Abstract
Highly active antiretroviral therapy (HAART) of human immunodeficiency virus-infected patients is associated with adverse effects, such as lipodystrophy and hyperlipidemia. The lipodystrophic syndrome is characterized by a peripheral lipoatrophy and/or fat accumulation in the abdomen and neck. In order to get insights into the physiopathological mechanisms underlying this syndrome, we treated mice with protease inhibitors (PIs) over a long period of time. Although atazanavir-treated mice presented the same circulating triglyceride concentration as control mice, lopinavir-ritonavir-treated mice rapidly became hypertriglyceridemic, with triglyceride levels of 200 mg/dl, whereas control and atazanavir-treated animals had triglyceride levels of 80 mg/dl. These results obtained with mice reproduce the metabolic disorder observed in humans. White adipose tissue (WAT) was analyzed after 8 weeks of treatment. Compared to the control or atazanavir treatment, lopinavir-ritonavir treatment induced a significant 25% weight reduction in the peripheral inguinal WAT depot. By contrast, the profound epididymal WAT depot was not affected. This effect was associated with a 5.5-fold increase in SREBP-1c gene expression only in the inguinal depot. Our results demonstrate that the long-term treatment of mice with PIs constitutes an interesting experimental model with which some aspects of the lipoatrophy induced by HAART in humans may be studied.
Highly active antiretroviral therapy (HAART) combines various protease inhibitors (PIs), nucleoside analogue reverse transcriptase inhibitors (NRTIs), and nonnucleoside analogue reverse transcriptase inhibitors (NNRTIs). Whereas HAART efficiently suppresses human immunodeficiency virus (HIV) replication, long-term treatment of HIV-infected patients has been associated with a lipodystrophic syndrome and metabolic complications, with one of the most common complications being hypertriglyceridemia (6). The HAART-associated lipodystrophy is characterized by peripheral fat wasting in the face and limbs and the accumulation of visceral fat, breast hypertrophy, and cervical fat pads (buffalo hump) (7-9). Metabolic complications are preferentially associated with PI treatment and appear much more rapidly than lipodystrophy (20). It is not known whether the latter is a long-term consequence of the metabolic disorder or whether it corresponds to distinct molecular mechanisms. Because of the lack of a pertinent experimental model, the pathogenesis and physiopathological mechanisms by which PIs, NRTIs, and NNRTIs cause HAART-associated lipodystrophy and metabolic disorders remain to be elucidated.
PIs, NRTIs, and NNRTIs can interfere with adipocyte differentiation and adipocyte-specific gene expression (16, 18, 22, 26-29). However, the reported effects are quite variable, positive or negative, depending on the molecule and on the experimental model studied. Furthermore, these cellular studies do not explain why HAART leads, in one case, to peripheral lipoatrophy and, in the other case, to abdominal hypertrophy of the adipose tissue. Short-term treatment (10 days) of mice evidenced a hyperlipidemic effect of the PI ritonavir (21). This effect correlated with the activation of SREBP-1c in liver tissue and white adipose tissues (WATs).
Because HAART is treatment with a combination of several drugs, it is impossible to discriminate the precise contribution of each antiretroviral compound. In order to get insights into the physiopathological mechanisms underlying the lipodystrophy syndrome, we treated mice with PIs over a long period. We found that the lopinavir (LPV)-ritonavir (RTV) treatment specifically reduces the peripheral inguinal WAT depots of adult treated mice.
MATERIALS AND METHODS
Animals and drug administration.
C57BL/6J male mice were housed on a 12-h light and 12-h dark schedule and had free access to water and food. Lopinavir-ritonavir in combination (LPV/r; ABT-378/r oral solution; Abbott Laboratories) or atazanavir (ATV; Reyataz; Bristol-Myers-Squibb Laboratories) was administered by oral gavage at 200 μl/animal once daily. Control mice received an equal volume of 0.5% hydroxymethyl cellulose through a pipette. Body weights were measured weekly.
The experiments were conducted according to standard ethical guidelines (European Union Guidelines on Animal Laboratory Care) and were approved by the Faculty de Medicine of Nice Ethical Committee.
Pharmacokinetic analysis.
C57BL/6J male mice were treated daily with atazanavir or lopinavir-ritonavir for 3 days. On the fourth day, the mice were anesthetized with pentobarbital (9 mg/mouse) and 500-μl blood samples were collected from the retro-orbital sinus at various times after the last gavage. The plasma PI concentrations were determined by high-pressure liquid chromatography with a reverse-phase column and a diode array detection detector; the limit of quantification was 50 ng/ml. The areas under the concentration-time curves (AUCs) for plasma were estimated by use of a noncompartmental analysis model (Winonlin 3.2 software).
Collection of tissues and serum analysis.
Following blood sampling, serum was isolated by centrifugation and was immediately stored at −80°C until analysis. Inguinal white adipose tissue, epididymal white adipose tissue, interscapular brown adipose tissue (BAT), and the liver were excised and weighed. For RNA isolation, samples of epididymal and inguinal white adipose tissues from the left side were placed on ice and were immediately homogenized with the TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA).
Triglycerides were quantified with a Triglycerides 100 biochemical kit (ABX Diagnostics, Montpellier, France). Glycemia was determined with Accu-Check active bands (Roche Diagnostics, Mannheim, Germany).
Adipose tissue histology.
The epididymal and inguinal white adipose tissue from the right side were formalin fixed and paraffin embedded. The sections were stained with hematoxylin-eosin-safranin for light microscopic examination.
Analysis of mRNA levels by real-time quantitative PCR.
Total RNA was prepared by using the TRIzol reagent (Invitrogen), and gene expression analysis was performed with an ABI Prism 7000 instrument (Applied Biosystem) and SYBR green reagents (Eurogentec, Seraing, Belgium), as already described (2). Briefly, cDNAs were synthesized from 2 μg of total RNA by using Superscript II reverse transcriptase (Invitrogen). Primer sets were designed according to the manufacturer's software. Samples contained 1× SYBR green master mixture, 0.5 μM primers, and 1/10 synthesized cDNA in a 25-μl volume. The PCR conditions were as follows: 10 min at 95°C and then 40 cycles of 15 s at 94°C, 30 s at 60°C, and 1 min at 72°C. We used 36B4 as the reference gene.
The primers and their sequences are as follows: primer FAS F, 5′-TGCTCCCAGCTGCAGGC-3′; primer FAS R, 5′-GCCCGGTAGCTCTGGGTGTA-3′; primer SREBP1c F, 5′-GGAGCCATGGATTGCACATT-3′; primer SREBP1c R, 5′-GCTTCCAGAGAGGAGGCCAG-3′; primer PPARγ F, 5′-CTGTTTTATGCTGTTATGGGTGAAA-3′; primer PPARγ R, 5′-GCACCATGCTCTGGGTCAA-3′; primer Adiponectin F, 5′-TCATGCCGAAGATGACGTTACT-3′; primer Adiponectin R, 5′-CCATCCAACCTGCACAAGTTC-3′; primer 36B4 F, 5′-TCCAGGCTTTGGGCATCA-3′; and primer 36B4 R, 5′-CTTTATCAGCTGCACATCACTCAGA-3′.
RESULTS
In order to set up an experimental model to study the HAART-associated lipodystrophy, control mice were compared to mice receiving two long-term intraperitoneal treatments: treatment with the classical combination of LPV/r, which is strongly involved in HAART, and treatment with the new compound ATV, which induces rare metabolic disorders in patients (12, 19).
Pharmacokinetic analysis.
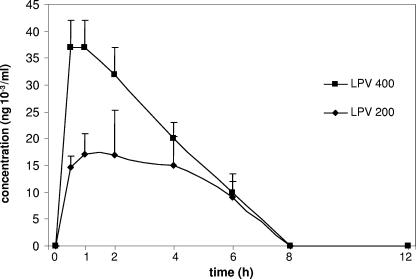
Pharmacokinetic analysis was performed to determine the dose regimen necessary to obtain circulating drug concentrations in mice similar to therapeutic levels in humans. Two LPV/r doses, 200/50 and 400/100 mg/kg of body weight (15- to 30-fold the doses therapeutic for humans, respectively), were given to the animals by oral gavage daily. On the fourth treatment day, the LPV and RTV concentrations were determined at 0, 2, 4, 6, 8, and 12 h after the last gavage. In humans, the AUC for lopinavir for a 12-h period is between 52 and 76 μg · h/ml (10) (14). Our results show that lopinavir was rapidly eliminated in mice, with the 200-mg/kg/day dose giving impregnation similar to that for human dosages and an AUC of 95 μg · h/ml AUC, whereas the impregnation by the 400-mg/kg/day dose was fourfold higher, with the AUC being 255 μg · h/ml (Fig. 1). Ritonavir was also eliminated rapidly; and its concentration peaked at 2 μg/ml for the 50-mg/kg dose, which is similar to that for human dosages (data not shown). Long-term treatment with the 400/100-mg/kg/day dose appeared to be highly toxic, leading to the death of most of the mice after a few weeks (data not shown). In the case of atazanavir, we also chose a concentration 15-fold higher than that used for humans and found that the 90-mg/kg/day dose gave a peak circulating concentration of 12 μg/ml after 1 h, which decreased rapidly (data not shown). The calculated AUC was 32.7 μg · h/ml, giving impregnation similar to that of human dosages (whose AUC is 28 μg · h/ml). Our results showed that the half-lives and steady-state levels of these molecules are shorter in mice than in humans. An important pharmaceutical parameter is the fraction of drug that binds to circulating proteins. Differences in this proportion between humans and mice could introduce a bias in the results. The association of LPV/r and ATV with plasma binding proteins in different species (including humans and mice) has been analyzed by others and is available on the European Medicine Agency website (http://www.emea.eu.int). Published results show no significant differences in protein binding between human and mice for these molecules. Therefore, we postulate that the impregnation and the proportion of active molecules that we obtained in treated mice are comparable to those reached in humans.
FIG. 1.
Pharmacokinetic analysis of lopinavir. Mice were treated daily for 4 days with LPV/r at either 200/50 mg (lopinavir dose, 200 mg) or 400/100 mg (lopinavir dose, 400 mg)/kg/day. At the indicated times after the last gavage, blood samples were analyzed by high-pressure liquid chromatography to determine drug concentrations. Values represent the means for three animals ± standard errors of the means.
LPV/r induces hypertriglyceridemia in mice.
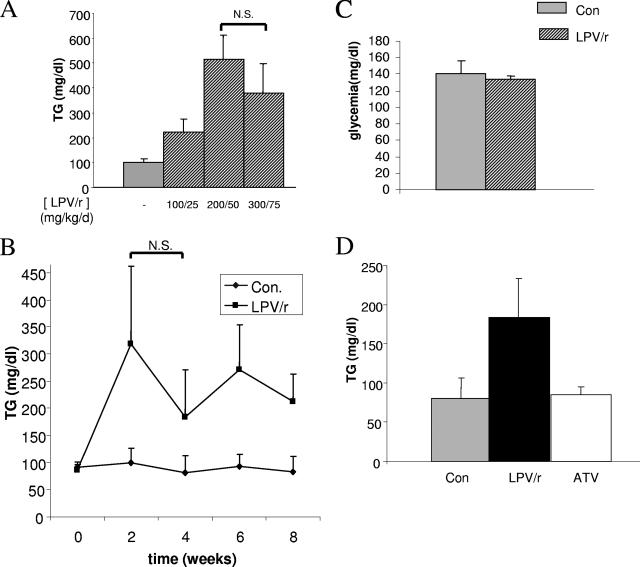
Retro-orbital blood samples were obtained from control male C57BL/6J mice and mice treated daily with three different doses of LPV/r (100/25, 200/50, and 300/75 mg/kg/day) for 2 weeks. The samples were analyzed for their glucose and triglyceride contents. The three LPV/r treatments induced hypertriglyceridemia in mice (2.2-, 5.1-, and 3.8-fold the triglyceride concentrations in control mice, respectively) after 2 weeks (Fig. 2A). This effect was stable over the time and persisted in animals treated for 8 weeks (Fig. 2B). Glycemia was measured in these animals, and no change was induced by the various LPV/r treatments (Fig. 2C for the 200/50-mg/kg/day dose and data not shown). Our results reproduce the dyslipidemia syndrome associated with HAART in humans and the observation that these effects are much more frequent and substantial than changes in glucose levels (25). The 200/50-mg/kg/day dose was used in the subsequent experiments. This dose did not appear to be toxic and induced reproducible hypertriglyceridemia.
FIG. 2.
Effect of treatments on triglyceridemia and glycemia. (A) Mice were treated daily for 2 weeks with increasing doses of the LPV/r (100/25, 200/50, or 300/75 mg/kg/day or control treatment), as indicated. Blood samples were analyzed for their triglyceride (TG) concentrations. The histogram shows the mean values for four animals per condition. (B) Mice were treated daily for 2 to 8 weeks, as indicated, with LPV/r at 200/50 mg/kg/day or with excipient alone (controls [Con]) and were analyzed for their triglyceride concentrations. The values are the means for 4 to 10 animals per group. (C) Mice were treated daily for 2 weeks with the LPV/r at 200/50 mg/kg/day or the excipient alone (controls), and glycemia was measured. The histogram shows the mean values for four animals per condition. (D) Mice were treated daily for 8 weeks with the LPV/r at 200/50 mg/kg/day, ATV at 90 mg/kg/day, or excipient alone (controls); and the triglyceride concentrations were measured. The histogram shows the mean values for 23 control animals, 17 LPV/r-treated animals, and 12 ATV-treated animals. Values are given as the means ± standard errors of the means. N.S., nonsignificant.
We then analyzed the metabolic parameters for mice treated for 8 weeks with either LPV/r (200/50 mg/day) or ATV (90 mg/kg/day) and compared them with the metabolic parameters for control treated mice. Whereas no change in glycemia was observed (data not shown), the LPV/r treatment induced hypertriglyceridemia (2.5-fold the triglyceride concentration of control or ATV-treated mice), confirming the absence of a metabolic effect of ATV (Fig. 2D).
LPV/r reduces the peripheral white adipose depot in mice treated for a long period.
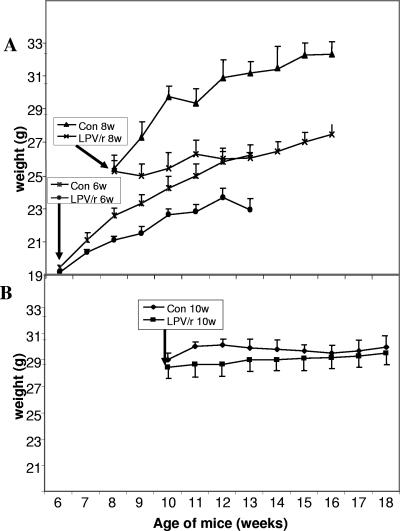
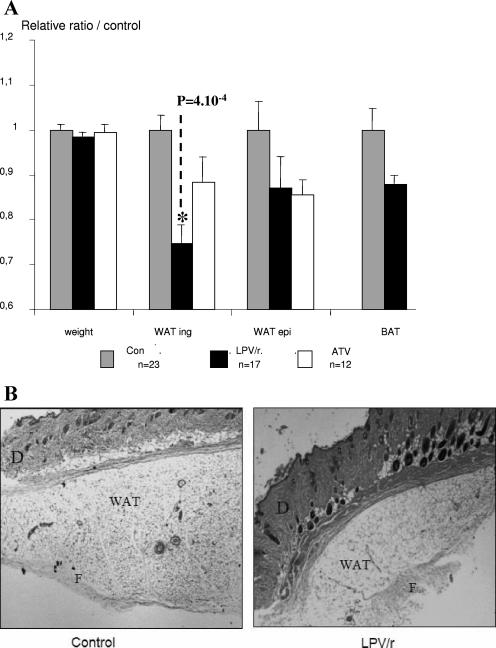
In preliminary experiments, 6- and 8-week-old mice were treated for 8 weeks with LPV/r at 200/50 mg/kg/day. At those ages, mice are still growing; and over a period of 8 weeks, the weights of the control mice increased from 20 to 27 g and from 25 to more than 30 g, respectively. In those mice, LPV/r treatment had a marked inhibitory effect on growth; the weights of the treated mice did not increase or increased much more slowly than those of the control mice (Fig. 3A). This effect of the 200/50-mg/kg LPV/r dose on young mice is probably related to the relatively toxic effect of LPV/r that was observed at higher doses. We then performed the experiments by starting with 10-week-old mice, and ATV-treated mice were included in those experiments. No effect of treatment on weight was noticed between the three groups of mice; the starting weight was about 30 ± 2 g for all the mice, and this value did not change significantly during the treatments (Fig. 3B and 4). After 8 weeks, the mice were killed and various tissues were carefully dissected and weighed. Two different groups of mice were treated under the same conditions, and the results for those mice are presented in Fig. 4A. Compared to the weight of the peripheral inguinal adipose depot of the control mice, the weight of the peripheral inguinal adipose depot was significantly reduced by 25% by the LPV/r treatment. A representative lipoatrophic effect of the LPV/r treatment on the peripheral inguinal adipose depot is presented in Fig. 4B. No significant change in the size of the adipocytes of LPV/r-treated animals was observed, suggesting that the lipoatrophic effect was due to a remodeling of the tissue rather than a cellular effect. By contrast, the epididymal fat depot and the brown adipose tissues were not significantly modified (Fig. 4A). Compared to the tissues of the control mice, ATV treatment did not induce significant changes in any of the tissues that we investigated (Fig. 4A).
FIG. 3.
Effects of LPV/r treatment on body weights of young and adult mice. Mice at the ages of 6 weeks (6w) or 8 weeks (8w) (A) or mice at the age of 10 weeks (10w) (B) were treated daily for 8 weeks with LPV/r at 200/50 mg/kg/day or received the control treatment (Con) and were weighed every week. Values are the means for 6 to 10 animals per group.
FIG. 4.
Effects of LPV/r and ATV on body and tissue weights. Ten-week-old mice were treated daily for 8 weeks with the LPV/r at 200/50 mg/kg/day or ATV at 90 mg/kg/day or received the control treatment (Con). (A) Mice were analyzed for their body weights; and their inguinal WAT (WAT ing), epididymal WAT (WAT epi), and BAT depots were dissected and weighed. Values are normalized to 1 for control animals ± the standard error of the mean. Inguinal WAT was significantly affected by the LPV/r treatment (P = 0.0004). (B) Representative histological analysis of inguinal WAT depots of either control or LPV/r-treated mice. D, dermis; F, fibrous trabecula. Magnification, ×10.
LPV/r induces SREBP-1c gene expression in the peripheral white adipose depot.
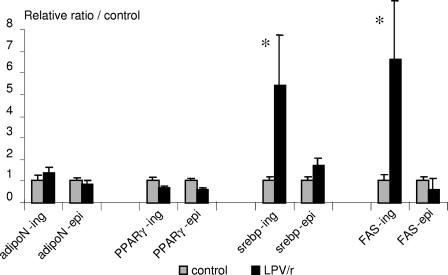
In order to study the molecular mechanisms involved in this tissue-specific effect, RNAs from both inguinal and epididymal fat depots were extracted and analyzed by real-time reverse transcription-PCR for the expression of genes for various adipocyte markers. Compared to the results for the controls, the results show that the expression of the genes for sterol regulatory element-binding protein-1c (SREBP-1c) and fatty acid synthase (FAS) was strongly induced (more than fivefold) in the inguinal WATs of the LPV/r-treated mice (Fig. 5). By contrast, in the epididymal fat pad, whose weight was not affected by LPV/r, the treatment did not significantly affect the expression of these genes. In both WAT depots, the levels of expression of the genes for the adiponectin and peroxisome proliferator-activated receptor gamma (PPARγ) adipogenic markers were not modified by the LPV/r treatment.
FIG. 5.
Expression of genes for adiponectin, PPARγ, SREBP-1c, and FAS in WAT. mRNAs from epididymal (epi) and inguinal (ing) WAT depots were extracted from control mice and from ATV- and LPV/r-treated mice and were analyzed by real-time reverse transcription-PCR with primers specific for the genes for adiponectin (adipoN), PPARγ, SREBP-1c (srebp), and FAS. Values were corrected for equal 36B4 expression and were normalized to 1 for the control animals ± standard error of the mean. *, P < 0.05.
DISCUSSION
In order to gain insight into the physiopathological mechanisms underlying the lipodystrophy syndrome, we treated mice with protease inhibitors for long periods. We found that the combination of lopinavir and ritonavir induces hypertriglyceridemia and specifically reduces the peripheral inguinal WAT depots of 10-week-old mice treated for 8 weeks. The metabolic disorder observed in our animal model reproduces the dyslipidemia observed in the humans. The HAART-associated lipodystrophic syndrome in humans is also reproduced in mice, but only partially. In particular, the peripheral lipoatrophy is effectively detected in mice at the level of inguinal WAT depots, whereas other tissues are not affected. However, this effect on mice does not completely match the observations reported for humans; for example, we did not notice any change at the level of the other WAT depots.
In a recently published study (11), the authors analyzed the effects of long-term ritonavir treatment on 5-week-old mice. They found that the drug induces a general lipoatrophy and exerts a potent blocking effect on the general growth of the animals. Similar to their findings, we also noticed that drug treatment of young animals, while they are still growing, led to potent inhibition of their growth. Furthermore, we observed that higher doses led to the rapid death of the animals. Therefore, it is possible that the effects observed in young animals may be related to an unspecific toxic effect rather than a potential for lipodystrophy. By contrast, when the treatment was started when the animals were adults (age, 10 weeks), the treatment did not affect their growth. Therefore, it is likely that the lipodystrophy in animals treated after puberty, when growth has slowed or stopped, is more relevant to the HAART-associated lipodystrophy described in adult humans.
Importantly, LPV/r reduces the peripheral white adipose tissue depot in mice treated for long periods without affecting the other fat depots. Compared to visceral adipose tissue, peripheral and subcutaneous adipose tissues display important differences in their biological characteristics and properties (for a review, see reference 15). These differences consist in different capacities of metabolic pathways and adipocyte differentiation potential. Whether the specific effect of LPV/r on peripheral tissue is due to one or several of these characteristics remains to be determined. Interestingly, potent activators of PPARγ, such as thiazolidinedione (TZD), increase the differentiation of subcutaneous preadipocytes into adipocytes but have no effect on the differentiation of visceral precursors into adipocytes (1). We found no effect of LPV/r on PPARγ expression, suggesting that the reduction of peripheral WAT by LPV/r is not due to PPARγ downregulation. However, one possibility to be tested is whether TZD could counteract the LPV/r-induced reduction of peripheral WAT by increasing adipogenesis in this tissue.
We found that the reduction in the peripheral WAT depot induced by LPV/r treatment is associated with an increase in the level of expression of the gene for the transcription factor SREBP-1c and of the gene for FAS, an SREBP-1c target. The levels of expression of the genes for the adipocyte markers PPARγ and adiponectin were not affected. Interestingly, in the same mice, regulation of these genes was not found in the epididymal WAT depot, whose weight was not modified by the drug treatment. SREBP-1c activation has largely been involved in the control of adipocyte differentiation, in the development of adipose tissue, and more recently, in HAART-associated lipodystrophy. Although SREBP-1c has been shown to promote adipocyte differentiation in vitro first (24), in vivo transgenic experiments demonstrated that its overexpression in WAT is associated with impaired adipogenesis and the lipoatrophic phenotype (13, 23). Furthermore, several studies have shown that PI treatment results in the regulation of SREBP-1c activity and, concomitantly, the inhibition of adipogenesis both in vitro (4, 5, 18) and in vivo (3, 21) in mice and humans. The effects of the PIs ritonavir and indinavir on SREBP1-c are complex; ritonavir increases the level of the mature nuclear active form of the protein both in vitro (18) and in mice (21), whereas indinavir decreases the total level of SREBP-1c protein expression and alters its subcellular localization (5). Because of its known function in the regulation of fatty acid synthesis, SREBP-1c activation could directly participate in the hypertriglyceridemia associated with PI treatment. Accordingly, our results showed that LPV/r induces both hypertriglyceridemia and increased levels of expression of the genes for SREBP-1c and FAS. Although the precise role of SREBP-1c in lipodystrophy is unclear (see the review by Nerurkar et al. [17]), our results strongly suggest that SREBP-1c gene expression is linked to lopinavir-induced lipoatrophy of peripheral WAT in mice. One can hypothesize that the high degree of variability of the lipodystrophy syndrome observed in humans reflects the various effects of PIs on the levels of expression and activities of the genes involved in adipogenesis, such as SREBP-1c. This hypothesis is strengthened by our results with atazanavir. In contrast to LPV/r, atazanavir does not induce either lipoatrophy of the peripheral WAT depot or changes in SREBP-1c gene expression. This absence of a drug effect on mice is in agreement with preliminary clinical observations. Since our experiments were performed with the combination of lopivanir and ritonavir, further experiments with each drug individually are required to determine whether the effects are due to lopinavir only or whether there is also a contribution of ritonavir. Likewise, since atazanavir is now also administered in association with ritonavir, the effects of this combination of drugs should be evaluated in studies with mice.
In conclusion, our results validated the use of mice as an experimental model to explore some aspects of the physiopathology of HAART-associated lipodystrophy in humans. In our experimental animal model, the treatment of adult mice seems to be a critical parameter required to obtain reliable effects. In order to get a model of disease closer to the human disease and to obtain evidence of additional clinical aspects relevant to HAART-associated lipodystrophy, it would be interesting to modify the treatment by increasing its duration and/or by adjusting the dose regimen so that the AUC further approaches the AUC in humans. Alternatively, testing of other PIs, alone or in combination, could also reveal interesting clinical aspects.
Acknowledgments
This work was supported by grants from the Agence Nationale de Recherches sur le SIDA and Ensemble contre le SIDA.
We thank F. Bost for its support.
Footnotes
Published ahead of print on 25 September 2006.
REFERENCES
- 1.Adams, M., C. T. Montague, J. B. Prins, J. C. Holder, S. A. Smith, L. Sanders, J. E. Digby, C. P. Sewter, M. A. Lazar, V. K. K. Chatterjee, and S. O'Rahilly. 1997. Activators of peroxisome proliferator-activated receptor gamma have depot-specific effects on human preadipocyte differentiation. J. Clin. Investig. 100:3149-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aouadi, M., K. Laurent, M. Prot, Y. Le Marchand-Brustel, B. Binetruy, and F. Bost. 2006. Inhibition of p38MAPK increases adipogenesis from embryonic to adult stages. Diabetes 55:281-289. [DOI] [PubMed] [Google Scholar]
- 3.Bastard, J. P., M. Caron, H. Vidal, V. Jan, M. Auclair, C. Vigouroux, J. Luboinski, M. Laville, M. Maachi, P. M. Girard, W. Rozenbaum, P. Levan, and J. Capeau. 2002. Association between altered expression of adipogenic factor SREBP1 in lipoatrophic adipose tissue from HIV-1-infected patients and abnormal adipocyte differentiation and insulin resistance. Lancet 359:1026-1031. [DOI] [PubMed] [Google Scholar]
- 4.Caron, M., M. Auclair, H. Sterlingot, M. Kornprobst, and J. Capeau. 2003. Some HIV protease inhibitors alter lamin A/C maturation and stability, SREBP-1 nuclear localization and adipocyte differentiation. AIDS 17:2437-2444. [DOI] [PubMed] [Google Scholar]
- 5.Caron, M., M. Auclair, C. Vigouroux, M. Glorian, C. Forest, and J. Capeau. 2001. The HIV protease inhibitor indinavir impairs sterol regulatory element-binding protein-1 intranuclear localization, inhibits preadipocyte differentiation, and induces insulin resistance. Diabetes 50:1378-1388. [DOI] [PubMed] [Google Scholar]
- 6.Carr, A., J. Miller, M. Law, and D. A. Cooper. 2000. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor-related lipodystrophy syndrome. AIDS 14:F25-F32. [DOI] [PubMed] [Google Scholar]
- 7.Carr, A., K. Samaras, S. Burton, M. Law, J. Freund, D. J. Chisholm, and D. A. Cooper. 1998. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS 12:F51-F58. [DOI] [PubMed] [Google Scholar]
- 8.Carr, A., K. Samaras, D. J. Chisholm, and D. A. Cooper. 1998. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet 351:1881-1883. [DOI] [PubMed] [Google Scholar]
- 9.Carr, A., K. Samaras, A. Thorisdottir, G. R. Kaufmann, D. J. Chisholm, and D. A. Cooper. 1999. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet 353:2093-2099. [DOI] [PubMed] [Google Scholar]
- 10.Corbett, A. H., M. L. Lim, and A. D. Kashuba. 2002. Kaletra (lopinavir/ritonavir). Ann. Pharmacother. 36:1193-1203. [DOI] [PubMed] [Google Scholar]
- 11.Goetzman, E. S., L. Tian, T. R. Nagy, B. A. Gower, T. R. Schoeb, A. Elgavish, E. P. Acosta, M. S. Saag, and P. A. Wood. 2003. HIV protease inhibitor ritonavir induces lipoatrophy in male mice. AIDS Res. Hum. Retrovir. 19:1141-1150. [DOI] [PubMed] [Google Scholar]
- 12.Haas, D. W., C. Zala, S. Schrader, P. Piliero, H. Jaeger, D. Nunes, A. Thiry, S. Schnittman, and M. Sension. 2003. Therapy with atazanavir plus saquinavir in patients failing highly active antiretroviral therapy: a randomized comparative pilot trial. AIDS 17:1339-1349. [DOI] [PubMed] [Google Scholar]
- 13.Horton, J. D., I. Shimomura, S. Ikemoto, Y. Bashmakov, and R. E. Hammer. 2003. Overexpression of sterol regulatory element-binding protein-1a in mouse adipose tissue produces adipocyte hypertrophy, increased fatty acid secretion, and fatty liver. J. Biol. Chem. 278:36652-36660. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 14.Hsu, A., J. Isaacson, S. Brun, B. Bernstein, W. Lam, R. Bertz, C. Foit, K. Rynkiewicz, B. Richards, M. King, R. Rode, D. J. Kempf, G. R. Granneman, and E. Sun. 2003. Pharmacokinetic-pharmacodynamic analysis of lopinavir-ritonavir in combination with efavirenz and two nucleoside reverse transcriptase inhibitors in extensively pretreated human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 47:350-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lafontan, M., and M. Berlan. 2003. Do regional differences in adipocyte biology provide new pathophysiological insights? Trends Pharmacol. Sci. 24:276-283. [DOI] [PubMed] [Google Scholar]
- 16.Lenhard, J. M., E. S. Furfine, R. G. Jain, O. Ittoop, L. A. Orband-Miller, S. G. Blanchard, M. A. Paulik, and J. E. Weiel. 2000. HIV protease inhibitors block adipogenesis and increase lipolysis in vitro. Antivir. Res. 47:121-129. [DOI] [PubMed] [Google Scholar]
- 17.Nerurkar, P. V., C. M. Shikuma, and V. R. Nerurkar. 2001. Sterol regulatory element-binding proteins and reactive oxygen species: potential role in highly active antiretroviral therapy (HAART)-associated lipodystrophy. Clin. Biochem. 34:519-529. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen, A. T., A. Gagnon, J. B. Angel, and A. Sorisky. 2000. Ritonavir increases the level of active ADD-1/SREBP-1 protein during adipogenesis. AIDS 14:2467-2473. [DOI] [PubMed] [Google Scholar]
- 19.Noor, M. A., R. A. Parker, E. O'Mara, D. M. Grasela, A. Currie, S. L. Hodder, F. T. Fiedorek, and D. W. Haas. 2004. The effects of HIV protease inhibitors atazanavir and lopinavir/ritonavir on insulin sensitivity in HIV-seronegative healthy adults. AIDS 18:2137-2144. [DOI] [PubMed] [Google Scholar]
- 20.Purnell, J. Q., A. Zambon, R. H. Knopp, D. J. Pizzuti, R. Achari, J. M. Leonard, C. Locke, and J. D. Brunzell. 2000. Effect of ritonavir on lipids and post-heparin lipase activities in normal subjects. AIDS 14:51-57. [DOI] [PubMed] [Google Scholar]
- 21.Riddle, T. M., D. G. Kuhel, L. A. Woollett, C. J. Fichtenbaum, and D. Y. Hui. 2001. HIV protease inhibitor induces fatty acid and sterol biosynthesis in liver and adipose tissues due to the accumulation of activated sterol regulatory element-binding proteins in the nucleus. J. Biol. Chem. 276:37514-37519. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 22.Roche, R., I. Poizot-Martin, C. M. Yazidi, E. Compe, J. A. Gastaut, J. Torresani, and R. Planells. 2002. Effects of antiretroviral drug combinations on the differentiation of adipocytes. AIDS 16:13-20. [DOI] [PubMed] [Google Scholar]
- 23.Shimomura, I., R. E. Hammer, J. A. Richardson, S. Ikemoto, Y. Bashmakov, J. L. Goldstein, and M. S. Brown. 1998. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 12:3182-3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tontonoz, P., J. B. Kim, R. A. Graves, and B. M. Spiegelman. 1993. ADD1: a novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol. Cell. Biol. 13:4753-4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsiodras, S., C. Mantzoros, S. Hammer, and M. Samore. 2000. Effects of protease inhibitors on hyperglycemia, hyperlipidemia, and lipodystrophy: a 5-year cohort study. Arch. Intern. Med. 160:2050-2056. [DOI] [PubMed] [Google Scholar]
- 26.Vernochet, C., S. Azoulay, D. Duval, R. Guedj, F. Cottrez, H. Vidal, G. Ailhaud, and C. Dani. 2005. Human immunodeficiency virus protease inhibitors accumulate into cultured human adipocytes and alter expression of adipocytokines. J. Biol. Chem. 280:2238-2243. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 27.Vernochet, C., D. S. Milstone, C. Iehle, N. Belmonte, B. Phillips, B. Wdziekonski, P. Villageois, E. Z. Amri, P. E. O'Donnell, R. M. Mortensen, G. Ailhaud, and C. Dani. 2002. PPARgamma-dependent and PPARgamma-independent effects on the development of adipose cells from embryonic stem cells. FEBS Lett. 510:94-98. [DOI] [PubMed] [Google Scholar]
- 28.Wentworth, J. M., T. P. Burris, and V. K. Chatterjee. 2000. HIV protease inhibitors block human preadipocyte differentiation, but not via the PPARgamma/RXR heterodimer. J. Endocrinol. 164:R7-R10. [DOI] [PubMed] [Google Scholar]
- 29.Zhang, B., K. MacNaul, D. Szalkowski, Z. Li, J. Berger, and D. E. Moller. 1999. Inhibition of adipocyte differentiation by HIV protease inhibitors. J. Clin. Endocrinol. Metab. 84:4274-4277. [DOI] [PubMed] [Google Scholar]