Abstract
Toxoplasmosis, caused by the protozoan Toxoplasma gondii, is medically important and distributed worldwide. Currently available medications are limited in terms of efficacy and side effects. We synthesized novel, nonacetal, hydrolytically stable derivatives of artemisinin and showed that they inhibit the replication of Toxoplasma gondii in cell culture.
Toxoplasma gondii is an apicomplexan protozoan of worldwide medical importance. Humans are infected by T. gondii through contact with feces from infected cats, by the consumption of undercooked meat from infected animals, or by transmission from infected mother to fetus. This parasite can cause systemic infection and widespread organ damage in immunocompromised individuals and neonates. Infection of immunocompetent adults can result in fever and adenopathy (11). Serological studies indicate that T. gondii could be associated with chronic neuropsychiatric diseases or behavioral abnormalities in some populations (1, 12).
Available medications for the prevention and treatment of toxoplasma infection show limited efficacy and have substantial side effects (5). Published studies have indicated that the naturally occurring 1,2,4-trioxane artemisinin and artemisinin derivatives such as artemether, originally developed for the treatment of malaria, have the ability to inhibit toxoplasma replication in vitro (2, 4, 6, 10). While these trioxanes have a number of advantages in terms of rapid action and low levels of toxicity, they are limited in terms of absorption, bioavailability, and short half-life (i.e., easy hydrolysis into toxic dihydroartemisinin) (9). Thus, we have designed and synthesized five C-10 nonacetal, hydrolytically stable derivatives of artemisinin (8) and measured their ability to inhibit the replication of T. gondii in vitro.
Artemisinin was obtained from Holley Pharmaceuticals Co., Inc., Fullerton, CA. Artemether was generously donated by Huiling Wang, University of Wuhan, China. Trimethoprim was purchased from Sigma Chemical Co. (St. Louis, MO). Chlorophenol red-β-d-galactopyranoside (Roche, Indianapolis, IN), 100 mM in 100 mM HEPES (pH 7.2), was stored frozen at −80°C. CellTiter 96 AQueous One Solution Reagent for determining cytotoxicity was purchased from Promega Corp., Madison, WI.
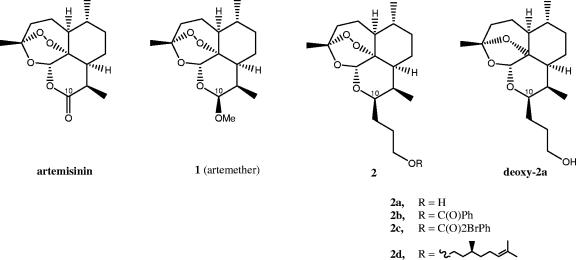
Structures of artemisinin and the derivatives are shown in Fig. 1. Trioxane C-10 primary alcohol 2a was prepared in 74% yield by hydroboration-oxidation of the corresponding known C-10 allyl trioxane. Esterification of primary alcohol 2a with benzoyl chloride led to benzoate ester 2b in 92% yield, and esterification with 2-bromobenzoyl chloride produced 2-bromobenzoate ester 2c in 86% yield. Deprotonation of the primary alcohol 2a using sodium hydride and then displacement of the bromide anion from citronelyl bromide gave citronelyl ether 2d in 55% yield. First, zinc-promoted deoxygenation of the known C-10 allyl trioxane and then hydroboration-oxidation produced dioxolane deoxy-2a in 72% yield. Each of these new lipid-soluble chromatographically purified artemisinin derivatives was fully characterized spectroscopically (proton and carbon-13 nuclear magnetic resonance, infrared, and high-resolution mass spectrometry). In contrast to artemether, these trioxane monomers are hydrolytically stable for at least 12 h even at 60°C in dimethyl sulfoxide-water at pH 7.4. Under these conditions, less than 5% decomposition was observed by proton nuclear magnetic resonance spectrometry.
FIG. 1.
Structures of artemisinin, artemether, and novel derivatives. Substitutions at R are as shown.
Normal human foreskin fibroblasts (American Type Culture Collection, Manassas, VA) were used to grow tachyzoites and to test compounds for activity and cytotoxicity. Cells were maintained in Dulbecco's modified Eagle medium (Gibco, Grand Island, NY) containing 10% fetal bovine serum (Atlas Biologicals, Fort Collins, CO), 25 mM HEPES (Gibco), 2 mM l-glutamine, 50 units of penicillin G per ml, and 50 μg of streptomycin sulfate per ml. A culture of the tachyzoites of Toxoplasma gondii strain 2F, which constitutively expresses cytoplasmic β-galactosidase and is derived from strain RH, was a gift from Vern Carruthers, University of Michigan Medical School.
Compounds were tested for in vitro efficacy against T. gondii and cytotoxicity by previously published methods (7). The median inhibitory dose (50% inhibitory dose [ID50]) and the median cytotoxic dose (50% toxic dose [TD50]) were calculated by extrapolation of the corresponding dose-response curve on a log-linear plot employing the portions of the curve that transected the 50% response point. For each compound, a therapeutic index (TI) was calculated by the formula TI = TD50/ID50.
The results of the toxoplasma inhibition and cytotoxicity assays are shown in Table 1. Artemether, 2b, 2c, and 2d all inhibited the toxoplasma at concentrations of less than 1 μg/ml. Somewhat less antitoxoplasma activity was noted with artemisinin and compound 2a, which inhibited toxoplasma replication at concentrations between 2 and 3 μg/ml. These values compare favorably to that of the antifolate positive-control compound trimethoprim, which inhibited toxoplasma replication at a concentration of 5.2 μg/ml. Nonperoxidic deoxy-2a showed virtually no inhibitory activity against the toxoplasma, with a TI of 1.2.
TABLE 1.
In vitro inhibition of T. gondii by artemisinin and synthetic derivatives
| Compounda | Mol wt | ID50
|
TD50
|
TI | ||
|---|---|---|---|---|---|---|
| μg/ml | μM | μg/ml | μM | |||
| Artemisinin | 282.34 | 2.3 | 8.0 | >320b | >1,130 | ≥243 |
| Artemether | 298.4 | 0.2 | 0.7 | 220 | 740 | 1,100 |
| 2d | 464.68 | 0.5 | 1.1 | 160 | 340 | 320 |
| 2c | 509.43 | 0.6 | 1.2 | >320b | >630 | ≥933 |
| 2b | 430.54 | 0.6 | 1.4 | >320b | >740 | ≥933 |
| 2a | 326.43 | 2.7 | 8.3 | 510 | 1,560 | 190 |
| Deoxy-2a | 310.43 | 173 | 560 | 200 | 640 | 1.2 |
| Trimethoprim | 290.3 | 5.2 | 17.9 | 60 | 210 | 12 |
The parent compound is shown first followed by the known derivative. The five new derivatives are listed in decreasing order of efficacy according to concentrations in μg/ml.
Toxicity endpoint was not reached; a value 1/4 log greater than the concentration tested was used to compute the TI.
In order to determine therapeutic indices and thus specific antitoxoplasma activity, we also measured the cytotoxicity of the test compounds. Artemisinin, 2a, 2b, and 2c showed little cytotoxicity at concentrations up to 510 μg/ml (compound 2a) or 320 μg/ml while the other artemisinin derivatives showed 50% cytotoxicity only at concentrations of >160 μg/ml. These degrees of cytotoxicity compare favorably with that of trimethoprim, with a TD50 of 60 μg/ml. The therapeutic indices of all of the compounds except deoxy-2a were thus approximately 10× to 100× more favorable than that of trimethoprim.
All of the compounds that showed inhibitory activity in our assays towards T. gondii have been shown to inhibit the replication of chloroquine-sensitive Plasmodium falciparum (NF 54) strains of malaria with ID50s from 5 to 30 nM (J. D'Angelo and G. Posner, unpublished data). And further, as expected (3), nonperoxidic deoxy-2a, which has virtually no antimalaria activity (J. D'Angelo and G. Posner, unpublished data), was also devoid of antitoxoplasma activity. These findings suggest that derivatives of artemisinin may affect similar pathways in toxoplasma and malarial organisms. The elucidation of these shared pathways should be the subject of additional investigations.
We have demonstrated that four new nonacetal derivatives of artemisinin have both increased antitoxoplasma activity and decreased cytotoxicity compared to trimethoprim, one of the antifolate compounds of relatively low toxicity that is used for the treatment of toxoplasma infection in humans. While other antifolate compounds, such as pyrimethamine, have increased antitoxoplasma activity, their toxicity generally precludes widespread usage, particularly over prolonged periods of administration (5). The availability of low-toxicity compounds capable of the prevention and treatment of T. gondii in humans would represent a major advance in the treatment of infections in immunocompromised individuals. Further, the availability of such compounds would also allow for clinical trials directed at defining the role of toxoplasma infections in human diseases.
Acknowledgments
We thank Theresa Shapiro, Andrew Rosenthal, and Dongpei Sang for valuable collaboration in obtaining in vitro antimalarial data.
This work was supported by the Theodore and Vada Stanley Foundation (L.J.-B. and R.Y.) and NIH grant AI 34885 (to G.H.P.).
Footnotes
Published ahead of print on 23 October 2006.
REFERENCES
- 1.Bachmann, S., J. Schröder, C. Bottmer, E. F. Torrey, and R. H. Yolken. 2005. Psychopathology in first-episode schizophrenia and antibodies to Toxoplasma gondii. Psychopathology 38:87-90. [DOI] [PubMed] [Google Scholar]
- 2.Berens, R. L., E. C. Krug, P. B. Nash, and T. J. Curiel. 1998. Selection and characterization of Toxoplasma gondii mutants resistant to artemisinin. J. Infect. Dis. 177:1128-1131. [DOI] [PubMed] [Google Scholar]
- 3.Brossi, A., B. Venugopalan, L. Dominguez Gerpe, H. J. C. Yeh, J. L. Flippen-Anderson, P. Bucks, X. D. Luo, W. Milhous, and W. Peters. 1988. Arteether, a new antimalarial drug: synthesis and antimalarial properties. J. Med. Chem. 31:645-650. [DOI] [PubMed] [Google Scholar]
- 4.Chang, H. R., C. W. Jefford, and J.-C. Pechère. 1989. In vitro effects of three new 1,2,4-trioxanes (pentatroxane, thiahexatroxane, and hexatroxanone) on Toxoplasma gondii. Antimicrob. Agents Chemother. 33:1748-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Georgiev, V. S. 1994. Management of toxoplasmosis. Drugs 48:179-188. [DOI] [PubMed] [Google Scholar]
- 6.Holfels, E., J. McAuley, D. Mack, W. K. Milhous, and R. McLeod. 1994. In vitro effects of artemisinin ether, cycloguanil hydrochloride (alone and in combination with sulfadiazine), quinine sulfate, mefloquine, primaquine phosphate, trifluoperazine hydrochloride, and verapamil on Toxoplasma gondii. Antimicrob. Agents Chemother. 38:1392-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones-Brando, L., E. F. Torrey, and R. Yolken. 2003. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr. Res. 62:237-244. [DOI] [PubMed] [Google Scholar]
- 8.Lin, A. J., D. L. Klayman, and W. K. Milhous. 1987. Antimalarial activity of new water-soluble dihydroartemisinin derivatives. J. Med. Chem. 30:2147-2150. [DOI] [PubMed] [Google Scholar]
- 9.O'Neill, P. M., and G. H. Posner. 2004. A medicinal chemistry perspective on artemisinin and related endoperoxides. J. Med. Chem. 47:2945-2964. [DOI] [PubMed] [Google Scholar]
- 10.Ou-Yang, K., E. C. Krug, J. J. Marr, and R. L. Berens. 1990. Inhibition of growth of Toxoplasma gondii by qinghaosu and derivatives. Antimicrob. Agents Chemother. 34:1961-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tenter, A. M., A. R. Heckeroth, and L. M. Weiss. 2000. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30:1217-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yolken, R. H., S. Bachmann, I. Ruslanova, E. Lillehoj, G. Ford, E. F. Torrey, and J. Schröder. 2001. Antibodies to Toxoplasma gondii in individuals with first-episode schizophrenia. Clin. Infect. Dis. 32:842-844. [DOI] [PubMed] [Google Scholar]