Abstract
We report the emergence of linezolid resistance (MICs of 16 to 32 mg/liter) in clonally related vancomycin-susceptible and -resistant Enterococcus faecium isolates from an intensive care unit patient after 12 days of linezolid therapy. Only linezolid-susceptible isolates of the same clone were detected at 28 days after termination of linezolid therapy.
Enterococci are part of the normal intestinal flora, but they can also cause serious infections of animals and humans. Approximately 90% of enterococcal infections are caused by Enterococcus faecalis, and 5% to 10% are caused by Enterococcus faecium (16). The percentage of enterococcal infections caused by E. faecium has increased in recent years, probably due to the broad spectrum of intrinsic and acquired antibiotic resistance and carriage of virulence genes by strains of this species (15). Glycopeptide resistance is mediated by the vanA and vanB gene clusters, which are located on transposons Tn1546 and Tn1547, respectively (4). Serious infections caused by vancomycin-resistant enterococci (VRE), whose incidence has been rising in recent years, are proving increasingly difficult to treat. Linezolid, the first of a new class of systemic antibacterial agents, the oxazolidinones, is among the first-line therapeutics against all VRE infections except endocarditis (18). Linezolid was licensed for clinical use in Europe in 2001. Enterococcal resistance to linezolid was first described in 1999 in the United States and later was sporadically detected in enterococci worldwide (10). In Germany, a surveillance study aimed at the detection of linezolid resistance among VRE in 2001 and 2002 found no linezolid-resistant enterococci (2). The first linezolid-resistant VRE in Germany was reported in 2004 (6). However, treatment failure due to emergence of linezolid resistance or reduced susceptibility of enterococci is rare (1, 13).
In the present paper, we report the isolation of linezolid-resistant E. faecium isolates from various specimens taken from a patient after 12 days of linezolid therapy.
The patient (a 76-year-old female) was transferred to our surgical intensive care unit for acute gastrointestinal bleeding 2 weeks after undergoing a pancreaticoduodenectomy (Whipple's procedure). On transfer the patient was mechanically ventilated and required circulatory support for septic shock. Antibiotic therapy was started with piperacillin-tazobactam (4.5 g three times a day intravenously [i.v.]) and ciprofloxacin (400 mg twice a day i.v.) (both continued for 12 days) plus fluconazole (400 mg once a day i.v.) (continued for 18 days). During the subsequent course, necrotizing pancreatitis persisted and several surgical interventions were performed. One month after the initial surgery, the remaining pancreatic tissue was resected and splenectomy performed. One week prior to this intervention, the first vancomycin-resistant but linezolid-sensitive E. faecium (VRLSE) strain was isolated, as well as a multidrug-resistant Pseudomonas aeruginosa strain. Both were repeatedly detected in intra-abdominal cultures, easy flow catheters, and urine, whereas blood cultures remained negative. Consequently, antibiotic treatment was changed to a combination of meropenem (1.0 g three times a day i.v.) and linezolid (600 mg twice a day i.v.) (continued for 12 days). Thereafter, microbiological swabs of the abdominal drainages revealed the presence of E. faecium isolates with resistance either to both vancomycin and linezolid (VRLRE) or to linezolid alone (VSLRE). However, in the absence of signs of inflammatory reaction or fever, antibiotic therapy was terminated despite the continuing presence of E. faecium and also of multidrug-resistant P. aeruginosa. MICs of antimicrobial agents, including vancomycin and linezolid, were determined with the Vitek II system. Susceptibilities to linezolid and vancomycin were also determined by Etest according to the manufacturer's recommendations and by broth microdilution (3). Follow-up microbiological swabs of the abdominal drainages up to 4 weeks after termination of linezolid therapy revealed the presence of VRLRE. Thereafter, E. faecium isolates with linezolid resistance were no longer detectable, whereas VRLSE persisted throughout the intensive care unit treatment period (Table 1). Three months after admission, the patient was transferred to a rehabilitation center in a stable condition.
TABLE 1.
Characteristics of Enterococcus faecium isolates
| Lane in Fig. 1 | Isolate | Time (days)a | MIC (mg/liter) ofb:
|
No. of mutated 23S rRNA alleles | PCR for:
|
MRA typec | MLST type | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP | ERY | TET | Q/D | TEC | VAN | LZD | vanA | vanB | esp | hyl | ||||||
| 1 | Va18647 | 0 | 32 | 8 | 1 | 0.5 | 32 | 32 | 2 | 0 | + | − | + | + | 1 | ST-18 |
| 2 | Va20971 | 12 | 32 | 8 | 1 | 0.5 | 32 | 32 | 16 | 3 | + | − | + | + | 1 | ST-18 |
| 3 | Va20972 | 12 | 32 | 1 | 1 | 0.5 | 0.5 | 1 | 16 | 3 | − | − | + | + | 1 | ST-18 |
| 4 | Va21282 | 16 | 32 | 1 | 1 | 0.5 | 0.5 | 1 | 32 | 3 | − | − | + | + | 1 | NTd |
| 5 | Va21523 | 20 | 32 | 1 | 1 | 0.5 | 1 | 1 | 16 | 3 | − | − | + | + | 1 | NT |
| 6 | Va24517 | 57 | 32 | 8 | 32 | 0.5 | 32 | 32 | 2 | 0 | + | − | + | + | 1 | NT |
Time after start of linezolid therapy when the corresponding strain was isolated (linezolid therapy was terminated after 12 days).
AMP, ampicillin; ERY, erythromycin; TET, tetracycline; Q/D, quinupristin-dalfopristin; TEC, teicoplanin; VAN, vancomycin; LZD, linezolid.
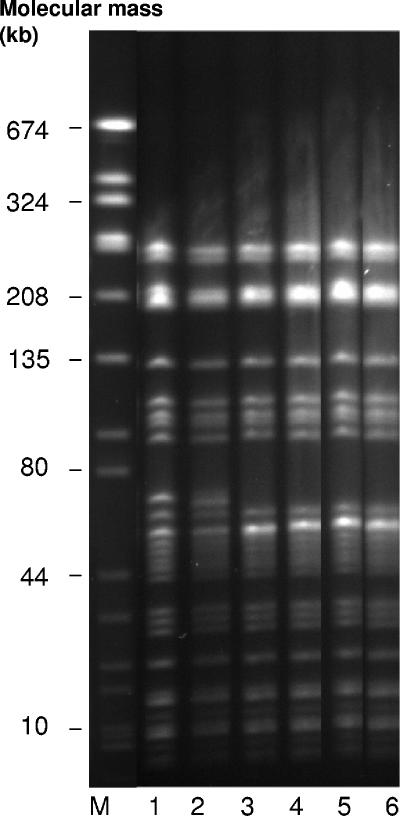
The difference in only one band in MRA patterns between the first two isolates and the following four isolates indicates the presence of the same clone in lanes 1 to 6 of Fig. 1 (see also MLST data).
NT, nontypeable.
Six representative enterococcal isolates, collected at different times and showing different resistance phenotypes (linezolid MICs, 2 to 32 mg/liter; vancomycin MICs, 1 to 32 mg/liter) (Table 1), were subjected to additional investigations. The six isolates were evaluated for their genetic relatedness by using SmaI macrorestriction analysis (MRA). Assessment of the MRA patterns according to international criteria for genotyping by MRA (11, 19) indicated both close relatedness and identity (Fig. 1, lanes 1 to 6). Additionally, we could show by multilocus sequence typing (MLST) (7) that these isolates belong to clonal complex 17 (CC-17) of E. faecium. They represent sequence type ST-18, which is a double-locus variant of ST-17, the founder of CC-17. E. faecium strains belonging to CC-17 are epidemic-virulent, hospital-adapted strains which have spread in hospitals worldwide (21), including German clinics (7).
FIG. 1.
SmaI macrorestriction patterns of Enterococcus faecium isolates. Lane M, molecular mass standard (Staphylococcus aureus ATCC 8325). The strains used are indicated in Table 1.
The presence of vanA and vanB genes in all isolates was determined by PCR as described elsewhere (7). The vanA gene was detected in all VRLSE and VRLRE isolates but not in the VSLRE isolates (Table 1). The VSLRE isolates were genotypically identical or closely related to the vancomycin-resistant isolates (Fig. 1), raising the possibility that the VSLRE isolates were segregants from VRLRE which had lost the vanA gene cluster. It is also conceivable, however, that glycopeptide-resistant and glycopeptide-sensitive E. faecium isolates with identical MRA patterns were present at the same time and that both developed linezolid resistance during linezolid therapy.
Linezolid resistance in enterococci is usually associated with a point mutation in the central region of domain V of the 23S rRNA gene leading to a nucleotide change from guanine (G) to uracil (U) at position 2576 in the 23S rRNA, the target of oxazolidinone antibiotics (8, 12). Identification of the resistance genotype is complicated by the presence of six 23S rRNA alleles in E. faecium. Although one mutated 23S rRNA allele seems to be sufficient to confer resistance, the number of mutated alleles correlates with an increase in the MICs of linezolid (8). In the E. faecium isolates examined, mutations in the 23S rRNA genes of three out of six 23S rRNA alleles led to linezolid resistance as determined by real-time PCR using two TaqMan probes as described elsewhere (20).
The rapid emergence of resistance to linezolid in our E. faecium isolates contradicts previous reports indicating that such resistance arises only after prolonged therapy with this antibiotic (5). Interestingly, no linezolid-resistant E. faecium could be detected in follow-up swabs 4 weeks after termination of linezolid therapy, although vancomycin resistance was still present (Fig. 1, lane 6). This observation is consistent with a recent report describing the reversion to susceptibility of a linezolid-resistant Staphylococcus aureus strain in a patient following termination of linezolid therapy (9).
In conclusion, because resistance to linezolid during therapy might occur rapidly, close monitoring of the strains' susceptibilities is advisable.
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Bethea, J. A., C. M. Walko, and P. A. Targos. 2004. Treatment of vancomycin-resistant Enterococcus with quinupristin/dalfopristin and high-dose ampicillin. Ann. Pharmacother. 38:989-991. [DOI] [PubMed] [Google Scholar]
- 2.Brauers, J., M. Kresken, D. Hafner, and P. M. Shah. 2005. Surveillance of linezolid resistance in Germany, 2001-2002. Clin. Microbiol. Infect. 11:39-46. [DOI] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing; 15th informational supplement, M100-S15, vol. 25, no. 1. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 4.Evers, S., R. Quintiliani, Jr., and P. Courvalin. 1996. Genetics of glycopeptide resistance in enterococci. Microb. Drug Resist. 2:219-223. [DOI] [PubMed] [Google Scholar]
- 5.Gonzales, R. D., P. C. Schreckenberger, M. B. Graham, S. Kelkar, K. DenBesten, and J. P. Quinn. 2001. Linezolid infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 357:1179. [DOI] [PubMed] [Google Scholar]
- 6.Halle, E., J. Padberg, S. Rosseau, I. Klare, G. Werner, and W. Witte. 2004. Linezolid-resistant Enterococcus faecium and Enterococcus faecalis isolated from a septic patient: report of first isolates in Germany. Infection. 32:182-183. [DOI] [PubMed] [Google Scholar]
- 7.Klare, I., C. Konstabel, S. Müller-Bertling, G. Werner, B. Strommenger, C. Kettlitz, S. Borgmann, B. Schulte, D. Jonas, A. Serr, A. M. Fahr, U. Eigner, and W. Witte. 2005. Spread of ampicillin/vancomycin-resistant Enterococcus faecium of the epidemic-virulent clonal complex-17 carrying the genes esp and hyl in German hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 24:815-825. [DOI] [PubMed] [Google Scholar]
- 8.Marshall, S. H., C. J. Donskey, R. Hutton-Thomas, R. A. Salata, and L. B. Rice. 2002. Gene dosage and linezolid resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob. Agents Chemother. 46:3334-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meka, V. G., H. S. Gold, L. Venkataraman, G. M. Eliopoulos, R. Moellering, Jr., and S. G. Jenkins. 2004. Reversion to susceptibility in a linezolid-resistant clinical isolate of Staphylococcus aureus. J. Antimicrob. Chemother. 54:818-820. [DOI] [PubMed] [Google Scholar]
- 10.Menichetti, F. 2005. Current and emerging serious Gram-positive infections. Clin. Microbiol. Infect. 11(Suppl. 3):22-28. [DOI] [PubMed] [Google Scholar]
- 11.Morrison, D., N. Woodford, S. P. Barrett, P. Sisson, and B. D. Cookson. 1999. DNA banding pattern polymorphism in vancomycin-resistant Enterococcus faecium and criteria of strain definition. J. Clin. Microbiol. 37:1084-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prystowsky, J., F. Siddiqui, J. Chosay, D. L. Shinabarger, J. Millichap, L. R. Peterson, and G. A. Noskin. 2001. Resistance to linezolid: characterization of mutations in rRNA and comparison of their occurrences in vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 45:2154-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raad, I. I., H. A. Hanna, R. Y. Hachem, T. Dvorak, R. B. Arbuckle, G. Chaiban, and L. B. Rice. 2004. Clinical-use-associated decrease in susceptibility of vancomycin-resistant Enterococcus faecium to linezolid: a comparison with quinupristin-dalfopristin. Antimicrob. Agents Chemother. 48:3583-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reference deleted.
- 15.Rice, L. B., L. Carias, S. Rudin, C. Vael, H. Goossens, C. Konstabel, I. Klare, S. R. Nallapareddy, W. Huang, and B. E. A. Murray. 2003. A potential virulence gene, hylEfm, predominates in Enterococcus faecium of clinical origin. J. Infect. Dis. 187:508-512. [DOI] [PubMed] [Google Scholar]
- 16.Ruoff, K. L., L. de la Maza, M. J. Murtagh, J. D. Spargo, and M. J. Ferraro. 1990. Species identities of enterococci isolated from clinical specimens. J. Clin. Microbiol. 28:435-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reference deleted.
- 18.Stevens, D. L., B. Dotter, and K. A. Madaras-Kelly. 2004. Review of linezolid: the first oxazolidinone antibiotic. Expert Rev. Anti-Infect. Ther. 2:51-59. [DOI] [PubMed] [Google Scholar]
- 19.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werner, G., B. Strommenger, I. Klare, and W. Witte. 2004. Molecular detection of linezolid resistance in Enterococcus faecium and Enterococcus faecalis by use of 5′ nuclease real-time PCR compared to a modified classical approach. J. Clin. Microbiol. 42:5327-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willems, R. J., J. Top, M. van Santen, D. A. Robinson, T. M. Coque, F. Baquero, H. Grundmann, and M. J. Bonten. 2005. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg. Infect. Dis. 11:821-828. [DOI] [PMC free article] [PubMed] [Google Scholar]