Abstract
The CS-023 concentration in plasma after administration by infusion to healthy volunteers at a dose of 700 mg was decreased, with a half-life of 1.7 h, and the cumulative urinary excretion was 59.4% of the dose. The total clearance, renal clearance, and volume of distribution were 8.12 liters/h, 4.14 liters/h, and 17.2 liters, respectively.
Many studies of the pharmacokinetics/pharmacodynamics of beta-lactams, including carbapenems, have revealed that the efficacy depends on the proportion of the dosing interval for which the serum concentration exceeds the MIC (2, 3, 7). A long plasma half-life allows more convenient dosing intervals while maintaining the same duration that the concentration exceeds the MIC.
CS-023 (RO4908463) (Fig. 1) is a novel 2-substituted 1-beta-methyl carbapenem with exceptional broad-spectrum activity against diverse hospital pathogens, including methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa (8, 9, 14), and with high stability of metabolism by dehydropeptidase I (8, 12). The aim of this study was to assess the pharmacokinetics of CS-023 in healthy volunteers.
FIG. 1.
Chemical structure of CS-023.
(Part of this work was presented at the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 27 to 30 September 2002 [J. Rennecke, T. Hirota, T. Shibayama, Y. Matsushita, S. Kuwahara, K. Püchler, A. Ruhland, and B. D. Drewelow, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-327, 2002].)
Approval for the study was obtained from the local ethics committee (Guy's Hospital Ethics Committee, London, United Kingdom), and all subjects gave written informed consent to participate in the study.
A separate group of eight healthy male Caucasian volunteers participated in each dose group (50, 100, 200, 350, 700, 1,400, or 2,100 mg), with six receiving CS-023 and two receiving a placebo (saline). CS-023 was administered intravenously as a 30-min infusion. Blood samples were drawn 15 min (during infusion), 30 min (at the end of infusion), 35 min, 45 min, and 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 h after the start of the infusion. Urine samples were collected 0 to 2, 2 to 4, 4 to 6, 6 to 8, 8 to 12, and 12 to 24 h after the start of the infusion. The urine samples were refrigerated and pooled until the end of the collection interval. The plasma and urine samples were stabilized by mixing in a 1:1 proportion with 0.5 M morpholinepropanesulfonic acid buffer (pH 7.4) and stored frozen at −70°C until assay.
Concentrations of CS-023 in plasma and urine were determined by a validated reverse-phase high-performance liquid chromatography method using a UV detector. The standard curves of the assay spanned a concentration range of 0.2 to 50 μg/ml for plasma or 2 μg/ml to 1,000 μg/ml for urine, with coefficients of correlation of 0.99 or greater. The bias and coefficient of variation of the quality control samples in the plasma assay (0.4, 4, and 40 μg/ml) ranged from −9.7% to −4.5% and from 1.2% to 9.2%, respectively. The bias and coefficient of variation of the quality control samples in the urine assay (4, 40, and 800 μg/ml) ranged from −6.4% to 0.5% and from 2.5% to 8.2%, respectively.
The protein binding ratios of CS-023 in CS-023-spiked serum (20 μg/ml and 100 μg/ml) were determined by a centrifugal ultrafiltration method (11) using an Amicon Centrifree micropartioning device (Millipore Co., MA).
The elimination half-life (t1/2), area under the plasma concentration-time curve extrapolated to infinity (AUC0-∞), and total clearance (CLtot) were calculated by the noncompartmental method, using the computer program WinNonlin (Standard Network Edition, version 1.5; Scientific Consulting, Inc., Apex, N.C.). The maximum plasma concentration (Cmax) was the maximum observed concentration in the plasma. The renal clearance (CLr) was obtained by multiplying the CLtot value by the cumulative urinary excretion ratio (Xu) of the dose within 24 h after administration. The volume of distribution at steady state (Vss) was calculated according to the equation Vss = (AUMC0-∞/AUC0-∞ − T/2) · CLtot, where AUMC0-∞ is the area under the moment curve, calculated using WinNonlin, and T is the duration of infusion. To assess the dose proportionality for Cmax and AUC0-∞, a power model of the form, pharmacokinetic parameter = a · Doseb, was applied using SAS System Release 8.2 (SAS Institute Inc., N.C.) software. If the 95% confidence interval for b contains unity, the relationship between the dose and the pharmacokinetic parameter is concluded to be dose proportional.
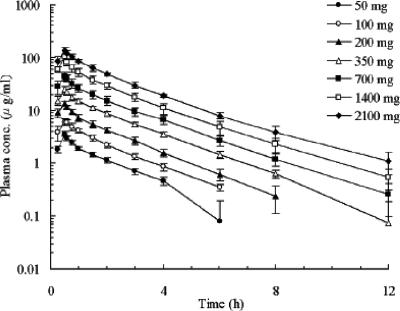
The plasma concentration-time profiles and the pharmacokinetic parameters are shown in Fig. 2 and Table 1, respectively. The mean Cmax and AUC0-∞ values increased significantly in proportion to dose, and the mean CLtot, Vss, and CLr values remained roughly constant over the dose range, suggesting the pharmacokinetics of CS-023 is linear up to 2,100 mg. The geometric means (coefficient of variation) of CLtot, Vss, and CLr for all the doses combined were 8.04 liters/h (12.9%), 16.2 liters (12.5%), and 4.87 liters/h (27.0%), respectively.
FIG. 2.
Plasma concentration-time profiles of CS-023 after administration by intravenous infusion for 30 min to healthy male Caucasian volunteers over a dose range of 50 to 2,100 mg. Each point represents the mean ± SD for six volunteers. The plasma concentrations were not detected at 24 h after the start of the infusion in all cases.
TABLE 1.
Pharmacokinetic parameters of CS-023 after administration by intravenous infusion for 30 min to healthy male Caucasian volunteers over a dose range of 50 to 2,100 mga
| Dose (mg) | Cmax(μg/ml) | AUC0-∞ (μg · h/ml) | t1/2 (h) | Vss (liters) | CLtot (liters/h) | CLr (liters/h) | Xu (% of dose) |
|---|---|---|---|---|---|---|---|
| 50 | 3.60 (10.8) | 6.22 (11.7) | 1.53 (13.7) | 15.9 (4.07) | 8.04 (12.7) | 5.23 (13.5) | 64.4 (4.0) |
| 100 | 6.14 (11.4) | 12.1 (12.8) | 1.56 (8.46) | 16.1 (14.5) | 8.24 (11.9) | 6.21 (13.3) | 74.1 (4.3) |
| 200 | 13.3 (10.2) | 23.4 (12.3) | 1.47 (9.59) | 15.8 (8.04) | 8.55 (14.0) | 4.38 (20.8) | 51.6 (6.7) |
| 350 | 24.6 (15.6) | 47.7 (8.91) | 1.63 (13.0) | 15.1 (15.1) | 7.33 (8.38) | 3.93 (26.2) | 54.9 (12.3) |
| 700 | 43.7 (18.0) | 86.3 (17.8) | 1.74 (15.8) | 17.2 (15.9) | 8.12 (15.0) | 4.14 (55.8) | 59.4 (24.0) |
| 1,400 | 101 (15.0) | 168 (16.7) | 1.87 (8.34) | 16.8 (12.4) | 8.36 (16.9) | 5.72 (21.7) | 68.6 (5.2) |
| 2,100 | 134 (13.8) | 273 (5.60) | 2.04 (17.5) | 16.8 (13.2) | 7.71 (5.84) | 5.36 (7.24) | 69.6 (3.5) |
Each value represents the geometric mean of values for six volunteers, with the corresponding geometric coefficient of variation (%) in parentheses.
CS-023 exhibited mean t1/2 values of between 1.48 h and 2.06 h. These values are higher than those reported for imipenem/cilastatin (0.93 h) (5) and meropenem (0.98 h) (1) but lower than that reported for ertapenem (4.9 h) (13). The mean Xu value was not dose related and ranged from 51.6 to 74.1% of the dose, which was comparable to those of imipenem/cilastatine (70%) (5) and meropenem (72%) (1) but was a little higher than that of ertapenem (42%) (13). The Vss values of CS-023 were around 16 liters, which is comparable to the volume of extracellular fluid, 18.2 liters (4). The mean serum binding ratios of CS-023 at concentrations of 20 μg/ml and 100 μg/ml were 9.5% (standard deviation [SD], ±5.4; n = 4) and 8.3% (SD, ±0.7; n = 4), respectively. The Vss values and the serum protein binding were similar to those of the carbepenems described above (15 to 20 liters and <10%, respectively) (10), except for ertapenem (5 liters and >95%, respectively) (13).
The high t1/2 value of CS-023 compared to those of imipenem and meropenem is likely to be attributable to the lack of renal tubular secretion of CS-023. Renal excretion is the main elimination pathway of CS-023, since around 70% of the dose was excreted in the urine in the intact form. The renal clearance of unbound CS-023 (CLr/plasma unbound fraction) was estimated to be 4.57 to 6.86 liters/h, and this value is closely comparable to the glomerular filtration rate (GFR), 7.50 liters/h, reported previously (4). This result suggests that net secretion is negligible in the renal handling of CS-023. On the other hand, renal clearance was reported to be larger than the GFR for imipenem and meropenem but was reduced to a value approximating the GFR by coadministration with probenecid, a typical inhibitor of the renal organic anion transport system, suggesting involvement of tubular secretion in their renal excretion (1, 5, 6). For ertapenem, it has been reported that the prolonged t1/2 value of about 5 h is due largely to the decreased renal clearance by high serum protein binding, more than 95% (13).
Single intravenous infusions of CS-023 up to 2,100 mg were considered safe and well tolerated. No serious adverse events and no clinically significant changes in electrocardiogram results, vital signs, or laboratory parameters were observed during the study.
In conclusion, the pharmacokinetics of CS-023 in healthy male Caucasian volunteers is characterized by a t1/2 value of nearly 2 h. CS-023 is currently under extensive clinical evaluation, in phase I studies in Japan and phase II studies in Europe and the United States, with an expectation of potent in vitro efficacy combined with a favorable safety profile being reflected clinically.
Acknowledgments
We gratefully acknowledge D. Amin of Guy's Hospital for conducting this clinical trial as the principal investigator.
Footnotes
Published ahead of print on 16 October 2006.
REFERENCES
- 1.Bax, R. P., W. Bastain, A. Featherstone, D. M. Wilkinson, M. Hutchison, and S. J. Haworth. 1989. The pharmacokinetics of meropenem in volunteers. J. Antimicrob. Chemother. 24(Suppl. A):311-320. [DOI] [PubMed] [Google Scholar]
- 2.Craig, W. A. 1997. The pharmacology of meropenem, a new carbapenem antibiotic. Clin. Infect. Dis. 24(Suppl. 2):S266-S275. [DOI] [PubMed] [Google Scholar]
- 3.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 4.Davies, B., and T. Morris. 1993. Physiological parameters in laboratory animals and humans. Pharm. Res. 10:1093-1095. [DOI] [PubMed] [Google Scholar]
- 5.Drusano, G. L., and H. C. Standiford. 1985. Pharmacokinetic profile of imipenem/cilastatin in normal volunteers. Am. J. Med. 78(Suppl. 6A):47-53. [DOI] [PubMed] [Google Scholar]
- 6.Drusano, G. L. 1986. An overview of the pharmacology of imipenem/cilastatin. J. Antimicrob. Chemother. 18(Suppl. E):79-92. [DOI] [PubMed] [Google Scholar]
- 7.Drusano, G. L. 2003. Prevention of resistance: a goal for dose selection for antimicrobial agents. Clin. Infect. Dis. 36(Suppl. 1):S42-S50. [DOI] [PubMed] [Google Scholar]
- 8.Kawamoto, I., Y. Shimoji, O. Kanno, K. Kojima, K. Ishikawa, E. Matsuyama, Y. Ashida, T. Shibayama, T. Fukuoka, and S. Ohya. 2003. Synthesis and structure-activity relationships of novel parenteral carbapenems, CS-023 (R-115685) and related compounds containing an amidine moiety. J. Antibiot. (Tokyo) 56:565-579. [DOI] [PubMed] [Google Scholar]
- 9.Koga, T., T. Abe, H. Inoue, T. Takenouchi, A. Kitayama, T. Yoshida, N. Masuda, C. Sugihara, M. Kakuta, M. Nakagawa, T. Shibayama, Y. Matsushita, T. Hirota, S. Ohya, Y. Utsui, T. Fukuoka, and S. Kuwahara. 2005. In vitro and in vivo antibacterial activities of CS-023 (RO4908463), a novel parenteral carbapenem. Antimicrob. Agents Chemother. 49:3239-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mouton, J. W., D. J. Touzw, A. M. Horrevorts, and A. A. Vinks. 2000. Comparative pharmacokinetics of the carbapenems: clinical implications. Clin. Pharmacokinet. 39:185-201. [DOI] [PubMed] [Google Scholar]
- 11.Pacifici, G. M., and A. Viani. 1992. Methods of determining plasma and tissue binding of drugs. Pharmacokinetics consequences. Clin. Pharmacokinet. 23:449-468. [DOI] [PubMed] [Google Scholar]
- 12.Shibayama, T., Y. Matsushita, N. Kikuchi, K. Kawai, T. Hirota, and S. Kuwahara. 2000. R-115685, a novel parenteral carbapenem: pharmacokinetics and metabolism in laboratory animals, abstr. F-1233. Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology. Washington, D.C.
- 13.Sundelof, J. G., R. Hajdu, C. J. Gill, R. Thompson, H. Rosen, and H. Kropp. 1997. Pharmacokinetics of L-749,345, a long-acting carbapenem antibiotic, in primates. Antimicrob. Agents Chemother. 41:1743-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomson, K. S., and E. S. Moland. 2004. CS-023 (R-115685), a novel carbapenem with enhanced in vitro activity against oxacillin-resistant staphylococci and Pseudomonas aeruginosa. J. Antimicrob. Chemother. 54:557-562. [DOI] [PubMed] [Google Scholar]