Abstract
The 15-mer dermaseptin S4 derivative S4(1-15) was recently shown to exhibit potent activity against oral pathogens associated with caries and periodontitis. Here, we investigated possible modes for improving the peptide's properties through systematic replacement of an N-terminal amino acid(s) with various fatty acids that modulate the peptide's hydrophobicity and/or charge. Deletion of 1 to 3 residues led to progressive loss of potency as assessed by MIC experiments performed on four test bacteria. Replacing the deleted amino acids with fatty acids most often resulted in potency recovery or improvement, as evidenced by lower MICs and faster bactericidal kinetics in culture media. Best results were obtained after replacement of the N-terminal dipeptide alanine-leucine with heptanoic (C7) or aminododecanoic (NC12) acid. Circular dichroism analysis correlated antibacterial properties to the peptide's secondary structure. MIC experiments and confocal laser scanning microscopy results indicated that C7-S4(3-15) and NC12-S4(3-15) were bactericidal to various oral pathogens, including those which are immobilized in a biofilm. C7-S4(3-15) performed similarly to or better than (depending on growth medium) IB-367, a peptide assessed in clinical trials for treatment of oral mucositis, reducing CFU counts by >3 log units within 2 min of incubation. Collectively, the data indicate that substitution of fatty acids for amino acids may be a useful strategy in revealing improved derivatives of known antimicrobial peptides and suggest the suitability of such compounds for controlling pathogens associated with oral diseases.
Ribosomally synthesized peptides with antimicrobial properties are produced by eukaryotes (6, 17) and prokaryotes (3, 47) and represent crucial components of their defense systems against invading microorganisms (25, 51). As part of the innate immunity, antimicrobial peptides (AMPs) can control viability of a vast range of pathogens, including encapsulated viruses (8, 27, 48), bacteria (4, 16, 44), intracellular parasites (7, 18, 23), and cancer cells (10, 33, 39). With the constant increase in microbial resistance to antibiotics, AMPs represent promising candidates for development of new antimicrobial therapeutics (20, 29). Multiple targets were proposed to account for the cytolytic properties of AMPs, including interference with structure and function of membranes (19, 49) and cytoplasmic components (5, 35). These nonspecific activities diminish a pathogen's ability to develop resistance mechanisms and thus represent an advantage of AMPs over conventional antibiotics. Other obvious advantages pertain to the peptide's relatively simple structure and broad spectrum of activity.
Dermaseptins are linear AMPs (12, 14, 24) whose lytic activity is mediated by interaction of N-terminal residues with the plasma membrane (30, 36). Previous structure-activity relationship studies yielded potent derivatives, of which the 15-mer dermaseptin S4 derivative S4(1-15) demonstrated the highest antibacterial activity (14), while a recent study showed the potent bactericidal effect of this derivative against oral bacteria (1).
N-terminal acylation of various dermaseptin S4 derivatives was shown to result in increased antimicrobial potency. Similar results were obtained with other AMPs (2, 12, 38). However, in various cases, acylation was shown to be deleterious to antimicrobial potency, especially when using long-chain acyls. Self-assembly in solution might at least partly explain this behavior (38, 53). To circumvent the risk of excessive hydrophobicity of acyl-peptides, we attempted in this study to limit hydrophobicity increase by exchanging the hydrophobic N-terminal amino acid residues with a fatty acid of moderate length (heptanoic [C7] or dodecanoic [C12] acid) or hydrophobicity (aminoheptanoic or aminododecanoic acid). This strategy was inspired by results obtained in previous investigations showing that truncation of 3 to 4 residues from the N terminus of the active sequence of various AMPs significantly hampered antimicrobial activity (14, 34, 52). We therefore tested the hypothesis that a combination of truncation and acylation strategies would play a compensational role.
In the present work, acyl moieties were conjugated to truncated derivatives of S4(1-15) in an attempt to maintain or improve the peptide's large-spectrum properties towards potential topical applications, such as in the treatment of oral mucositis. Oral mucositis is a painful condition and a major cause of morbidity in cancer patients, resulting from the cytotoxic effects of radiation or chemotherapy on rapidly dividing epithelial cells of the oropharyngeal mucosa (31). This therapy alters the ecological balance, thereby allowing some of the resident oral flora, which consists of hundreds of microbial species normally harmless to healthy people, to initiate a pathogenic process (13). Opportunistic pathogens are also thought to exacerbate this condition, leading to ulceration and inflammation of the buccal and sublingual mucosae (13, 31). Several antimicrobial agents were clinically evaluated as treatments for oral mucositis, including the protegrin analog IB-367, which is currently undergoing phase 3 clinical trials (46). The reasons for selecting IB-367 for potential treatment of oral mucositis included its rapid microbicidal activity, broad-spectrum activity, potency in saliva, low prospects for inducing resistance, and potent activity against antibiotic-resistant organisms (9).
MATERIALS AND METHODS
Peptides.
Peptides were synthesized by a solid-phase method, applying 9-fluorenylmethyloxy carbonyl active ester chemistry with an Applied Biosystems model 433A peptide synthesizer. 4-Methylbenzhydrylamine resin (Novabiochem, Darmstadt, Germany) was used to obtain amidated peptides. The acylated analogs were prepared by covalent linking of the peptides' amino termini to lauric acid as described previously (38). The crude peptides were purified to ≥95% chromatographic homogeneity by reverse-phase high-performance liquid chromatography (HPLC) (Alliance-Waters). Purification and refolding of IB-367, which contains cysteine residues, were performed basically according to a procedure described by Harwig et al. (21) and repurified by HPLC as described above, and the β-sheet content was confirmed by circular dichroism (CD). All purified peptides were subjected to electrospray mass spectrometry (Micromass ZQ; Waters) to confirm their composition and stored as a lyophilized powder at −20°C. Prior to being tested, fresh solutions were prepared in water (10 mM acetate buffer for IB-367), briefly vortexed, sonicated, centrifuged, and then diluted in the appropriate medium. Buffers were prepared with bidistilled water. Polymyxin B, rifampin, and piperacillin were obtained from Sigma. All other reagents were analytical grade.
MICs.
MICs presented in Table 1 were determined using microdilution susceptibility tests as described previously (38). Briefly, 100 μl peptide solution was mixed with 100 μl bacterial suspension (approximately 5 × 105 CFU/ml) in LB medium (0.5% yeast extract, 1% Bacto tryptone, 0.5% NaCl). Antibacterial activity was assessed after overnight incubation at 37°C against the following strains: Bacillus cereus (ATCC 11778), Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 35218), and Pseudomonas aeruginosa (ATCC 9027). MICs against oral bacteria presented in Table 2 were determined using microdilution susceptibility tests against the following bacterial strains: Streptococcus mutans (ATCC 27351), Actinomyces viscosus (ATCC 43146), and Streptococcus salivarius (ATCC 25975). These bacteria were incubated in brain heart infusion (BHI) (Difco, Maryland) in an atmosphere enriched with 5% CO2 at 37°C. Actinobacillus actinomycetemcomitans (ATCC 29523) was incubated in 0.5% yeast extract, 1.5% Bacto tryptone, 0.75% d-glucose, 0.25% NaCl, 0.075% l-cysteine, 0.05% sodium thioglycolate, and 4% NaHCO3 in 5% CO2 at 37°C.
TABLE 1.
List of compounds investigated and their properties
| Peptide or antibiotic | Hb | Qc | MIC (μM)d
|
|||
|---|---|---|---|---|---|---|
| B. cereus | S. aureus | E. coli | P. aeruginosa | |||
| S4(1-15)a | 48 | 5 | 3 | 9 ± 3 | 3 | 6 |
| S4(2-15) | 47 | 5 | 4.5 ± 1.5 | 18 ± 7 | 4.5 ± 1.5 | 12.5 |
| NC7-S4(2-15) | 53 | 5 | 2 ± 1 | 9 ± 3 | 6 | 1.5 |
| NC12-S4(2-15) | 56 | 5 | 3 | 3 | 6 | 4.5 ± 1.5 |
| C7-S4(2-15) | 60 | 4 | 3 | 3 | 12.5 | 3 |
| C12-S4(2-15) | 69 | 4 | 18 ± 7 | 9 ± 3 | 25 | 12.5 |
| S4(3-15) | 44 | 5 | >50 | >50 | 37 ± 13 | 25 |
| NC7-S4(3-15) | 49 | 5 | 4.5 ± 1.5 | 18 ± 7 | 12.5 | 3 |
| NC12-S4(3-15) | 53 | 5 | 3 | 3 | 3 | 4.5 ± 1.5 |
| C7-S4(3-15) | 57 | 4 | 3 | 2 ± 1 | 6 | 2 ± 1 |
| C12-S4(3-15) | 68 | 4 | 18 ± 7 | 9 ± 3 | 18 ± 7 | 12.5 |
| S4(4-15) | 34 | 5 | >50 | >50 | >50 | >50 |
| NC7-S4(4-15) | 45 | 5 | >50 | >50 | >50 | >50 |
| NC12-S4(4-15) | 49 | 5 | 9 ± 3 | 12.5 | 6 | 6 |
| C7-S4(4-15) | 56 | 4 | 50 | 37 ± 13 | 12.5 | 25 |
| C12-S4(4-15) | 67 | 4 | 9 ± 3 | 9 ± 3 | 9 ± 3 | 9 ± 3 |
| S4(5-15) | 38 | 4 | >50 | >50 | >50 | >50 |
| NC7-S4(5-15) | 45 | 4 | >50 | >50 | >50 | >50 |
| NC12-S4(5-15) | 50 | 4 | 50 | 50 | 50 | 50 |
| C7-S4(5-15) | 59 | 3 | 50 | 50 | 25 | 25 |
| C12-S4(5-15) | 72 | 3 | 4.5 ± 1.5 | 3 | 6 | 6 |
| IB-367 | 45 | 4 | NDe | 3 | 4.5 ± 1.5 | 19 ± 6 |
| Polymyxin B | 42 | 5 | ND | >50 | 2 | 0.75 |
| Rifampin | ND | ND | ND | <0.1 | 7.5 | 12.5 |
| Piperacillin | ND | ND | ND | 50 | 5.5 | 12.5 |
Reference peptide (ALWKTLLKKVLKAAA-amide).
H, hydrophobicity, defined as the percentage of acetonitrile in which the peptide is eluted from a C18 HPLC column.
Q, charge at physiological pH.
Lowest peptide concentration that inhibits bacterial growth after 24 h of incubation in LB medium at 37°C. MICs represent means ± standard deviations obtained from at least two independent experiments performed in duplicate. Lack of a standard deviation reflects consistency. Decimal values were rounded up to the nearest half unit for simplicity.
ND, not determined.
TABLE 2.
MICs of S4(1-15) derivatives against oral bacteria
| Peptide | MIC (μM)a
|
|||
|---|---|---|---|---|
| S. mutans | A. viscosus | A. actinomycetemcomitans | S. salivarius | |
| NC12-S4(3-15) | 8 | 16 | 16 | >32 |
| C7-S4(3-15) | 8 | 16 | 32 | >32 |
Lowest peptide concentration that inhibits bacterial growth after 24 h of incubation in BHI, as detailed in Materials and Methods. Values represent results of triplicate experiments. Decimal values were rounded up to the nearest half unit for simplicity.
Light scattering.
The aggregation properties were investigated by static light scattering measurements as detailed previously (24). Peptides at an initial concentration of 50 μM were successively diluted in 0.4 ml of phosphate-buffered saline (PBS) at room temperature, and light scattering was recorded. The static light scattering signal is proportional to the number of aggregated molecules and the size of the aggregate. Therefore, the slope is indicative of the aggregation tendency of the peptides, where a slope value above unity indicates the presence of micellar form.
Time-kill experiments.
Bactericidal kinetics was assessed as described previously (50). Briefly, 100 μl of suspended bacteria at 2 × 106 to 4 × 106 CFU/ml of culture medium was added to a final volume of 1 ml culture medium containing 0 or 8 μM of each peptide. After 0, 5, 15, 30, 60, and 120 min of exposure to peptide, samples taken were subjected to serial 10-fold dilutions in 0.87% saline, from which 20-μl aliquots were plated on LB agar plates. The plates were incubated at 37°C for 24 h, and colonies were counted to determine bactericidal kinetics of the peptides. Fungicidal kinetics was assessed similarly, with the following exceptions. Thirty microliters of suspended clinically isolated Candida albicans CAF3-1 (15) at 4 × 105 CFU/ml was added to a final volume of 300 μl. Peptide concentration was 100 μM. Culture medium was yeast extract-peptone-dextrose (1% yeast extract, 2% Bacto peptone, 2% d-glucose, and 2% agarose for yeast extract-peptone-dextrose agar) supplemented with uridine at 50 mg/liter. Incubation temperature was 30°C.
CD spectra were recorded on a model π*-180 CDF spectrometer (Applied Photophysics), using a QS Hellma quartz cell of 2-mm path length at 25°C, between 190 and 250 nm at a scanning speed of 13.5 nm/min. The CD spectrum was scanned for peptide samples (100 μM) that were dissolved in PBS, in the presence or absence of 2 mM POPC:POPG (3:1) liposomes (where POPC is 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine and POPG is 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol; both provided by Sigma). Liposomes were prepared as described previously (42). Minor contributions of circular differential scattering were eliminated by subtracting the CD spectrum of buffer and liposomes without peptide. CD data represent average values from three separate recordings.
Microbicidal effect in saliva was determined as described previously (31). Briefly, fresh human saliva from healthy volunteers was mixed 1:1 with a dermaseptin analog (sterile water), IB-367 (10 mM sodium acetate buffer [pH 5]), or 10 mM sodium acetate buffer (pH 5) (negative control), and the mixture was incubated at 37°C without aeration. The numbers of viable CFU were determined after 0, 2, 8, and 16 min of exposure to peptide by subjecting culture samples to serial 10-fold dilution in 0.87% saline, from which 20-μl aliquots were plated on Trypticase soy agar plates (Biolife, Milan, Italy) containing 10% fetal bovine serum (Biological Industries, Israel). The plates were incubated at 37°C for 24 h, and the colonies were enumerated to determine the microbicidal effect of the drug. The same assay was also applied using LB agar plates for CFU determination. In this case, the culture exposed to IB-367 was sampled at 0, 5, and 15 min of incubation, as was the control group.
Biofilm assays.
Bacteria (Streptococcus mutans and Actinomyces viscosus) from frozen cultures were incubated in BHI (Difco, Maryland) in an atmosphere enriched with 5% CO2 for 18 h in 37°C. Biofilms were generated by placing 10-μl aliquots of the above-described overnight culture in each well of an eight-well sterile microscopy chamber (ibidi, Munich, Germany) for an hour, after which an additional 300 μl BHI supplemented with 2% sucrose was added. After 18 h of incubation, the supernatant fluid was aspirated and the peptides, at a final concentration of 140 μg/ml, were added in BHI. After an additional incubation for 18 h at 37°C and 5% CO2, the supernatant fluid was aspirated. The biofilms were washed and stained with a Live:Dead BacLight bacterial viability kit (Molecular Probes, Inc., Oregon) under dark conditions, according to the manufacturer's instructions, and immediately analyzed using confocal laser scanning microscopy (Zeiss, Germany) at different biofilm depths. The Live:Dead BacLight kit consists of Syto 9 and propidium iodide (excitation/emission maxima are 480/530 nm and 520/580 nm, respectively), which stain viable bacteria in green and those with damaged membranes in red (11, 40).
RESULTS
Screen for improved potency.
Antibacterial activity was routinely assessed in terms of MIC against two gram-positive bacteria (B. cereus and S. aureus) and two gram-negative bacteria (E. coli and P. aeruginosa). Table 1 summarizes the MICs and physical properties, such as net positive charge and hydrophobicity, of the reference peptide S4(1-15) (ALWKTLLKKVLKAAA-amide) and its derivatives. For comparison purposes, Table 1 also lists the properties of two antimicrobial peptides (IB-367 and polymyxin) and two conventional antibiotics (rifampin and piperacillin) which were assayed under identical conditions.
Whereas the reference peptide S4(1-15) displayed potent and broad-spectrum activity, loss of alanine in position 1 (Ala1) affected antibacterial potency in a mild but noticeable manner, whereby MICs increased by about twofold.
Substitution of various fatty acids for Ala1 resulted in a complex pattern of behavior without an apparent common denominator. Thus, substitutions with aminoheptanoyl (NC7) and aminododecanoyl (NC12) derivatives, which progressively increased the peptide's hydrophobicity (but not the charge), resulted in recovery of potency against B. cereus and P. aeruginosa and even improved potency against S. aureus as well as against E. coli (although to a lesser extent). A different outcome was observed with the corresponding nonaminated acyls, which further increased the hydrophobicity while reducing the charge. Substitution of a dodecanoyl derivative (C12) for Ala1 was rather deleterious to potency except against S. aureus, unlike the less hydrophobic derivative (C7), which rather enhanced potency except against E. coli.
Deletion of Leu2 further diminished potency, while substitution of the fatty acids for Leu2 resulted in a reminiscent pattern, as observed with substitution for Ala1. It is noteworthy, however, that this series uncovered two acyl derivatives, NC12-S4(3-15) and C7-S4(3-15), that, in addition to recovering potency, displayed enhanced potency by up to threefold against S. aureus and P. aeruginosa.
Deleting 3 or 4 residues from the peptide's N terminus led to inactivity in the tested concentrations (up to 50 μM). While some acyl derivatives succeeded in maintaining potency of the parent peptide, most did not lead to full recovery or enhance it, with the exception of the dodecanoyl (C12) derivative. Unlike its longer peptide analogs (14- and 13-mer peptides), whose dodecanoylation led to the least active compounds among the various acyls, the dodecanoylated 12-mer analog C12-S4(4-15) restored nearly all activities while the dodecanoylated 11-mer anlog C12-S4(5-15) was the most active among the acyl derivatives, displaying a MIC against S. aureus threefold lower than that of the 15-mer reference peptide.
Self-assembly.
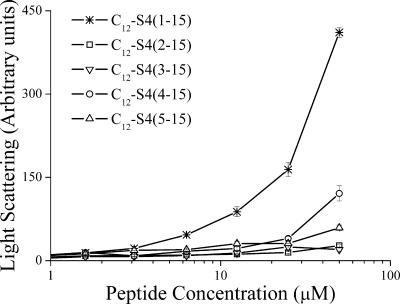
As aggregation of hydrophobic peptides (24) and lipopeptides (2, 38) can occur in aqueous solutions and affect their potency, the organizational properties (light scattering) of various acylated and nonacylated derivatives were compared in PBS. Figure 1 shows the outcome for representative peptides, the most hydrophobic compounds, the dodecanoylated derivatives. The outcome suggested that dodecanoyl conjugation to the reference peptide S4(1-15) led to self-assembly at a low micromolar concentration, but all of the dodecanoyl-substituted derivatives behaved as monomers at the relevant active concentrations.
FIG. 1.
Peptide self-assembly in PBS, as investigated by static light scattering measurements. Intensity of scattered light is plotted against total peptide concentrations. Plotted values represent the means ± standard deviations obtained from 10 measurements.
Characterization of C7-S4(3-15), a representative substituted derivative.
Out of the listed derivatives, C7-S4(3-15) was the smallest molecule that displayed the highest potency against the four bacterial strains. This compound was therefore compared with the parent peptide S4(1-15) in terms of bactericidal kinetics and secondary structure. In addition, C7-S4(3-15) was assessed for its ability to affect a polymicrobial population, such as the oral microflora. Finally, C7-S4(3-15) was also tested for its ability to affect bacteria within a biofilm.
Microbicidal kinetics in culture medium.
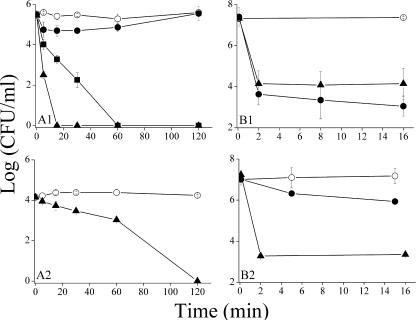
The bactericidal properties of S4(1-15) and C7-S4(3-15) were compared with those of IB-367 at a concentration of 8 μM against a representative strain of P. aeruginosa (ATCC 9027). Panel A1 of Fig. 2 demonstrates that both dermaseptin derivatives have rapid bactericidal kinetics, although C7-S4(3-15) managed to reduce the CFU count by >5 log units within 15 min of incubation whereas S4(1-15) required a longer time (60 min). Under the same conditions, IB-367 initially displayed some bactericidal effect (for about 60 min), which eventually dissipated after a longer exposure time to bacteria.
FIG. 2.
Bactericidal kinetics in culture medium and in human saliva. (A) Bacterial suspensions of P. aeruginosa (panel A1) or C. albicans (panel A2) were added to culture medium containing 0, 8 μM (panel A1), or 100 μM (panel A2) peptide concentration. Bacteria were sampled at indicated times after exposure to peptide. (B) Bactericidal kinetics against polymicrobial flora in saliva. The final peptide concentration was 131.5 μM (panel B1) or 100 μM (panel B2). Samples taken at indicated time intervals were plated on Trypticase soy agar plates containing 10% fetal bovine serum (panel B1), on LB agar plates (panels A1 and B2), or on yeast extract-peptone-dextrose plates containing 50 mg/liter uridine (panel A2) for CFU counts after overnight incubation. Plotted values represent the means ± standard deviations obtained from at least two independent experiments performed in duplicate. Symbols: open circle, control; filled circle, IB-367; filled square, S4(1-15); filled triangle, C7-S4(3-15).
C. albicans is an important opportunistic oral pathogen, causing mild to severe lesions in immunocompromised or critically ill patients. As shown in panel A2 of Fig. 2, C7-S4(3-15) displayed a significant fungicidal effect.
Circular dichroism.
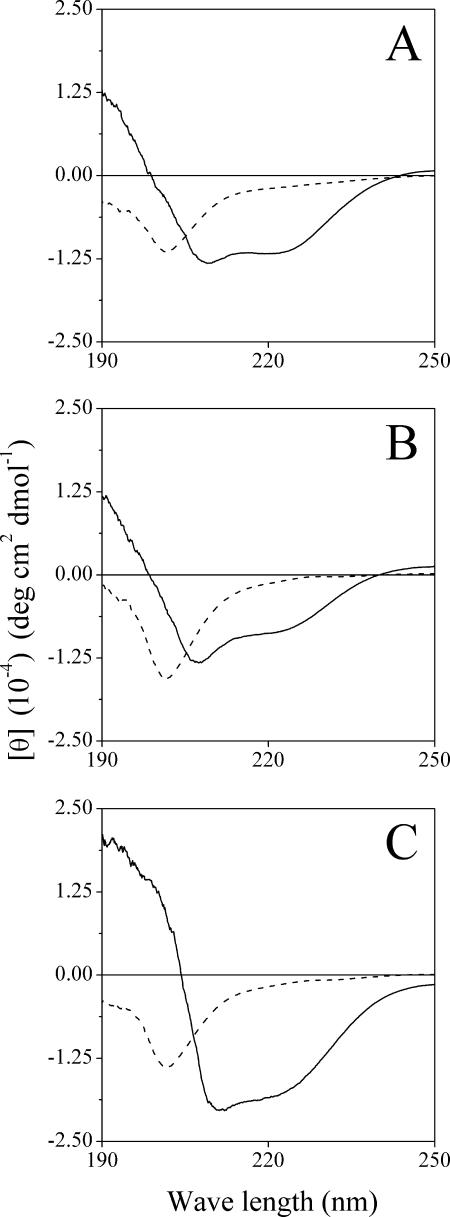
A global indication for structural differences was obtained using CD measurement in PBS in the presence of POPC:POPG liposomes. In PBS alone, all peptides had unordered structure (Fig. 3). In the presence of liposomes, S4(1-15) displayed an ellipticity profile typical of an α-helix, as characterized by double minima at 208 and 222 nm (Fig. 3A). The truncated derivative S4(3-15) displayed reduced helical content (Fig. 3B), which was restored (and further enhanced) with C7-S4(3-15) (Fig. 3C). Extent of helicity correlated with antibacterial potency, inasmuch as C7-S4(3-15) demonstrated the highest potency and helicity while S4(3-15) displayed the lowest potency and helicity.
FIG. 3.
Effect of truncation and substitution on peptide secondary structure. CD spectra were measured for peptide samples (100 μM) dissolved in PBS alone (dashed line) or containing POPC:POPG (3:1) (solid line). Plotted lines represent average values from three separate recordings. (A) S4(1-15), (B) S4(3-15), (C) C7-S4(3-15). θ, mean residue molar ellipticity; deg, degrees.
Oral microflora.
We further examined the ability of C7-S4(3-15) to affect oral microflora in saliva. The potency of the dermaseptin derivative was compared to that of IB-367 according to an assay used by Mosca et al. (31). The outcome is summarized in panel B1 of Fig. 2. The control group (saliva mixed 1:1 with acetate buffer) exhibited no antimicrobial effect, while C7-S4(3-15) displayed rapid bactericidal kinetics, reducing by 3 log units the levels of the endogenous oral microflora within 2 min. Note that under the same conditions NC12-S4(3-15) also performed similarly (data not shown). The kinetics of IB-367, which in our hands was similar to that previously published (31), was essentially equivalent to that of the dermaseptin derivatives although less potent under different experimental conditions (Fig. 2, panel B2).
Table 2 displays the peptide MICs determined against four additional bacteria that are highly associated with supragingival oral diseases (26). As both S. mutans and A. viscosus were sensitive to the derivatives C7-S4(3-15) and NC12-S4(3-15), antibacterial potency was further challenged under biofilm conditions.
Biofilm.
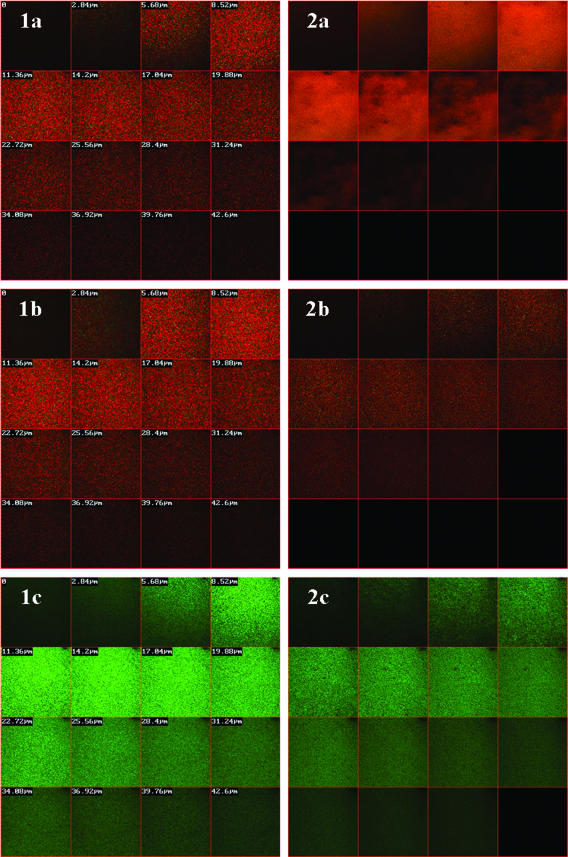
C7-S4(3-15) and NC12-S4(3-15) were further subjected to additional testing to evaluate their effectiveness against oral biofilm, since this environment reflects in vivo conditions in the oral cavity (28). Figure 4 displays the peptide effect over immobilized S. mutans and A. viscosus, two facultative aerobic bacteria highly associated with dental caries. Since one of the unique characteristic of biofilms is a low diffusion rate of agents across the biofilm (43), we tested peptide exposure to immobilized bacteria (embedded in a biofilm) for 18 h to allow substantial diffusion of the peptide across the biofilm layers in order to assess the potential of these peptides to affect bacterial viability even at deep layers of the biofilm. As shown in Fig. 4, the red color observed throughout the entire depth of both biofilms (compared to control biofilms cultured in the absence of peptide) indicated that both C7-S4(3-15) and NC12-S4(3-15) demonstrated bactericidal effect reaching substantial biofilm depths.
FIG. 4.
Bactericidal effect over bacterial biofilm. Mature biofilms were incubated overnight in the presence of sucrose and 140 μg/ml (∼100 μM) peptide. Following incubation, samples were stained for live (green)/dead (red) bacteria and observed with confocal laser scanning microscopy, recording cross sections into the depth of the biofilm. Column 1, S. mutans; column 2, A. viscosus. Row a, treatment with C7-S4(3-15); row b, treatment with NC12-S4(3-15); row c, control treatment.
DISCUSSION
By use of structure-activity relationship tools, this work showed that various acyls, differing in charge and length, can replace N-terminal amino acid sequences. This study has initially established that loss of a few amino acids dramatically affects antibacterial potency of the reference peptide, while short acyl derivatives display, in various instances, similar or improved potencies. Inactivity of the truncated analogs can be linked to altered physical properties, including reduced charge, hydrophobicity, and structure, that are critical for activity of AMPs in general (5, 34, 37, 45) and of dermaseptins in particular (24, 38, 41). Recovery of potency after acyl conjugation is apparently linked to the ability of the successful acyl to restore these parameters or to enhance them. This seems to be accomplished through a compensative mechanism whereby a large increase in hydrophobicity can compensate for a loss of charge [compare, for instance, NC12-S4(3-15) and C7-S4(3-15)]. CD data showed a correlation between active peptides and α-helical structure that was enhanced or reduced, respectively, for the more active or the less active derivatives. Helical structure was shown to stabilize amphipathic organization, conceivably via interaction of the acyl chain with the hydrophobic face of the helix, which in turn affects peptide interaction with the plasma membrane (42).
Two additional observations were particularly intriguing. (i) Dodecanoyl substitutions led to a peculiar behavior in that they hampered potency at first [e.g., in C12S4(2-15)] but ended up enhancing potency [e.g., in C12S4(5-15)]. It is possible that the latter compound reached an optimal charge/hydrophobicity ratio. The concept that there is an optimal hydrophobicity window for potent activity is supported by various studies (22, 38, 53). Based on light scattering measurements, we excluded the possibility that self-assembly would account for the reduced potency of the dodecanoylated longer peptide analogs. (ii) The fact that the 11-mer dodecanoyl derivative was more potent than the 15-mer reference peptide suggests that dodecanoic acid might enable potency recovery after further reduction in the peptide length, eventually with longer fatty acids. Both of these issues remain unsolved in this study and warrant full additional investigation.
The two most potent derivatives issued from this study, C7- and NC12-S4(3-15), demonstrated bactericidal effect over biofilm growth of oral bacteria and therefore might be effective in treatment of oral disorders associated with bacterial biofilms. C7-S4(3-15) also displayed a fungicidal effect, as assessed with C. albicans. Moreover, C7-S4(3-15) was shown to have antimicrobial potency in saliva similar to that of IB-367, a peptide currently undergoing clinical trials for the treatment of oral mucositis. Incidentally, the observed differences (as shown in Fig. 2, panels B1 and B2) probably reflect medium-mediated selection of microbial populations, meaning that serum-containing medium encourages growth of certain bacterial strains which are not likely to develop in another medium and vice versa.
For successful treatment of oral mucositis, the preferred antimicrobial agent should include properties such as a broad-spectrum activity that is achieved despite a short contact time with the pathogen and the ability to remain uncompromised by saliva components. From these points of view, acylated dermaseptin derivatives such as C7-S4(3-15) and NC12-S4(3-15) have the potential to conform with the criteria while having other intrinsic advantages over the 17-mer nonlinear IB-367, namely, advantages pertaining to amino acid composition, which simply translate to production ease and cost. Acylation also endows peptides with resistance to proteolysis (38). As bacteria were previously shown to be unable to develop strains resistant to long or short dermaseptin S4 derivatives (32), the acylated derivatives presented here are likely to behave in a similar manner.
In conclusion, the data collectively support the concept that a strategy based on combined truncation and acylation may be useful in revealing improved derivatives of known antimicrobial peptides.
Acknowledgments
This research was supported by the Israel Science Foundation (grant no. 387/03).
C. albicans strain CAF3-1 was kindly supplied by D. Kornitzer of the Ruth and Bruce Rappaport Faculty of Medicine, Technion—Israel Institute of Technology, Israel.
Footnotes
Published ahead of print on 16 October 2006.
REFERENCES
- 1.Altman, H., D. Steinberg, Y. Porat, A. Mor, D. Fridman, M. Friedman, and G. Bachrach. 2006. In vitro assessment of antimicrobial peptides as potential agents against several oral bacteria. J. Antimicrob. Chemother. 58:198-201. [DOI] [PubMed] [Google Scholar]
- 2.Avrahami, D., and Y. Shai. 2002. Conjugation of a magainin analogue with lipophilic acids controls hydrophobicity, solution assembly, and cell selectivity. Biochemistry 41:2254-2263. [DOI] [PubMed] [Google Scholar]
- 3.Baba, T., and O. Schneewind. 1998. Instruments of microbial warfare: bacteriocin synthesis, toxicity and immunity. Trends Microbiol. 6:66-71. [DOI] [PubMed] [Google Scholar]
- 4.Brogden, K. A. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3:238-250. [DOI] [PubMed] [Google Scholar]
- 5.Brogden, K. A., M. Ackermann, P. B. McCray, Jr., and B. F. Tack. 2003. Antimicrobial peptides in animals and their role in host defences. Int. J. Antimicrob. Agents 22:465-478. [DOI] [PubMed] [Google Scholar]
- 6.Brown, K. L., and R. E. Hancock. 2006. Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol. 18:24-30. [DOI] [PubMed] [Google Scholar]
- 7.Chalk, R., H. Townson, and P. J. Ham. 1995. Brugia pahangi: the effects of cecropins on microfilariae in vitro and in Aedes aegypti. Exp. Parasitol. 80:401-406. [DOI] [PubMed] [Google Scholar]
- 8.Chang, T. L., J. Vargas, Jr., A. DelPortillo, and M. E. Klotman. 2005. Dual role of α-defensin-1 in anti-HIV-1 innate immunity. J. Clin. Investig. 115:765-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, J., T. J. Falla, H. Liu, M. A. Hurst, C. A. Fujii, D. A. Mosca, J. R. Embree, D. J. Loury, P. A. Radel, C. C. Cheng, L. Gu, and J. C. Fiddes. 2000. Development of protegrins for the treatment and prevention of oral mucositis: structure-activity relationships of synthetic protegrin analogues. Biopolymers 55:88-98. [DOI] [PubMed] [Google Scholar]
- 10.Chen, J., X. M. Xu, C. B. Underhill, S. Yang, L. Wang, Y. Chen, S. Hong, K. Creswell, and L. Zhang. 2005. Tachyplesin activates the classic complement pathway to kill tumor cells. Cancer Res. 65:4614-4622. [DOI] [PubMed] [Google Scholar]
- 11.Chiang, P., and L. L. Burrows. 2003. Biofilm formation by hyperpiliated mutants of Pseudomonas aeruginosa. J. Bacteriol. 185:2374-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dagan, A., L. Efron, L. Gaidukov, A. Mor, and H. Ginsburg. 2002. In vitro antiplasmodium effects of dermaseptin S4 derivatives. Antimicrob. Agents Chemother. 46:1059-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnelly, J. P., L. A. Bellm, J. B. Epstein, S. T. Sonis, and R. P. Symonds. 2003. Antimicrobial therapy to prevent or treat oral mucositis. Lancet Infect. Dis. 3:405-412. [DOI] [PubMed] [Google Scholar]
- 14.Feder, R., A. Dagan, and A. Mor. 2000. Structure-activity relationship study of antimicrobial dermaseptin S4 showing the consequences of peptide oligomerization on selective cytotoxicity. J. Biol. Chem. 275:4230-4238. [DOI] [PubMed] [Google Scholar]
- 15.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedrich, C. L., D. Moyles, T. J. Beveridge, and R. E. Hancock. 2000. Antibacterial action of structurally diverse cationic peptides on gram-positive bacteria. Antimicrob. Agents Chemother. 44:2086-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710-720. [DOI] [PubMed] [Google Scholar]
- 18.Giacometti, A., O. Cirioni, F. Barchiesi, F. Caselli, and G. Scalise. 1999. In-vitro activity of polycationic peptides against Cryptosporidium parvum, Pneumocystis carinii and yeast clinical isolates. J. Antimicrob. Chemother. 44:403-406. [DOI] [PubMed] [Google Scholar]
- 19.Hallock, K. J., D. K. Lee, and A. Ramamoorthy. 2003. MSI-78, an analogue of the magainin antimicrobial peptides, disrupts lipid bilayer structure via positive curvature strain. Biophys. J. 84:3052-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hancock, R. E., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harwig, S. S., A. Waring, H. J. Yang, Y. Cho, L. Tan, and R. I. Lehrer. 1996. Intramolecular disulfide bonds enhance the antimicrobial and lytic activities of protegrins at physiological sodium chloride concentrations. Eur. J. Biochem. 240:352-357. [DOI] [PubMed] [Google Scholar]
- 22.Kluver, E., S. Schulz-Maronde, S. Scheid, B. Meyer, W. G. Forssmann, and K. Adermann. 2005. Structure-activity relation of human β-defensin 3: influence of disulfide bonds and cysteine substitution on antimicrobial activity and cytotoxicity. Biochemistry 44:9804-9816. [DOI] [PubMed] [Google Scholar]
- 23.Krugliak, M., R. Feder, V. Y. Zolotarev, L. Gaidukov, A. Dagan, H. Ginsburg, and A. Mor. 2000. Antimalarial activities of dermaseptin S4 derivatives. Antimicrob. Agents Chemother. 44:2442-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kustanovich, I., D. E. Shalev, M. Mikhlin, L. Gaidukov, and A. Mor. 2002. Structural requirements for potent versus selective cytotoxicity for antimicrobial dermaseptin S4 derivatives. J. Biol. Chem. 277:16941-16951. [DOI] [PubMed] [Google Scholar]
- 25.Lehrer, R. I. 2004. Primate defensins. Nat. Rev. Microbiol. 2:727-738. [DOI] [PubMed] [Google Scholar]
- 26.Liljemark, W. F., and C. Bloomquist. 1996. Human oral microbial ecology and dental caries and periodontal diseases. Crit. Rev. Oral Biol. Med. 7:180-198. [DOI] [PubMed] [Google Scholar]
- 27.Lorin, C., H. Saidi, A. Belaid, A. Zairi, F. Baleux, H. Hocini, L. Belec, K. Hani, and F. Tangy. 2005. The antimicrobial peptide dermaseptin S4 inhibits HIV-1 infectivity in vitro. Virology 334:264-275. [DOI] [PubMed] [Google Scholar]
- 28.Marsh, P. D. 2004. Dental plaque as a microbial biofilm. Caries Res. 38:204-211. [DOI] [PubMed] [Google Scholar]
- 29.Mor, A. 2000. Peptide-based antibiotics: a potential answer to raging antimicrobial resistance. Drug Dev. Res. 50:440-447. [Google Scholar]
- 30.Mor, A., and P. Nicolas. 1994. The NH2-terminal α-helical domain 1-18 of dermaseptin is responsible for antimicrobial activity. J. Biol. Chem. 269:1934-1939. [PubMed] [Google Scholar]
- 31.Mosca, D. A., M. A. Hurst, W. So, B. S. Viajar, C. A. Fujii, and T. J. Falla. 2000. IB-367, a protegrin peptide with in vitro and in vivo activities against the microflora associated with oral mucositis. Antimicrob. Agents Chemother. 44:1803-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navon-Venezia, S., R. Feder, L. Gaidukov, Y. Carmeli, and A. Mor. 2002. Antibacterial properties of dermaseptin S4 derivatives with in vivo activity. Antimicrob. Agents Chemother. 46:689-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papo, N., A. Braunstein, Z. Eshhar, and Y. Shai. 2004. Suppression of human prostate tumor growth in mice by a cytolytic D-, L-amino acid peptide: membrane lysis, increased necrosis, and inhibition of prostate-specific antigen secretion. Cancer Res. 64:5779-5786. [DOI] [PubMed] [Google Scholar]
- 34.Park, C. B., K. S. Yi, K. Matsuzaki, M. S. Kim, and S. C. Kim. 2000. Structure-activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: the proline hinge is responsible for the cell-penetrating ability of buforin II. Proc. Natl. Acad. Sci. USA 97:8245-8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patrzykat, A., C. L. Friedrich, L. Zhang, V. Mendoza, and R. E. Hancock. 2002. Sublethal concentrations of pleurocidin-derived antimicrobial peptides inhibit macromolecular synthesis in Escherichia coli. Antimicrob. Agents Chemother. 46:605-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pouny, Y., D. Rapaport, A. Mor, P. Nicolas, and Y. Shai. 1992. Interaction of antimicrobial dermaseptin and its fluorescently labeled analogues with phospholipid membranes. Biochemistry 31:12416-12423. [DOI] [PubMed] [Google Scholar]
- 37.Powers, J. P., and R. E. Hancock. 2003. The relationship between peptide structure and antibacterial activity. Peptides 24:1681-1691. [DOI] [PubMed] [Google Scholar]
- 38.Radzishevsky, I. S., S. Rotem, F. Zaknoon, L. Gaidukov, A. Dagan, and A. Mor. 2005. Effects of acyl versus aminoacyl conjugation on the properties of antimicrobial peptides. Antimicrob. Agents Chemother. 49:2412-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Risso, A., E. Braidot, M. C. Sordano, A. Vianello, F. Macri, B. Skerlavaj, M. Zanetti, R. Gennaro, and P. Bernardi. 2002. BMAP-28, an antibiotic peptide of innate immunity, induces cell death through opening of the mitochondrial permeability transition pore. Mol. Cell. Biol. 22:1926-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rolland, S. L., J. F. McCabe, C. Robinson, and A. W. Walls. 2006. In vitro biofilm formation on the surface of resin-based dentine adhesives. Eur. J. Oral Sci. 114:243-249. [DOI] [PubMed] [Google Scholar]
- 41.Shalev, D. E., A. Mor, and I. Kustanovich. 2002. Structural consequences of carboxyamidation of dermaseptin S3. Biochemistry 41:7312-7317. [DOI] [PubMed] [Google Scholar]
- 42.Shalev, D. E., S. Rotem, A. Fish, and A. Mor. 2006. Consequences of N-acylation on structure and membrane binding properties of dermaseptin derivative K4-S4-(1-13). J. Biol. Chem. 281:9432-9438. [DOI] [PubMed] [Google Scholar]
- 43.Steinberg, D. 2000. Studying plaque biofilms on various dental surfaces, p. 353-370. In Y. H. An and R. J. Friedman (ed.), Handbook of bacterial adhesion: principles, methods, and applications. Humana Press, Totowa, N.J.
- 44.Toke, O. 2005. Antimicrobial peptides: new candidates in the fight against bacterial infections. Biopolymers 80:717-735. [DOI] [PubMed] [Google Scholar]
- 45.Tossi, A., L. Sandri, and A. Giangaspero. 2000. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 55:4-30. [DOI] [PubMed] [Google Scholar]
- 46.Trotti, A., A. Garden, P. Warde, P. Symonds, C. Langer, R. Redman, T. F. Pajak, T. R. Fleming, M. Henke, J. Bourhis, D. I. Rosenthal, E. Junor, A. Cmelak, F. Sheehan, J. Pulliam, P. Devitt-Risse, H. Fuchs, M. Chambers, B. O'Sullivan, and K. K. Ang. 2004. A multinational, randomized phase III trial of iseganan HCl oral solution for reducing the severity of oral mucositis in patients receiving radiotherapy for head-and-neck malignancy. Int. J. Radiat. Oncol. Biol. Phys. 58:674-681. [DOI] [PubMed] [Google Scholar]
- 47.van Belkum, M. J., J. Kok, G. Venema, H. Holo, I. F. Nes, W. N. Konings, and T. Abee. 1991. The bacteriocin lactococcin A specifically increases permeability of lactococcal cytoplasmic membranes in a voltage-independent, protein-mediated manner. J. Bacteriol. 173:7934-7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu, Z., F. Cocchi, D. Gentles, B. Ericksen, J. Lubkowski, A. Devico, R. I. Lehrer, and W. Lu. 2005. Human neutrophil α-defensin 4 inhibits HIV-1 infection in vitro. FEBS Lett. 579:162-166. [DOI] [PubMed] [Google Scholar]
- 49.Yamaguchi, S., T. Hong, A. Waring, R. I. Lehrer, and M. Hong. 2002. Solid-state NMR investigations of peptide-lipid interaction and orientation of a β-sheet antimicrobial peptide, protegrin. Biochemistry 41:9852-9862. [DOI] [PubMed] [Google Scholar]
- 50.Yaron, S., T. Rydlo, D. Shachar, and A. Mor. 2003. Activity of dermaseptin K4-S4 against foodborne pathogens. Peptides 24:1815-1821. [DOI] [PubMed] [Google Scholar]
- 51.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]
- 52.Zasloff, M., B. Martin, and H. C. Chen. 1988. Antimicrobial activity of synthetic magainin peptides and several analogues. Proc. Natl. Acad. Sci. USA 85:910-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zelezetsky, I., U. Pag, H. G. Sahl, and A. Tossi. 2005. Tuning the biological properties of amphipathic alpha-helical antimicrobial peptides: rational use of minimal amino acid substitutions. Peptides 26:2368-2376. [DOI] [PubMed] [Google Scholar]