Abstract
Drug-induced inhibition of fungal growth is used in the diagnostic laboratory to predict therapeutic efficacy but is relatively slow, and determination of endpoints can be problematic. Nuclear magnetic resonance (NMR) spectroscopy identifies the metabolic complement of microorganisms while monitoring utilization of constituents of the incubation medium. This technique may provide a rapid and objective indicator of antifungal effects. We evaluated the effects of caspofungin, amphotericin B (AMB), and voriconazole on metabolic profiles of yeast species cultured in RPMI-2% glucose-morpholinepropanesulfonic acid buffer in microtiter plates in a proof-of-principle study. 1H NMR spectra were obtained using Bruker NMR spectrometers at 1H frequencies of 600 and 360 MHz. Metabolites were identified by two-dimensional correlation NMR spectra, and relative peak integrals were calculated from one-dimensional 1H NMR spectra. MICs were determined by a modification of the Clinical and Laboratory Standards Institute broth microdilution method M27-A. Utilization of glucose and branched-chain and aromatic amino acid substrates was accompanied by fungal production of acetate, acetaldehyde, ethanol, formate, fumarate, glycerol, lactate, pyruvate, and succinate. Clear-cut metabolic endpoints indicating a greater than 50% reduction in substrate utilization and fungal metabolite production which correlated with MICs were noted at 16 and 24 h for all three drugs. At 8 h, reductions of greater than 50% for selected metabolites were noted for caspofungin and AMB. Direct NMR-based observation of metabolic alterations in yeast cultures reveals changes in key metabolic pathways and should be evaluated formally as a rapid technique for determining susceptibility to antifungal drugs.
The effects of drugs on fungi are measured in the diagnostic laboratory by growth inhibition using standardized methods including broth microdilution or agar based-methods such as the E-test. These aim to predict therapeutic efficacy by generating a quantitative result expressed as the MIC of the drug. Clinical breakpoints have been established in invasive yeast infections for fluconazole, itraconazole, and amphotericin B (AMB) (for a review, see reference 18) and voriconazole (13). The main drawbacks of these tests, which are simple and relatively cheap, are the long turnaround time due to reliance on fungal growth as the endpoint (up to 72 h) and interpretation of the trailing endpoints that occur particularly with azole drugs.
High-throughput analytical technologies such as nuclear magnetic resonance (NMR) spectroscopy are used to determine the metabolic composition of complex mixtures and are applicable to living cells. Such a “metabolic profiling” approach, combined with analysis using multivariate statistical tools, enabled spectral profiles from yeasts of medical importance to be distinguished and the respective species to be identified (2, 6, 7, 8). Changes in the metabolic profiles of Cryptococcus neoformans and Candida species that were induced by osmotic or heat stress and different culture media have also been identified using NMR spectroscopy (2, 6, 8).
In the present study the effects of antifungal drug pressure on the metabolic profiles of a range of yeast species were evaluated in broth culture, which allowed simultaneous monitoring of the utilization of constituents of the incubation medium and production of fungal metabolites. Large-volume broth cultures were used initially to establish that the major yeast fermentation product, ethanol, was detectable by NMR spectroscopy and to establish the time course of production. Subsequently, a microtiter broth dilution format was used, since this is readily available in clinical and microbiological research laboratories, can be automated, and allows direct comparison with standard MICs. Candida species sensitive and resistant to caspofungin, clinical isolates, and a standard ATCC strain of Candida albicans were tested against caspofungin. AMB and voriconazole were included as representative antifungal drugs with different modes of action. We show that direct NMR-based observation of metabolic alterations in suspensions of fungi is reproducible, precedes detectable inhibition of fungal growth by conventional means, and invites formal investigation as a platform for rapid determination of susceptibility to antifungal drugs.
(This work was presented in part as a poster at the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy, 16 to 19 December 2005, Washington, D.C. [abstract M-1615].)
MATERIALS AND METHODS
Yeast strains.
The provenance of the yeast species used in this study is summarized in Table 1.
TABLE 1.
Provenance of Candida strains
| Study and isolate | Species | Source/commenta |
|---|---|---|
| Preliminary broth dilution study | ||
| 01-132-1299 | C. albicans | Clinical isolate, Westmead Hospital, Sydney, Australia |
| 01-1741796 | C. albicans | Clinical isolate, Westmead Hospital, Sydney, Australia |
| 01-234-0858 | C. albicans | Clinical isolate, Westmead Hospital, Sydney, Australia |
| Y123 | Clavispora lusitaniae | MSD, source unknown |
| 97-291-0929 | C. lusitaniae | Clinical isolate, Westmead Hospital, Sydney, Australia |
| 99-167-0481 | C. lusitaniae | Clinical isolate, Westmead Hospital, Sydney, Australia |
| 00-049-3110 | Trichosporon beigelii | Clinical isolate, Westmead Hospital, Sydney, Australia |
| Microbroth dilution study | ||
| ATCC 90028 | Candida albicans | |
| Y105 | C. albicans | MSD, heterozygous for semidominant mutation in C. albicans FKSb; reduced susceptibility to caspofungin |
| Y111 | C. albicans | MSD, clinical (blood) isolate, reduced susceptibility to caspofungin |
| TS3 | C. albicans | Clinical isolate, Westmead Hospital, Sydney, Australia |
| 03-226-3110 | C. albicans | Clinical isolate, Westmead Hospital, Sydney, Australia |
| Y114 | Candida glabrata | MSD, clinical (blood) isolate from Chile |
| 03-257-1430 | C. glabrata | Clinical (blood) isolate, Westmead Hospital, Sydney, Australia |
| Y117 | Candida krusei | MSD, clinical (blood) isolate, Buffalo, New York |
| Y118 | C. krusei | MSD, clinical (stool) isolate, Germany; reduced susceptibility to caspofungin |
| 9235 | C. parapsilosis | Clinical (blood) isolate, CIDMLS |
| 9236 | C. parapsilosis | Clinical (blood) isolate, CIDMLS |
| 8336 | C. guilliermondii | Clinical (blood) isolate, CIDMLS |
| 8333 | C. guilliermondii | Clinical (blood) isolate, CIDMLS |
MSD, Merck, Sharp and Dohme; CIDMLS, Centre for Infectious Diseases and Microbiology Laboratory Services, Westmead Hospital, Sydney, Australia.
Antifungal agents.
Caspofungin pure powder (Merck Research Laboratories, Rahway, NJ) was dissolved in sterile water. Stock solutions of AMB powder (Apothecon; Bristol-Myers Squibb, Princeton, NJ) and voriconazole pure powder (Pfizer, Sandwich, Kent, United Kingdom) were made up in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO). Serial dilutions of drug in RPMI 1640 (Sigma-Aldrich), supplemented with 2% (wt/vol) glucose and buffered at pH 7 with 0.165 M 3-(N-morpholino)propanesulfonic acid (MOPS; Sigma-Aldrich), or supplemented medium alone were dispensed in 100-μl aliquots into 96-well, flat-bottomed microtiter plates (Linbro; ICN Biomedicals and Reagents, Sydney, NSW, Australia). Plates were stored at −70°C for a maximum of 3 weeks before use.
Broth microdilution MICs.
MICs were determined by a modification of the reference method of the Clinical and Laboratory Standards Institute (CLSI) (11). Isolates were recovered from stock and cultured on Sabouraud's dextrose agar (Difco, Sparks, MD) at 35°C for 48 h. The isolates were subcultured, incubated for 24 h at 35°C, and adjusted to a concentration of 0.5 × 103 to 2.5 × 103/ml in supplemented RPMI medium. Aliquots (100 μl) were dispensed into microtiter wells containing antifungal drug. Growth and sterility controls were included in each run. MICs were determined at 24 h visually and by measurement of optical density at a wavelength of 530 nm in a spectrophotometer. Experiments were performed in duplicate. Endpoints were defined as the lowest concentration of caspofungin or voriconazole that caused prominent growth reduction (≥50% reduction by spectrophotometer reading) and, for AMB, the lowest concentration that caused complete inhibition of growth on visual inspection (≥80% by spectrophotometer reading).
Effect of amphotericin B on the time course of ethanol production by yeast species with different susceptibilities to amphotericin B.
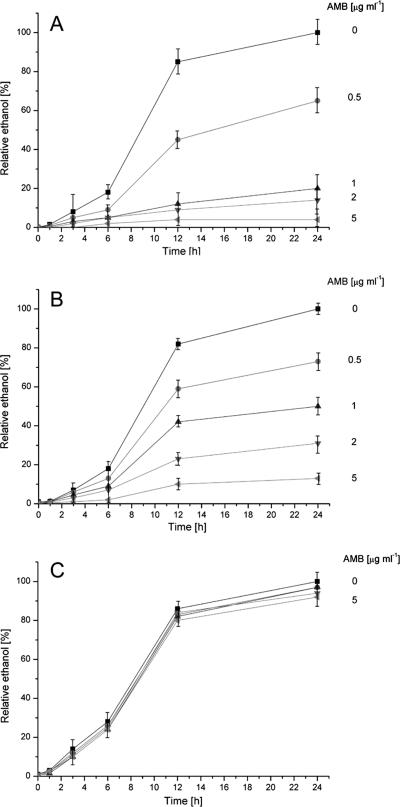
Large-volume broth cultures were used initially to establish that the major yeast fermentation product, ethanol, is detectable by NMR spectroscopy and for time course studies of the effect of different concentrations of AMB on ethanol production. Six clinical yeast strains were tested (Table 1). Four were susceptible to AMB (three of C. albicans and one of Clavispora lusitaniae, 99-167-0481, MIC = 0.06 to 0.12 μg ml−1), one had decreased susceptibility (C. lusitaniae, 97-291-0929, MIC = 0.5 μg ml−1), and one was resistant (Trichosporon beigelii, MIC = 4 μg ml−1). An inoculum density of 2 × 107 ml−1 was prepared in 20 ml yeast-nitrogen broth with 2% (wt/vol) glucose and buffered at pH 7 with 165 mM MOPS. A constant volume of DMSO (0.4 ml) containing AMB to result in a final concentration of 0, 0.1, 0.5, 1, 2, and 10 μg ml−1 was added to the suspension at zero time. Suspensions were incubated for 24 h at 35°C in a water bath with shaking at 30 rpm. A volume of 0.4 ml was withdrawn after 1, 3, 6, 12, and 24 h and transferred to a 5-mm NMR tube (Wilmad, Buena, NJ) containing 0.1 ml phosphate-buffered saline (PBS; ICN Biomedicals, Aurora, OH) made up in 99.5% (vol/vol) deuterated water (D2O; Australian Nuclear Science and Technology Organization, Lucas Heights, NSW, Australia). One-dimensional 1H NMR spectra were acquired using a Bruker Avance NMR spectrometer equipped with a 5-mm [1H, 13C] inverse-detection dual-frequency probe operating at 360.1 MHz. Acquisition parameters were as follows: repetition time, 3 s; 4,096 data points; 32 transients; and spectral width, 3,600 Hz. D2O was used for the field frequency lock. Water suppression was performed by a selective-excitation field gradient method. Chemical shift referencing was performed by setting the center of the spectrum to 4.64 ppm (the nominal position of the water resonance with respect to tetramethylsilane in PBS-D2O at 37°C). All spectra from each strain (different time points as well as AMB concentrations) were acquired using the same receiver gain in order to allow relative quantification. Relative ethanol concentration was compared for the various time points, and range of AMB concentrations of a particular strain (see Fig. 1), by setting the integral for the ethanol resonance at 1.18 ppm of the control culture ([AMB] = 0 mM) at the endpoint (t = 24 h) to 100%. Experiments on each yeast strain were performed in duplicate.
FIG. 1.
Relative quantification and reproducibility of ethanol synthesis (integral of the resonance at 1.15 to 1.20 ppm) by various yeast strains in the presence of AMB. Yeasts were incubated for 24 h with 0, 0.5, 1, 2, and 5 μg/ml of AMB. Integrals were quantified relative to the ethanol signal in the yeast suspension without AMB after 24 h (for each strain). Ethanol was quantified after 1, 3, 6, 12, and 24 h. (A) Mean values and standard deviations (error bars) from three C. albicans isolates (two cultures per isolate) with MICs between 0.06 and 0.12 μg/ml. (B) Mean values and standard deviations (error bars) from one C. lusitaniae isolate (97-291-0929, two cultures) with a MIC of 0.5 μg/ml. (C) Mean values and standard deviations (error bars) from one T. beigelii isolate (two cultures) with a MIC of 4 μg/ml.
Broth microdilution samples and NMR spectroscopy.
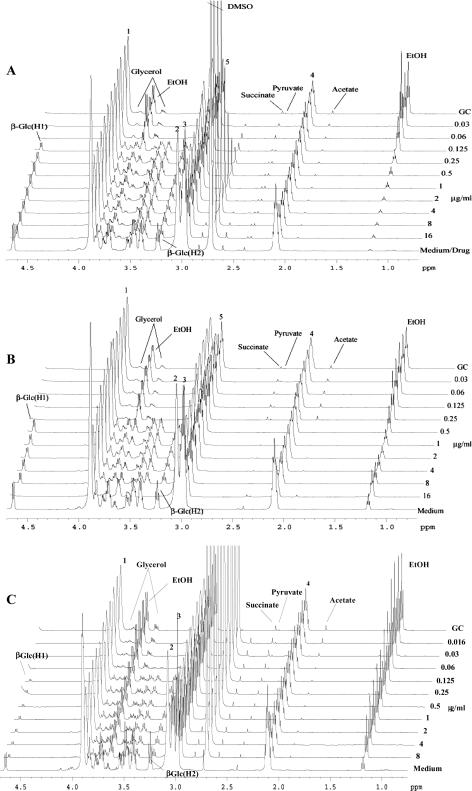
Preliminary experiments indicated that incubation of drugs with fungal inocula at least 1,000× higher than those used for determination of MICs (CLSI method [11]) or 10× higher than that proposed by the Antifungal Susceptibility Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST [4]) resulted in obvious utilization of glucose and other nutrients from the medium and higher concentrations of fungal metabolites when visualized in the NMR spectra (see Fig. 2). When standard inocula (CLSI method) were used, no utilization of glucose was detected and only small amounts of ethanol were produced after 24 h of incubation (not shown). Inocula adjusted using 0.5 or 2.0 McFarland standards yielded similar results that were reproducible (not shown). An inoculum density of 1 × 106 to 5 × 106 ml−1 (equivalent to a 0.5 McFarland standard) was used subsequently, except where otherwise indicated. Cultures were incubated at 35°C for 8, 16, or 24 h in a moist environment. Experiments were performed in duplicate.
FIG. 2.
Representative 1H NMR spectra of samples of C. albicans ATCC 90028 incubated with AMB (A), caspofungin (B), and voriconazole (C). Drug concentrations are indicated on the right. GC is the growth control; the spectrum of medium alone is at the bottom (for panel A, well 1 contained 16 μg/ml drug in addition to medium). The numbered resonances correspond to the MOPS buffer as follows: 1, O—(CH2)2-(CH2)2-N-CH2-CH2-CH2-SO3−Na+; 2, O—(CH2)2-(CH2)2-N-CH2-CH2-CH2-SO3−Na+; 3, O—(CH2)2-(CH2)2-N-CH2-CH2-CH2-SO3−Na+; 4, O—(CH2)2-(CH2)2-N-CH2-CH2-CH2-SO3−Na+; 5, O—(CH2)2-(CH2)2-N-CH2-CH2-CH2-SO3−Na+. The large peak at 2.7 ppm in panels A and C is due to DMSO used to solubilize the AMB and voriconazole. Concentration-dependent changes in fungal metabolite production (e.g., ethanol [EtOH] at 1.18 ppm) and glucose utilization (resonances at 3.24 and 4.7 ppm) are clearly seen.
The contents of each microtiter well (200 μl) and PBS-D2O (300 μl) were transferred into 5-mm NMR tubes. Spectra were acquired at a 1H observation frequency of 600.13 MHz (Bruker DRX-600) and temperature of 298 K. Chemical shifts were referenced to that of ethanol (1H, δ 1.18), and D2O provided a field frequency lock. Typically, 64 transients were collected over a spectral width of 6,410 Hz with a relaxation delay of 1 s and an acquisition time of 3 s. A line-broadening function of 1.0 Hz was applied to all spectra prior to Fourier transformation. Signal assignment was facilitated for representative samples by acquisition of a suite of two-dimensional hetero- and homonuclear NMR spectra, namely, 1H-1H correlation spectroscopy (using magic-angle gradients) (21), 1H-13C heteronuclear single quantum coherence, and 1H-13C heteronuclear multibond correlation experiments.
Determination of MEPs.
Stack plots of NMR spectra were constructed to show the effect of drug pressure on utilization of nutrients from the medium and production of fungal metabolites. Indicative metabolic endpoints (MEPs) could be determined from spectral peaks as the lowest concentration of drug at which glucose assimilation, or fungal metabolite production, was inhibited by at least 50%. To obtain a quantifiable endpoint, integrals were calculated for resonances of acetaldehyde (1H δ 9.63 to 9.69); acetate and arginine (1H δ 1.89 to 1.94); ethanol (1H δ 1.15 to 1.20 and δ 3.61 to 3.69); formate (1H δ 8.44 to 8.46); fumarate (1H δ 6.51 to 6.53); α-glucose (1H δ 5.21 to 5.26 [H1]); β-glucose (1H δ 4.62 to 4.67 [H1], δ 3.21 to 3.28 [H2]); glutamine (1H δ 2.42 to 2.49); isoleucine, leucine, and valine methyl resonances (1H δ 0.92 to 1.03); lactate (1H δ 1.31 to 1.35); leucine plus arginine (1H δ 1.61 to 1.78); phenylalanine (1H δ 7.31 to 7.34); pyruvate (1H δ 2.36 to 2.38); succinate (1H δ 2.39 to 2.42); and tyrosine (1H δ 7.18 to 7.22). Peak integrals were determined for these metabolites and were normalized and calculated relative to the corresponding integrals in the medium-only sample. To determine the most suitable endpoint indicative of reproducible and substantial changes in fungal metabolism, designated the MEP, drug concentrations resulting in at least 25%, 33%, 50%, and 80% inhibition of utilization of the principal substrate in the medium, glucose, were calculated from graphed data as the percent difference from the growth control. Similarly, drug concentrations resulting in inhibition of metabolite production by at least 25%, 33%, 50%, and 80% were calculated as the percent difference from the sterility control. The most suitable MEP was determined to be the lowest drug concentration corresponding to a change of >50% from the sterility control.
RESULTS
Effect of AMB on fungal production of ethanol.
Incubation of 2 × 107 ml−1 yeast cells with 103 to 105 times the inoculum used for MIC determination resulted in a more rapid metabolic response to AMB. Large amounts of ethanol were evident in the NMR spectra. Figure 1 shows the rate of ethanol synthesis in suspensions of six different yeast strains (12 cultures) over 24 h in the presence of 0 to 5 μg ml−1 of AMB. The standard deviations of the mean ethanol concentrations of three C. albicans strains with MICs similar to those of AMB (Fig. 1A) are small, confirming the reproducibility of the method. A similar curve was obtained using the sensitive strain of C. lusitaniae (99-167-0481; not shown). Ethanol synthesis by the resistant strain of T. beigelii was almost unaffected by even the highest concentration of AMB (Fig. 1C). The strain of Clavispora lusitaniae (MIC = 0.5 μg ml−1) showed a decrease in ethanol synthesis with increasing amounts of AMB (Fig. 1B). Although the decrease was evident after 3 h, statistical significance was achieved after 12 h. This was even more evident for isolates with lower MICs (Fig. 1A), where statistically significant, lower values for the ethanol integrals were obtained after 6 h. In general, highly susceptible isolates (MIC of <0.12 μg/ml) showed a strong response to even small concentrations of AMB, whereas isolates with an increased MIC of >0.5 μg ml−1 showed a more moderate response (Fig. 1).
Effects of caspofungin, AMB, and voriconazole on cultures of Candida albicans.
The metabolic profiles of 24-h cultures of C. albicans strain ATCC 90028 were identified using the suite of two-dimensional NMR techniques (see Materials and Methods). The spectra show resonances from the buffer MOPS and from metabolites including acetaldehyde, acetate, ethanol, formate, fumarate, glucose, glutamine, glycerol, isoleucine, lactate, leucine, phenylalanine, pyruvate, succinate, tyrosine, and valine. The drug solvent DMSO (δ 1H 2.73) was evident in spectra from wells containing AMB and voriconazole.
The most prominent changes in metabolite concentrations following incubation of C. albicans ATCC 90028 with AMB, caspofungin, and voriconazole were observed in the δ 0.7- to 4.7-ppm region of the 1H NMR spectra (Fig. 2). Active yeast metabolism was evident at an AMB concentration of 0.03 μg ml−1 (Fig. 2A): the spectrum showed depletion of glucose, phenylalanine (7.31 to 7.34 ppm; not shown), tyrosine (7.18 to 7.22 ppm; not shown), isoleucine, leucine, and valine and production of significant quantities of acetate, ethanol, glycerol, lactate, and succinate as well as increased levels of acetaldehyde. In contrast, at AMB concentrations of 0.125 μg ml−1 and above, the production of fungal metabolites (acetate, acetaldehyde, ethanol, glycerol, lactate, and succinate) and utilization of glucose and amino acids were much reduced, consistent with inhibition of fungal metabolism and an indicative MEP of 0.125 μg ml−1. The corresponding MIC (CLSI modified; 2% glucose) of AMB was 0.125 μg ml−1.
Similar metabolic changes were evident for C. albicans ATCC 90028 at a caspofungin concentration of 0.5 μg ml−1 (the indicative MEP; Fig. 2B); however, lactate levels were not elevated. These metabolic changes were consistent with the MIC of caspofungin at 0.5 μg ml−1. Unlike with AMB, there was a paradoxical increase in glucose utilization and ethanol production at the highest concentration of caspofungin (16 μg ml−1). This was also seen as an increase in growth of this Candida strain in the standard MIC susceptibility plates. In Fig. 2C it can be seen that glucose utilization and metabolite production are inhibited at a voriconazole concentration of 0.125 μg ml−1 (MIC, 0.125 μg ml−1).
For AMB and caspofungin, relatively sharp changes occurred over a narrow range of drug concentrations (e.g., Fig. 2A and 2B), whereas with voriconazole, more gradual changes were noted (Fig. 2C).
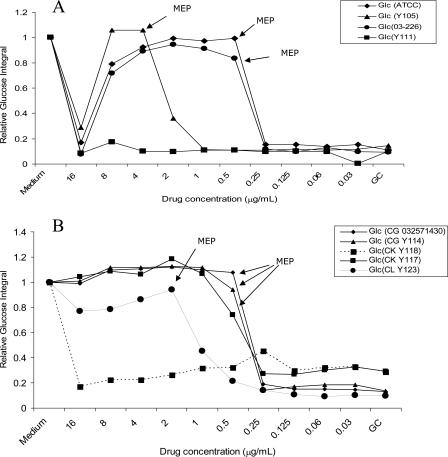
Comparative MEPs of caspofungin incubated with different Candida species for 24 h.
Graphs of the relative integrals for glucose from four strains of C. albicans (Fig. 3A) and five strains of other Candida species (Fig. 3B) incubated for 24 h with caspofungin allowed MEPs for each isolate to be readily determined. Three of the four isolates of C. albicans with measurable MICs of caspofungin showed a paradoxical effect at the highest drug concentration (16 μg/ml). This was paralleled by an increase in growth in the MIC studies of the same strains. A less marked effect was evident at 16 μg/ml with C. lusitaniae (Y123). Y111 and Y118 represent strains that were resistant to caspofungin by this method, as the substrate was utilized across the entire range of drug concentrations, i.e., no inhibition of metabolism was evident and hence the MEP was recorded as greater than the highest drug concentration tested (16 μg/ml).
FIG. 3.
MEP determinations for caspofungin based on the NMR spectral integrals for glucose. (A) C. albicans (four isolates). (B) Candida krusei (Y117 and Y118), C. glabrata (Y114 and 03-257-1430), and C. lusitaniae (Y123).
Comparison of MEPs with MICs of antifungal drugs determined at 24 h of incubation.
On retesting of all isolates, MEPs were reproducible to within 1 drug dilution (not shown). Internally consistent values for MEPs were obtained using integrals calculated for different metabolites (not shown). MEPs were compared with MICs (Table 2) determined by the standard CLSI method (kindly performed by David Ellis, Adelaide, South Australia) or, in the case of two recent clinical isolates (CA032263110 and CG032571430), by E-test and by the modified CLSI method (see Materials and Methods). MEPs and MICs were generally within 1 dilution of each other. The strain Y111 (with reduced susceptibility to caspofungin; Table 1) had a MEP of >16 μg/ml and a MIC (CLSI) of 2 μg/ml but a MIC of 8 μg/ml when determined in RPMI supplemented with 2% glucose as used for MEP determination, rather than the standard 0.2%.
TABLE 2.
Comparison of MEPs and MICs using NMR spectral integrals calculated for ethanol and glucose resonancesa
| Drug | Isolate | Species | Glucose | EtOH | MIC modifiedb | MIC (CLSI)c |
|---|---|---|---|---|---|---|
| Caspofungin | Y105 | C. albicans | 4 | 4 | 1 | 2 |
| 03-226-3110 | C. albicans | 0.5 | 1 | 0.5 | ND | |
| Y111 | C. albicans | >16 | >16 | 8 | 2 | |
| 03-257-1430 | C. glabrata | 0.5 | 1 | 0.5 | ND | |
| Y114 | C. glabrata | 0.5 | 1 | 1 | 0.125 | |
| Y118 | C. krusei | >16 | >16 | ND | >16 | |
| Y117 | C. krusei | 0.5 | 1 | 1 | 0.25 | |
| Y123 | C. lusitaniae | 2 | 2 | 2 | 0.5 | |
| ATCC 90028 | C. albicans | 0.5-1 | 1 | 0.5 | 0.125 | |
| 9235 | C. parapsilosis | ND | 2.0 | 2.0 | 0.5 | |
| 9236 | C. parapsilosis | ND | 2.0 | 1.0 | 0.5 | |
| 8336 | C. guilliermondii | ND | 2.0 | 1.0 | 0.5 | |
| 8333 | C. guilliermondii | ND | 2.0 | 1.0 | 0.5 | |
| AMB | TS3 | C. albicans | 0.06 | 0.125 | 0.125 | ND |
| Y118 | C. krusei | 0.25 | 0.25 | 0.25 | 1.0 | |
| ATCC 90028 | C. albicans | 0.125 | 0.25 | 0.125 | 0.5 | |
| Voriconazole | TS3 | C. albicans | 0.125 | 0.25 | 0.125 | 0.125 |
| Y118 | C. krusei | 2.0 | NCEP | 0.5 | 1.0 | |
| ATCC 90028 | C. albicans | 0.125 | 0.125 | 0.125 | 0.03 |
Abbreviations: EtOH, ethanol; ND, not done; NCEP, no clear endpoint.
CLSI modified (2% glucose), read at 24 h.
MIC measured as prominent inhibition of growth at 48 h.
Time course of the effect of antifungal agents on profiles of selected metabolites.
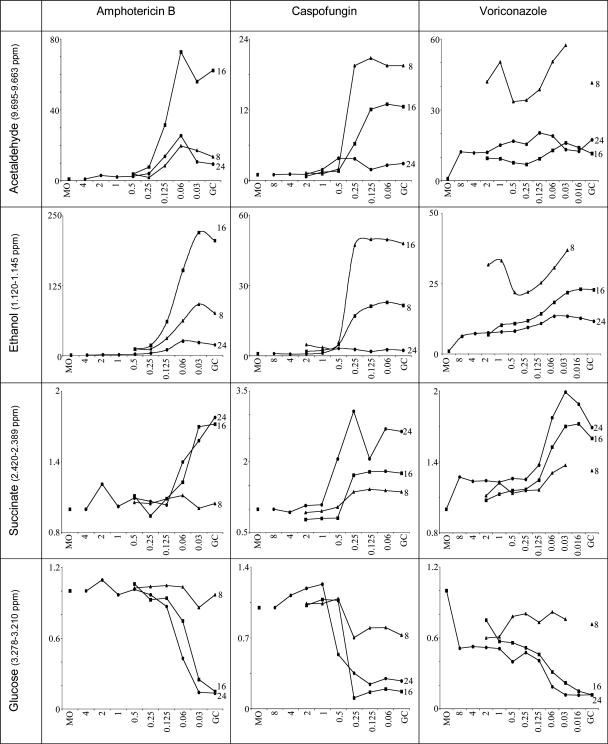
To test the hypothesis that drug-induced metabolic changes are evident before visible inhibition of fungal growth, we performed time course studies. Relative integrals of acetaldehyde, ethanol, β-glucose, and succinate resonances obtained from NMR spectra representing 8, 16, and 24 h of incubation with drug concentrations spanning the MICs of AMB, caspofungin, and voriconazole and C. albicans strain ATCC 90028 are shown in Fig. 4. At 16 and 24 h, compared with drug-free growth controls, clear-cut, consistent changes in metabolite concentrations of at least 50% were noted for all three drugs. Metabolic changes were readily discerned at 16 h and were comparable with (or, for ethanol and caspofungin, greater than) those in the 24-h cultures. Glucose consumption at 8 h fell by a maximum of approximately 30% in cultures containing AMB and caspofungin, and no consumption could be discerned in cultures containing voriconazole. MEPs similar to those determined at 16 and 24 h were calculable in 8-h cultures containing caspofungin and AMB by using acetaldehyde and ethanol (Fig. 4). For voriconazole at 8 h, only ethanol appeared to be reduced; however, the magnitude of change was less than 30% from the sterility control level. The slope of this graph paralleled the slopes for voriconazole-associated changes in ethanol production at 16 and 24 h (Fig. 4). The corresponding drug concentrations for the ≥50% endpoints were the same or 1 drug concentration higher than if expressed as a change of at least 25% or 30%. We defined the MEP as the lowest drug concentration associated with a ≥50% change in the amount of metabolite.
FIG. 4.
Time course of the effect of antifungal agents on profiles of selected metabolites. The figure shows changes in acetaldehyde, ethanol, and succinate production and glucose consumption by C. albicans ATCC 90028 incubated with amphotericin B, caspofungin, or voriconazole for 24 h (•), 16 h (▪), or 8 h (▴). The x axis shows the drug concentration (μg/ml); the y axis shows the relative integral. MO, medium only; GC, growth control. Each graph displays results from one of duplicate experiments.
DISCUSSION
Metabolite profiling for measurement of antifungal activity.
Metabolite profiling yielded objective endpoints (MEPs). These were internally consistent for multiple metabolites, the most abundant of which represented the mixed fermentation products expected during growth under anaerobic conditions in the presence of excess carbon substrate (in this case, 2% glucose). The method was substantially faster than, yet yielded results that correlated with, conventional broth dilution MICs. This may have been due in part to the higher fungal inoculum and relatively high (2%; 11.1 mM) glucose concentration chosen to optimize the balance between production of fungal metabolites and utilization of glucose.
The CLSI method contains 2 g liter−1 of glucose (rather than 20 g liter−1) and an inoculum of ∼2 × 103 ml−1 (rather than 1 × 106 to 5 × 106 ml−1) and specifies that MICs are to be read after 48 h of incubation. The methodology for testing fermentative yeasts proposed in the EUCAST method utilizes RPMI containing 20 g liter−1 glucose, an inoculum density of 0.5 × 105 to 2.5 × 105 ml−1, and an incubation time of 24 h (4). A recent interlaboratory comparison (5) of MICs of four azole drugs, including voriconazole, by the CLSI and AFST-EUCAST methodologies revealed generally lower MICs by the AFST-EUCAST method. The difference was attributed in part to the higher concentration of glucose in the latter method. However, there is also evidence that this higher concentration of glucose enhances fungal growth without significantly changing MICs of AMB, flucytosine, ketoconazole, and fluconazole, determined by the CLSI method (for a review, see reference 18).
Rapid determination of antifungal susceptibility is of increasing clinical relevance with the increase in resistance of certain Candida species to antifungal drugs and the unpredictable resistance of some (e.g., Candida glabrata and fluconazole or itraconazole) (14). Twenty-four hours is the time proposed in the AFST-EUCAST methodology (4). Satisfactory 24-h visual endpoints (rather than the currently agreed 48 h) for MICs of triazole drugs were obtained by the CLSI M27-A2 method in a recent study, although some strains, especially Candida dubliniensis, grew relatively slowly, requiring MICs to be read at 48 h (5). Twenty-four-hour endpoints for fluconazole and itraconazole correlated better with sterol quantification than did 48-h endpoints (1), as did the outcome of fluconazole treatment in a mouse model of invasive candidiasis (17). In a large study by Odds et al. (12) results of caspofungin susceptibility testing by the CLSI M27-A2 method were compared in 17 laboratories and revealed that the most consistent endpoints relied upon prominent growth reduction at 24 h in RPMI containing 0.2% glucose. This method was validated in a recent study by Pfaller et al. (15). Although growth in 0.2% glucose gave the best distinction between isolates with “normal” MICs and those with FKS1 gene mutations that were known to have reduced susceptibility to caspofungin, reproducible results were also obtained in RPMI containing 2% glucose when read visually or using a spectrophotometer (12).
In an attempt to shorten incubation times further, several groups have investigated endpoints based on parameters other than cell growth and correlated them with standard MICs. Examples include enzymatic measurements of glucose utilization (3, 19), detection of cell damage by flow cytometry using fluorescent probes such as carboxyfluorescein diacetate (10) and 2-chloro-4-(2,3-dihydro-3-methyl-(benzo-1,3-thiazol-2-yl)-methylidene)-1-phenyl-quinolinium iodide (FUN-1) (16), and reduction of intracellular ATP levels (9). Significant drug effects were generally noted, however, within 6 to 8 h using FUN-1, and a time as short as 1 h yielded results that correlated with MICs. In our preliminary study, AMB suppressed fungal ethanol synthesis by susceptible yeast strains after 6 h. In the broth microdilution cultures, drug-induced changes in NMR spectra were evident within 8 h and were maximal after 16 and 24 h of incubation. Changes at 8 h were convincing for the small number of isolates studied with AMB and caspofungin, resulting in a >50% change in acetaldehyde and ethanol, but with voriconazole only a 25 to 30% change in ethanol was noted. These results, and in particular whether this effect of voriconazole is significant, require validation by testing a larger number of isolates. Notably, the endpoint for enzymatic measurement of glucose utilization for susceptibility testing of Candida species chosen by Chen et al. required a reduction of only 10% (3).
Paradoxical metabolite production at high concentrations of caspofungin.
We observed paradoxical increases in both glucose utilization and metabolite production by three of the four strains of C. albicans at high concentrations of caspofungin (16 μg ml−1), which were paralleled by an increase in growth of the same strains in the MIC studies. This paradoxical effect has been reported previously with caspofungin in approximately 16% of C. albicans isolates (20). Although the mechanism of this effect is unknown, it is not due to selection of a resistant subpopulation of cells. Furthermore, the effect is not specific to the class of drug, since it was not observed with another echinocandin drug, micafungin (20). It has been proposed that high concentrations of caspofungin regulate calcineurin and protein kinase C signaling pathways that affect functionally redundant cellular events but which are important in caspofungin resistance (22).
Potential role of metabolite profiling for antifungal susceptibility testing.
Metabolic profiling of Candida species provides a wealth of phenotypic information on the effect of drug pressure on fungal metabolic pathways. This study shows that the application of NMR spectroscopic techniques to determine the metabolic composition of suspensions of yeast incubated with a range of drugs is a potentially valuable method for antifungal susceptibility testing because of its rapidity, which at 16 h is considerably less than the 48 h specified in the current CLSI reference method M27-A2. Whether the NMR-based method and platform are suitable for use in large diagnostic reference laboratories or in research and development facilities with high-throughput testing requirements requires validation in a large study. Notably, ultrashielded NMR spectrometers with a small magnetic field footprint (<2 meter2) are available commercially, so that they could be integrated into established microbiological reference or research and development laboratories. The platform is robust, i.e., suitable for use by nonexpert technologists. Sample throughput is rapid (∼5 min per sample) and can be automated. Setup (equipment) costs are relatively high, but running costs are very low due to minimal operator time and reagent costs and the negligible sample processing involved compared with standard methods, and more so compared with the DNA-based methods under development for identification of drug resistance.
In conclusion, direct NMR-based observation of metabolite alterations in yeast cultures reveals key metabolic pathways. A larger study to evaluate its use as a rapid method for testing susceptibility to antifungal drugs is indicated.
Acknowledgments
We are grateful to David Ellis for performing the CLSI broth microdilution susceptibility tests on our fungal strains and to Ronda Plummer for performing the NMR spectroscopy on the Candida parapsilosis and Candida guilliermondii isolates.
This project was funded by a Medical School Grant from Merck, Sharp and Dohme, Pty Ltd. The work of T.C.S. is supported by an NSW Department of Health Infrastructure grant and a Centre of Clinical Research Excellence grant from the National Health and Medical Research Council of Australia (no. 264625). The work of P.W.K. is supported by a Discovery grant from the Australian Research Council. U.H. was supported by a project grant from the NHMRC (no. 153805).
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Arthington-Skaggs, B. A., H. Jradi, T. Desai, and C. J. Morrison. 1999. Quantitation of ergosterol content: novel method for determination of fluconazole susceptibility of Candida albicans. J. Clin. Microbiol. 37:3332-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bubb, W. A., L. C. Wright, M. Cagney, R. T. Santangelo, T. C. Sorrell, and P. W. Kuchel. 1999. Heteronuclear NMR studies of metabolites produced by Cryptococcus neoformans in culture media: identification of possible virulence factors. Magn. Reson. Med. 42:442-453. [DOI] [PubMed] [Google Scholar]
- 3.Chen, J., Z. Wan, and R. Li. 2004. Modified colorimetric assay for susceptibility testing of azole antifungal drugs against Candida species. J. Clin. Microbiol. 42:1790-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuenca-Estrella, M., C. B. Moore, F. Barchiesi, J. Bille, E. Chryssanthou, D. W. Denning, J. P. Donnelly, F. Dromer, B. Dupont, J. H. Rex, M. D. Richardson, B. Sancak, P. E. Verweij, and J. L. Rodriguez-Tudela. 2003. Multicenter evaluation of the reproducibility of the proposed antifungal susceptibility testing method for fermentative yeasts of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST). Clin. Microbiol. Infect. 9:467-474. [DOI] [PubMed] [Google Scholar]
- 5.Espinel-Ingroff, A., F. Barchiesi, M. Cuenca-Estrella, M. A. Pfaller, M. Rinaldi, J. L. Rodriguez-Tudela, and P. E. Verweij. 2005. International and multicenter comparison of EUCAST and CLSI M27-A2 broth microdilution methods for testing susceptibilities of Candida spp. to fluconazole, itraconazole, posaconazole, and voriconazole. J. Clin. Microbiol. 43:3884-3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Himmelreich, U., T. E. Dzendrowskyj, C. Allen, S. Dowd, R. Malik, B. P. Shehan, P. Russell, C. E. Mountford, and T. C. Sorrell. 2001. Cryptococcomas distinguished from gliomas with MR spectroscopy: an experimental rat and cell culture study. Radiology 220:122-128. [DOI] [PubMed] [Google Scholar]
- 7.Himmelreich, U., R. L. Somorjai, B. Dolenko, H. M. Daniel, and T. C. Sorrell. 2005. A rapid screening test to distinguish between Candida albicans and Candida dubliniensis using NMR spectroscopy. FEMS Microbiol. Lett. 251:327-332. [DOI] [PubMed] [Google Scholar]
- 8.Himmelreich, U., R. L. Somorjai, B. Dolenko, O. C. Lee, H. M. Daniel, R. Murray, C. E. Mountford, and T. C. Sorrell. 2003. Rapid identification of Candida species by using nuclear magnetic resonance spectroscopy and a statistical classification strategy. Appl. Environ. Microbiol. 69:4566-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kretschmar, M., T. Nichterlein, P. Kuntz, and H. Hof. 1996. Rapid detection of susceptibility to fluconazole in Candida species by a bioluminescence assay of intracellular ATP. Diagn. Microbiol. Infect. Dis. 25:117-121. [DOI] [PubMed] [Google Scholar]
- 10.Liao, R. S., R. P. Rennie, and J. A. Talbot. 2002. Comparative evaluation of a new fluorescent carboxyfluorescein diacetate-modified microdilution method for antifungal susceptibility testing of Candida albicans isolates. Antimicrob. Agents Chemother. 46:3236-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard NCCLS document M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 12.Odds, F. C., M. Motyl, R. Andrade, J. Bille, E. Canton, M. Cuenca-Estrella, A. Davidson, C. Durussel, D. Ellis, E. Foraker, A. W. Fothergill, M. A. Ghannoum, R. A. Giacobbe, M. Gobernado, R. Handke, M. Laverdiere, W. Lee-Yang, W. G. Merz, L. Ostrosky-Zeichner, J. Peman, S. Perea, J. R. Perfect, M. A. Pfaller, L. Proia, J. H. Rex, M. G. Rinaldi, J. L. Rodriguez-Tudela, W. A. Schell, C. Shields, D. A. Sutton, P. E. Verweij, and D. W. Warnock. 2004. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J. Clin. Microbiol. 42:3475-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller, M. A., D. J. Diekema, J. H. Rex, A. Espinel-Ingroff, E. M. Johnson, D. Andes, V. Chaturvedi, M. A. Ghannoum, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, P. Troke, T. J. Walsh, and D. W. Warnock. 2006. Correlation of MIC with outcome for Candida species tested against voriconazole: analysis and proposal for interpretive breakpoints. J. Clin. Microbiol. 44:819-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaller, M. A., S. A. Messer, L. Boyken, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2004. Geographic variation in the susceptibilities of invasive isolates of Candida glabrata to seven systemically active antifungal agents: a global assessment from the ARTEMIS Antifungal Surveillance Program conducted in 2001 and 2002. J. Clin. Microbiol. 42:3142-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller, M. A., L. Boyken, R. J. Hollis, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2006. In vitro susceptibilities of Candida spp. to caspofungin: four years of global surveillance. J. Clin. Microbiol. 44:760-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pina-Vaz, C., S. Costa-de-Oliveira, A. G. Rodrigues, and A. Espinel-Ingroff. 2005. Comparison of two probes for testing susceptibilities of pathogenic yeasts to voriconazole, itraconazole, and caspofungin by flow cytometry. J. Clin. Microbiol. 43:4674-4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rex, J. H., P. W. Nelson, V. L. Paetznick, M. Lozano-Chiu, A. Espinel-Ingroff, and E. J. Anaissie. 1998. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob. Agents Chemother. 42:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rex, J. H., M. A. Pfaller, T. J. Walsh, V. Chaturvedi, A. Espinel-Ingroff, M. A. Ghannoum, L. L. Gosey, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, and D. W. Warnock. 2001. Antifungal susceptibility testing: practical aspects and current challenges. Clin. Microbiol. Rev. 14:643-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riesselman, M. H., K. C. Hazen, and J. E. Cutler. 2000. Determination of antifungal MICs by a rapid susceptibility assay. J. Clin. Microbiol. 38:333-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens, D. A., M. Espiritu, and R. Parmar. 2004. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob. Agents Chemother. 48:3407-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Zijl, P., A. O'Neil, M. Johnson, S. Mori, and R. E. Hurd. 1995. Magic-angle-gradient double-quantum-filtered COSY. J. Magn. Reson. 113A:265-270. [Google Scholar]
- 22.Wiederhold, N. P., D. P. Kontoyiannis, R. A. Prince, and R. E. Lewis. 2005. Attenuation of the activity of caspofungin at high concentrations against Candida albicans: possible role of cell wall integrity and calcineurin pathways. Antimicrob. Agents Chemother. 49:5146-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]