daily in the active chambers using a highly sensitive hydrophone. After 3 days, the catheter segments were rinsed and cut into two halves. One half was subjected to sonication at 20 kHz and 3 to 4 W output (model 550 sonicator; Fisher Scientific) to shed the biofilm off the catheter. The overall bioburden on catheter surfaces was assayed by plate counts on blood agar of removed biofilm mass from 3-cm sections of the catheters. Other sections were left intact for biofilm assessment by scanning electron microscopy (SEM).
Preparing catheter samples for SEM.
Catheter samples were fixed in 4% buffered formaldehyde (Frutarom, Israel) and rinsed four times with phosphate-buffered saline (GIBCO). Critical drying was performed with ethanol at concentrations increasing from 25% to 100% in double distilled water. The samples were dried in a critical point dryer (Bio-Rad C.P.D 750), mounted on metal stubs, and coated with a gold layer. Three different points were examined in each catheter by SEM at three magnifications: ×500, ×1,000, and ×3,500.
Catheters removed from rabbit urinary bladders were sectioned into 1-cm-long fragments of the body, balloon, and tip of each catheter and processed for SEM as indicated above. The outer and inner surfaces were evaluated separately at three different magnifications, ×500, ×1,000 and ×3,500, from four different animals in each experimental group.
Evaluation of SAW effects on microbial biofilm formation on urinary catheters in rabbits.
The animal studies were approved by the Animal Care and Welfare Committee of the Israel Ministry of Health. A single piezo actuator was attached to the extracorporeal portion of 10Fr siliconized latex Foley urethral catheter bodies (Unoplast), sterilized with 70% ethanol, and dried. New Zealand White rabbits, 3 to 4 months old and weighing 3.5 to 5.5 kg, were anesthetized with a mixture of 1:1 ketamine (25 mg/ml) and xylazine (20 mg/ml) (0.7 ml/kg of body weight). The perineal region was disinfected with 70% ethanol and antiseptic povidone iodine, the catheters were inserted through the meatus, and the internal balloon was inflated with 3 to 4 ml sterile saline. The rabbits were dressed with a coat-like harness attached to an overhead wire which ran across the top of the cage, enabling limited forward backward movements while preventing the rabbits from pulling out the catheters. A sterile collecting bag was connected to the catheter and replaced daily when urine samples were collected. The extracorporeal portion of the catheter was attached to swing-like devices hanging from the ceiling. These devices allowed free mobility of the catheter with movements of the rabbit and prevented friction with the cage floor, premature catheter detachment, and excessive contamination with feces.
Following catheterization, the piezo elements were activated with power from an alternating current source and remained active throughout the full duration of the experiments (7 days in one experiment, up to 8 days in a second experiment, and 9 days in a third experiment). Catheters showing markedly decreased or no urine output for 12 h were unblocked using sterile flexible wires. Urine was collected once daily in a sterile manner from the bag throughout the experiment, serial dilutions were performed in PBS, 100 μl was dispersed evenly on blood agar plates (Hylabs, Rehovot, Israel), and bacterial counts were performed after 24 h. Animals that developed bacteriuria of >105 CFU/ml were excluded in accord with Animal Care Committee requirements.
Induction of guinea pig erythrocyte aggregation by mannose receptor-specific adhesion of uropathogenic E. coli bacteria bearing type 1 pili.
A strain of uropathogenic E. coli bacteria bearing type 1 pili and displaying the FimH lectin was selected from clinical isolates at the microbiology laboratory of the Sheba Medical Center. The bacteria were analyzed for the ability to form biofilms and the ability to induce guinea pig red blood cell (RBC) aggregation. Bacteria (109/ml) were applied to a 4% guinea pig erythrocyte suspension in saline (0.9% NaCl) in 50-mm Miniplast petri dishes to which a single SAW actuator has been attached at the external bottom surface of the plates. d-Mannose at a final concentration of 50 mM was used to confirm mannose receptor specificity of the interaction with FimH. The plates were monitored microscopically at room temperature after 15 min, 1 h, and 3 hours for the effects of SAW on bacterial adhesion-mediated aggregation and photographed with a Nikon digital camera.
Statistical analyses.
The two-tailed Student t test was used for determination of statistical significance with a P of <0.05 as a cutoff.
RESULTS
Prevention of microbial biofilm formation by surface acoustic waves.
We examined the effects of low-energy SAW on biofilm formation by four common clinically relevant types of microorganisms on several types of surfaces, including 16Fr urinary catheters to which actuators were attached. Bacterial bioburden on catheter surfaces, measured by plate counts, revealed marked reductions in the biofilm loads formed on surfaces of SAW-treated catheters ranging from −0.75 to −1.22 log10 (P ≤ 0.05, n = 3) relative to controls (Table 1).
TABLE 1.
Bioburden analyses (CFU/cm2) of microbial biofilms developing on 16Fr Foley catheters treated with SAWa
| Microbial species | Bioburden (CFU/cm2) of microbial biofilm developing on 16Fr Foley catheter
|
SDb | Log reductionc | P valuec | |
|---|---|---|---|---|---|
| Control | SAW-treated | ||||
| Escherichia coli | 4.07E+04 | 6.55E+03 | 1.84E+05 | −0.79 | 0.009 |
| Candida albicans | 1.52E+04 | 9.02E+02 | 1.13E+04 | −1.22 | 0.050 |
| Proteus mirabilis | 6.90E+04 | 4.87E+03 | 1.20E+05 | −1.15 | 0.001 |
| Enterococcus faecalis | 4.42E+04 | 7.78E+03 | 2.26E+05 | −0.75 | 0.015 |
Three-centimeter sections were prepared from each catheter, sonicated at 20 KHz and 3 to 4 W in two 30-second pulses to shed the catheter-associated biofilms and disperse them in solution for titration. Microbial counts correspond to overall load on 3-cm-long catheter sections.
Standard deviations refer to differences in bacterial loads between the control and the SAW-treated in the three repetitions of each analysis.
Log reduction values and P values compare the bioburdens for control and SAW-treated catheters.
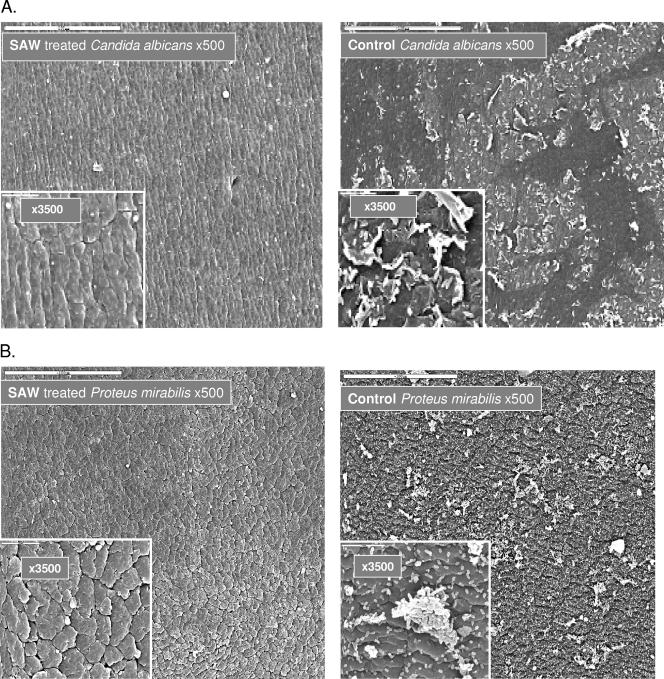
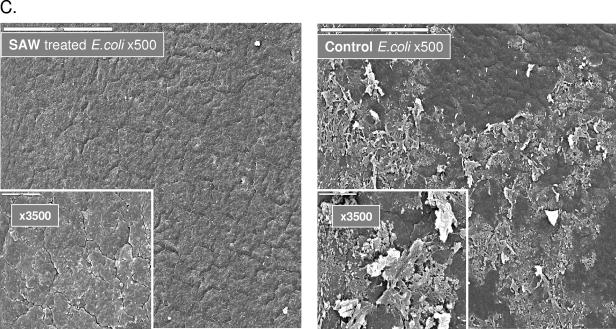
Other segments of these catheters were examined by scanning electron microscopy, and results obtained with Candida albicans, Proteus mirabilis, and E. coli are presented in Fig. 2. The SAW treatment effectively reduced biofilm formation, leaving catheters virtually clean of adherent microorganisms, irrespective of the types of bacteria that were examined. Similar prevention of microbial cell adhesion and biofilm formation was also noted on glass rod surfaces attached with piezo actuators (data not shown), indicating that these element-generated elastic waves can be adjusted to prevent microbial adhesion and biofilm formation on surfaces with different consistencies and shapes.
FIG.2.
Scanning electron microscopic analyses of external surfaces of SAW-vibrated 16Fr urinary catheter segments, on which several types of bacteria were passed in culture. Catheter segments, 6 cm long in 25-ml tissue culture flasks (Corning, N.Y.), were attached to a piezo resonator that generated acoustic pressure amplitudes ranging from 0.16 kPa at the edge of the catheter to 0.21 kPa at the center. Fresh media containing 103 CFU/ml of several types of bacteria (from ATCC) were pumped continuously from chemostats at 0.5 ml/min and a temperature of ∼30°C for 3 days. The segments were fixed in 4% buffered formaldehyde, rinsed four times with PBS, and dehydrated incrementally with 25% to 100% aqueous ethanol gradients. Following drying in a Bio-Rad C.P.D 750 critical point dryer, the samples were mounted on metal stubs and coated with a gold layer, and three different areas on each catheter were examined by SEM. Surfaces of SAW-treated catheters (left panels) are compared to nontreated controls (right panels).
Surface acoustic waves interfere with adhesion of planktonic microorganisms to cellular surfaces.
Our analyses of mechanisms by which SAW interfere with bacterial biofilm formation focused on the hypothesis that SAW target the adhesion of planktonic bacteria to surfaces, the first step in the biofilm formation process. To evaluate the effects of SAW on bacterial adhesion, we used the mannose receptor-specific adhesion of uropathogenic E. coli bacteria to guinea pig erythrocytes as a model; the adhesion occurs via type 1 pili, FimH lectin and culminates in RBC aggregation (22). In this system, bacterial adhesion occurs rapidly, can be easily monitored microscopically in real time, and enables an easy and accurate monitoring of the reversibility of the acoustic wave effect upon cessation of the treatment.
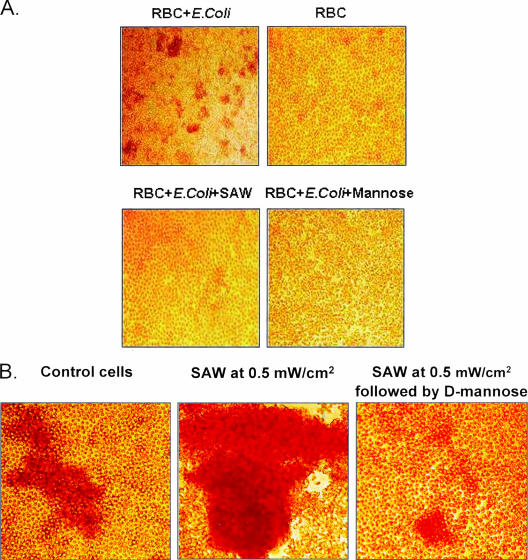
Vibration energy-generating actuators were attached to the external bottom surfaces of 50-mm Miniplast petri dishes in which uropathogenic E. coli bacteria were cocultured with guinea pig RBC. Power intensities of 0.1 and 0.2 mW/cm2, generating vibration frequencies of 95 kHz and 220 kHz with acoustic pressure amplitudes of 0.1 and 0.22 kPa, respectively (equivalent to those measured on the tip and body of the urinary catheter), were applied. RBC aggregation mediated by bacterial adhesion was monitored; it became detectable in control dishes 12 min ± 3 min after administration of the bacteria and was monitored for hours. Figure 3A shows that SAW effectively prevented RBC aggregation at these two power intensity outputs throughout the follow-up time. The findings support our hypothesis that SAW interfere with lectin-mediated adhesion of planktonic bacteria to substrates.
FIG. 3.
(A) Prevention of guinea pig RBC aggregation induced by adhesion of type 1 pilus-positive E. coli bacteria. Surface acoustic waves at a power intensity of 0.2 mW/cm2 are shown to effectively prevent mannose receptor-specific adhesion of bacteria to RBC and their subsequent aggregation. Specificity was confirmed with 50 mM d-mannose. (B) Enhancement of E. coli-induced guinea pig RBC aggregation by high-energy SAW. Surface acoustic waves applied at a power intensity of 0.5 mW/cm2 are shown to enhance mannose receptor-specific bacterial adhesion to RBC. The samples in panels A and B were photographed 3 h after administration of bacteria and initiation of treatment with SAW. Exceedingly large RBC aggregates formed, as shown in Fig. 3B (middle panel), which were susceptible to dissociation with d-mannose (Fig. 3B, right panel).
We deactivated the SAW treatment and continued to monitor the plates with time-lapse photography. Guinea pig erythrocyte aggregation resumed 10 min ± 4 min after SAW termination, a rate similar to RBC aggregation in control plates (12 min ± 3 min; difference not significant). These findings indicate that inhibition of RBC aggregation by SAW is mechanical, readily reversible following SAW deactivation, and does not diminish the functionality of the FimH lectin on fimbriae. The bacterial mechanism for adhesion to RBC and other cells is thus not damaged by SAW. Once aggregation has taken place, RBC aggregates could no longer be dissociated by resumption of the SAW treatment (not shown), although it was reversed by d-mannose.
We next examined the correlation between levels of SAW energy that were applied and E. coli-induced RBC aggregation. SAW activated with 0.05 to 0.20 mW/cm2 effectively prevented RBC aggregation (Fig. 3A); however, increasing the output to beyond a 0.35-mW/cm2 threshold converted the inhibition into a significant enhancement of bacterial attachment. Exceedingly large RBC aggregates formed as shown in Fig. 3B, which were susceptible to dissociation with d-mannose (Fig. 3B) and gradually dissolved upon cessation of the SAW treatment (not shown). Hence, SAW applied at power intensities beyond approximately 0.35 mW/cm2 can activate FimH force sensor activity in a manner similar to force sensor activation seen when shear force is applied to uropathogenic E. coli bacteria (22).
Prevention of microbial biofilm formation on urinary catheters with acoustic nanowave actuators in an animal model in vivo.
The ultimate preclinical determination of whether SAW-generating piezo actuators can interfere with microbial biofilm formation on urinary catheters in clinical settings is in animal studies. We inserted 10Fr Foley catheters attached with a piezo actuator at the extracorporeal portion of the catheter into the urinary bladders of male rabbits in a sterile manner. The devices were activated for up to 9 days in four of eight tested rabbits (in three separate experiments). Urine samples were collected daily, the bacterial load was titrated, and time to bacteriuria was determined. Urine samples from rabbits with SAW-treated catheters remained sterile for 5, 7, and 9 days (26 cumulative days of sterile urine) despite the extensive contamination of the perineal area with feces. Furthermore, the bacteriuria that did develop in some rabbits was mostly of low titers, whereas three of four control rabbits developed bacteriuria of >106 CFU/ml within 2 or 3 days and the fourth had a titer of >108 CFU/ml on day 7. The average number of days to development of urinary tract infection, defined as bacteriuria of >105 CFU/ml, was 7.3 ± 1.3 days for the SAW-treated animals versus 1.5 ± 0.6 days in the nontreated controls (P < 0.0009 by two-tailed Student's t test; n = 4) (Table 2).
TABLE 2.
Time to bacteriuria in rabbits with 10Fr Foley catheters and SAW-generating piezo actuatorsa
| Rabbit | Bacterial titer (CFU/ml) on:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | |
| SAW-treated rabbits | |||||||||
| 163 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 229 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 265 | 0 | 0 | 0 | 0 | 0 | 104 | 106 | ||
| 143 | 0 | 0 | 0 | 0 | 0 | 3 × 103 | 2 × 104 | ||
| Control rabbits | |||||||||
| 28 | 0 | 1.4 × 107 | 5 × 107 | ||||||
| 31 | 4 | 104 | 5 × 103 | 104 | 0 | 0 | 8 × 108 | 2.5 × 108 | |
| 150 | 0 | 6 | 4 × 106 | 108 | |||||
| 144 | 70 | 106 | 5 × 106 | ||||||
Rabbits had 10Fr Foley catheters inserted. The catheters were attached to SAW-generating piezo actuators at the extracorporeal body of the catheters. Animals that developed bacteriuria were removed and their participation in the experiments was terminated due to limitations imposed by the Animal Welfare and Care Committee.
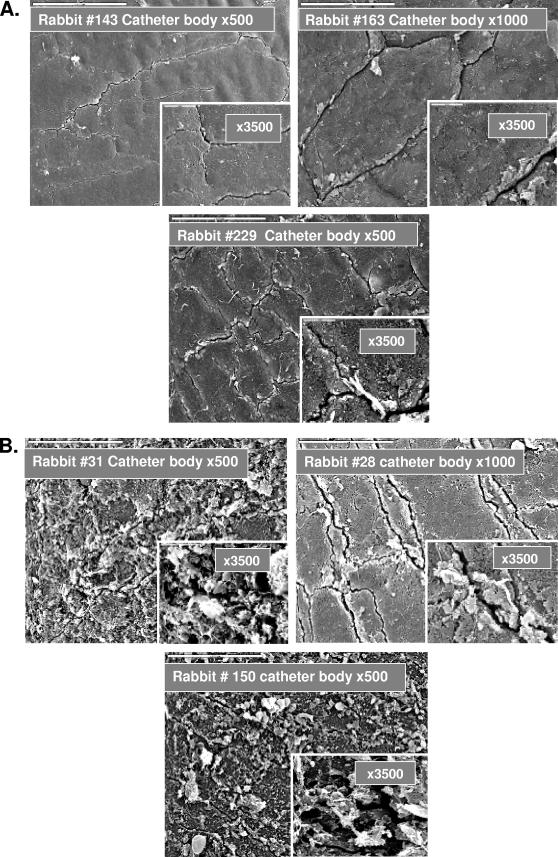
At the end of the experiments, the animals were sacrificed, the bladder and urethra were cut open, and the catheters were removed carefully, avoiding disruption of the biofilms. Biofilm content was examined by SEM. Analyses of the internal surfaces of recovered catheters revealed strong inhibition of bacterial biofilm formation on the surfaces of catheters treated with SAW (Fig. 4A). In contrast, control group catheters were covered with various densities of microbial biofilms despite the shorter durations of catheterization (in two of the animals, the catheters were in place for only 3 or 4 days) (Fig. 4B).
FIG. 4.
SEM analyses of the inner surfaces of catheters recovered from rabbit bladders following treatment with SAW in vivo. Catheters were removed from rabbit urinary bladders, sectioned (into body, balloon, and tip), and processed for SEM as described in the legend to Fig. 2. (A) SAW-treated animals and (B) control animals.
Evaluation of the integrity of mucous membranes by histological and ultrastructural analyses in all control and SAW-treated animals revealed that the treatment with SAW did not produce any histopathological changes. Furthermore, uroepithelial integrity was found to be less affected by trauma and better conserved in the SAW-treated animals than in the controls (data not shown).
DISCUSSION
The remarkable flexibility by which microorganisms adapt to changing environments or become insulated from environmental hazards has been the core of shortcomings in the ability of chemical approaches to prevent microbial biofilm formation on implanted medical devices. Efforts to eradicate biofilms therefore include mechanical approaches, which thus far have mainly been aimed at increasing the penetration of antibiotics into microbial colonies (3, 18).
We have contemplated utilization of mechanical vibration energy to interfere with early events in the biofilm development process—the adhesion of planktonic microorganisms to surfaces. By preventing adhesion, we sought to abort their subsequent firm attachment to substrates (1), gene expression reprogramming, and synthesis of the corresponding protein products that transform the lifestyle of microorganisms from the planktonic to sessile form (2, 4, 19). We also speculate that chaotic microstreaming produced in fluids by the ongoing vibrations hampers the development of coherent concentration-dependent gradients of quorum-sensing molecules. Disruption of such gradients is likely to interfere with cell-cell communications between microorganisms, virulence factor production, and other postattachment biofilm developmental processes. The outcome is prevention of colony differentiation and biofilm formation (7, 10, 16).
We show that low-energy elastic acoustic waves transmitted directly to extracorporeal portions of implanted medical devices can interfere effectively with attachment of planktonic microorganism to surfaces and prevent biofilm formation for extended time intervals. The mechanical nature of this treatment implies that the elastic waves must be powered continuously throughout the duration of device implantation to prevent attachment of planktonic bacteria. Disruption of the vibration energy is found to promote renewed adhesion of bacteria to these surfaces, indicating that the effects of SAW are readily reversible and do not diminish the functionality of bacterial adhesion mechanisms. For example, the fimbrial FimH lectin of uropathogenic E. coli allowed attachment of E. coli to guinea pig RBC following disruption of SAW.
A unique feature of this approach is the effectiveness of minute power intensities in preventing bacterial attachment to substrates. Analyses of mannose receptor-mediated adhesion of E. coli to guinea pig erythrocytes reveal that power densities ranging from 0.05 to 0.20 mW/cm2 with amplitudes of ≤3 nm completely prevent erythrocyte aggregation. In contrast, SAW intensities of >0.35 mW/cm2 generate opposite effects, inducing strong FimH-mediated adhesion of the bacteria and enhanced RBC aggregation (Fig. 3B). This response to high SAW intensities bears similarities to the response of these bacteria to shear stress. Under stress, the FimH lectin has been reported to act as a force sensor switching bacterial loose adhesion into a firm attachment (22). Application of high-SAW power intensities to E. coli bacteria cocultured with guinea pig RBC also yielded a similar type of switching to enhanced erythrocyte aggregation.
We propose the following hypothesis to explain the low-energy SAW-mediated biofilm prevention phenomenon. Attraction or repulsion of bacteria in the 10-nm range near surfaces is an outcome of van der Waals and hydrophobic attraction forces being counteracted by electrostatic repulsion (6). This phenomenon known as the Z potential of the surface varies with the distance from the interface. SAW-induced elliptical vibrations affect the surface and are transmitted through the surrounding fluid media, causing the bacteria to vibrate with the same frequency. The amplitude of bacterial vibration is smaller than that of the surface, is governed by Stoke's law, and results in a relative velocity of bacteria respective to the surface (16). When the SAW-generated bacterial vibration amplitudes are smaller than the Z-potential repulsive zone, an overall net repulsion occurs, preventing bacterial attachment. This is the hallmark of SAW. Increasing the bacterial vibration amplitudes to values exceeding the Z-potential repulsion zone results in a net attraction force, promoting the adhesion of bacteria. Such SAW intensities also activate bacterial docking and force sensor activities, and this synergism can elicit the increased adhesion of bacteria which we noted at the higher SAW intensities.
The studies which show that SAW reduces biofilm bioburden on catheter segments in suspensions with several gram-negative and -positive bacteria as well as fungi indicate that the action of SAW is efficacious against a broad spectrum of microorganisms and not limited to selected groups. The studies in rabbits demonstrate the feasibility of delaying catheter-associated urinary tract infections with SAW. Conditioning films encrusted with proteins, electrolytes, and other organic molecules that develop on urinary catheters shortly after their insertion (23) do not appear to interfere with biofilm prevention by SAW. The absence of any detectable adverse effects from treatments with SAW suggest that this system may potentially be attached to a variety of indwelling medical devices, including endotracheal tubes and central venous or peritoneal dialysis catheters. The entire medical device industry, including prosthetic joints and others, is likely to benefit from this approach.
Acknowledgments
We are grateful to Naomi Bahat of the Faculty of Agriculture of Hebrew University for her assistance in performance of SEM analyses.
Footnotes
Published ahead of print on 28 August 2006.
REFERENCES
- 1.An, Y. H., R. B. Dickinson, and R. J. Doyle. 2000. Mechanisms of bacterial adhesion and pathogenesis of implant and tissue infections, p. 1-21. In Y. H. An and R. J. Friedman (ed.), Handbook of bacterial adhesion: principles, methods and applications. Humana Press, Totowa, N.J.
- 2.Brozel, V. S., G. M. Strydom, and T. E. Cloete. 1995. A method for the study of de novo protein synthesis in Pseudomonas aeruginosa after attachment. Biofouling 8:195-210. [Google Scholar]
- 3.Carmen, J. C., B. L. Roeder, J. L. Nelson, R. L. Ogilvie, R. A. Robison, G. B. Schaalje, and W. G. Pitt. 2005. Treatment of biofilm infections on implants with low-frequency ultrasound and antibiotics. Am. J. Infect. Control 33:78-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies, D. G., A. M. Chakrabarty, and G. G. Geesey. 1993. Exopolysaccharide production in biofilms: substratum activation of alginate gene expression by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 59:1181-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donskoj, L. B., O. K. Keller, and G. S. Kratysh. 1982. Ultrazvukovije elektrotechnologiceskije ustanovki. In Energija, p. 149-192. Energiosdat, St. Petersburg, Russia.
- 6.Gristina, A. G. 1987. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science 237:1588-1595. [DOI] [PubMed] [Google Scholar]
- 7.Hastings, J. W., and K. H. Nealsen. 1977. Bacterial bioluminescence. Annu. Rev. Microbiol. 31:549-595. [DOI] [PubMed] [Google Scholar]
- 8.Leighton, T. G. 1997. The acoustic bubble, p. 526-528. Academic Press, New York, N.Y.
- 9.Lewis, K. 2001. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li, Y. H., N. Tang, M. B. Aspiras, P. C. Lau, J. H. Lee, R. P. Ellen, and D. G. Cvitkovitch. 2002. A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J. Bacteriol. 184:2699-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mah, T. F., and G. A. O'Toole. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34-39. [DOI] [PubMed] [Google Scholar]
- 12.Mahenthiralingam, E., M. E. Campbell, and D. P. Speert. 1994. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect. Immun. 62:596-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maki, D. G., and L. A. Mermel. 1998. Infections due to infusion therapy, p. 689-724. In J. V. Bennet and P. S. Brachman (ed.), Hospital infections, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 14.Maki, D. G., and P. A. Tambyah. 2001. Engineering out the risk of infection with urinary catheters. Emerg. Infect. Dis. 7:342-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pesci, E. C., and B. H. Iglewski. 1997. The chain of command in Pseudomonas quorum sensing. Trends Microbiol. 5:132-135. [DOI] [PubMed] [Google Scholar]
- 16.Qian, Z., R. D. Sagers, and W. G. Pitt. 1999. Investigation of the mechanism of the bioacoustic effect. J. Biomed. Mater. Res. 44:198-205. [DOI] [PubMed] [Google Scholar]
- 17.Raad, I. 1998. Intravascular-catheter-related infections. Lancet 351:893-898. [DOI] [PubMed] [Google Scholar]
- 18.Rediske, A. M., B. L. Roeder, J. L. Nelson, R. L. Robison, G. B. Schaalje, R. A. Robison, and W. G. Pitt. 2000. Pulsed ultrasound enhances the killing of Escherichia coli biofilms by aminoglycoside antibiotics in vivo. Antimicrob. Agents Chemother. 44:771-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sauer, K., and A. K. Camper. 2001. Characterization of phenotypic changes in Pseudomonas putida in response to surface-associated growth. J. Bacteriol. 183:6579-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schierholz, J. M., and J. Beuth. 2001. Implant infections: a haven for opportunistic bacteria. J. Hosp. Infect. 49:87-93. [DOI] [PubMed] [Google Scholar]
- 21.Thibon, P., X. Le Coutour, R. Leroyer, and J. Fabry. 2000. Randomized multi-centre trial of the effects of a catheter coated with hydrogel and silver salts on the incidence of hospital-acquired urinary tract infections. J. Hosp. Infect. 45:117-1124. [DOI] [PubMed] [Google Scholar]
- 22.Thomas, W. E., E. Trintchina, and E. V. Sokurenko. 2002. Bacterial adhesion to target cells enhanced by shear force. Cell 109:913-923. [DOI] [PubMed] [Google Scholar]
- 23.Trautner, B. W., and R. O. Darouich. 2004. Catheter-associated infections: pathogenesis affects prevention. Arch. Intern. Med. 164:842-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Victorov, I. A. 1981. Surface sound waves in solids, p. 5-10. Nauka Publishing, Moscow, Russia.
- 25.Whiteley, M., K. M. Lee, and E. P. Greenberg. 1999. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:13904-13909. [DOI] [PMC free article] [PubMed] [Google Scholar]