Abstract
The spread of plasmid-mediated quinolone resistance determinants QnrA and QnrS was evaluated in a collection of 186 extended-spectrum β-lactamase (ESBL)-positive enterobacterial isolates from 2002 to 2005 and 185 nalidixic acid-resistant strains isolated during the first 6 months of 2005 at the Bicêtre hospital, France. Out of these 186 ESBL-positive isolates, 2.2 and 1.6% carried a QnrA1 and a QnrS1 determinant, respectively. The ESBLs associated with QnrA1 were VEB-1, SHV-12, and CTX-M-1, whereas those associated with QnrS1 were TEM-52, SHV-12, and CTX-M-1. Among the 185 nalidixic acid-resistant strains isolated in 2005, 0.5 and 2.7% had a QnrA1 determinant and a QnrS1 determinant, respectively. The genetic environments of the qnrA1 gene differed but were always associated with sul1 type integrons. In contrast, qnrS1 genes were not embedded in class 1 integrons but located often (but not systematically) downstream of the insertion sequence ISEcl2 on plasmids that often carried a novel β-lactamase gene, blaLAP-1. This is the first study identifying the QnrS resistance determinant in Europe and indicating that this determinant might also be widespread.
Quinolone resistance in Enterobacteriaceae mostly results from chromosomal mutations in genes coding for DNA gyrase and topoisomerase IV and changes in outer membrane and efflux proteins or in their regulatory mechanisms (26). Recent findings indicate that plasmid-mediated resistance mechanisms might also play a significant clinical role related to pentapeptide proteins of the Qnr family (16). Plasmid-mediated resistance to quinolone (related to protein QnrA) was reported first in 1998 for a Klebsiella pneumoniae isolate from the United States (14). QnrA protects DNA gyrase and topoisomerase IV from the inhibitory activity of quinolones (29, 30). It confers resistance to nalidixic acid and increases the MICs of fluoroquinolones four- to eightfold (13, 14). QnrA-positive isolates were identified in the United States and then in Canada, Asia, Australia, Turkey, and Europe (11, 13, 15, 20-22, 25, 32). In addition to QnrA, two plasmid-mediated quinolone resistance determinants, QnrB and QnrS, have been identified more recently (7, 9). QnrB-like determinants were identified in Citrobacter koseri, Escherichia coli, Enterobacter cloacae, and Klebsiella pneumoniae from the United States and India (9). The QnrS1 determinant was identified in a Shigella flexneri isolate from Japan and in an E. cloacae isolate from Vietnam (7, 19), whereas the QnrS2 variant was identified in a non-Typhi Salmonella isolate from the United States (5). QnrA1 and QnrS1 determinants share 59% amino acid identity, whereas both determinants share 40% amino acid identity with QnrB1. The association of the QnrA determinant with different clavulanic acid-inhibited expanded-spectrum β-lactamases (ESBLs) (Ambler class A [1]), such as VEB-1 (13, 15, 20, 21), SHV-7 (31), CTX-M-9 (31), and CTX-M-14 (2), has been reported repeatedly (16). In contrast, the qnrS-positive S. flexneri and E. cloacae isolates expressed the narrow-spectrum penicillinase TEM-1 (7, 19).
The aim of the study was to evaluate the prevalence of QnrA and QnrS determinants (the nucleotide sequence of qnrB was not available at the time of the study) in a collection of enterobacterial isolates recovered at the Bicêtre hospital, in a suburb of Paris, France. That collection was made of ESBL-producing strains recovered during a 3.5-year period (from January 2002 to June 2005) and of the most recently isolated nalidixic acid-resistant enterobacterial isolates (from January to June 2005). Our goal was also to analyze and compare the genetic backgrounds of the qnrA and qnrS genes.
(The results of this study were presented in part at the 16th European Congress of Clinical Microbiology and Infectious diseases, 2006, Nice, France.)
MATERIALS AND METHODS
Bacterial isolates.
The enterobacterial isolates included in the study were identified by using the API20E system (bioMérieux SA, Marcy-l'Etoile, France). Electrocompetent E. coli DH10B (GIBCO BRL, Life Technologies, Cergy Pontoise, France) was used as the recipient strain in cloning experiments, whereas azide-resistant E. coli J53 was used as the recipient strain in conjugation experiments (21). E. coli Lo and E. cloacae 287, carrying the qnrA1 and qnrS1 genes, respectively, were used as a positive control (13, 19).
Susceptibility testing and screening for ESBL-producing strains.
The antibiotic susceptibility of the enterobacterial isolates was determined first by the disk diffusion method on Mueller-Hinton agar plates with β-lactam and non-β-lactam antibiotic-containing disks (Sanofi Diagnostics Pasteur, Marnes-La-Coquette, France). ESBL detection was performed as described previously (10). MICs were determined for selected β-lactams as described previously (18) or using Etest strips for quinolones and were interpreted according to the guidelines of the CLSI (3).
PCR amplification, cloning, and sequencing.
Under standard PCR conditions (27), a series of primers was used for detection of Ambler class A β-lactamase genes (1). Detection was performed for β-lactamase genes encoding TEM, SHV, CTX-M, PER, VEB, and GES as described previously (6). Screening of the qnrA-like genes was performed using primers QnrA and QnrB (13) and of the qnrS gene with primers qnrSA1 and qnrSA2 (19).
Based on the nucleotide sequences identified next to the qnrS gene in plasmid p287HN2 (19), primer Pre-QnrS1 (5′-CTGATAACACTTCAACCATC-3′) and primer Pre-QnrS2 (5′-TCGTTTTATAAATTTGAGCG-3′) were designed to PCR amplify in combination with primers qnrSA2 and qnrSA1 at the 5′ and 3′ ends of the qnrS gene, respectively. PCR amplification of the quinolone resistance determinant regions of the chromosome-encoded gyrA and parC subunits in these qnrA- and qnrS-positive E. cloacae isolates was performed as previously described (13). Sequencing reactions were performed using an automated sequencer (ABI 377; Applied Biosystems, Foster City, Calif.). The nucleotide and deduced protein sequences were analyzed with software available from the Internet.
PFGE.
Pulsed-field gel electrophoresis (PFGE) analysis was performed to compare QnrS-positive E. cloacae isolates by using the BamHI restriction enzyme, according to the manufacturer's instructions (Bio-Rad), as described previously (18). Electrophoresis was performed using a CHEF DRII apparatus (Bio-Rad). The chromosomal fingerprints were compared by eye and assigned to PFGE types and subtypes (28).
Hybridization.
Plasmid DNA hybridizations were performed as described by Sambrook et al. (27), followed by a Southern transfer of an agarose gel containing plasmid DNA of qnrA1- and qnrS1-positive isolates onto a nylon membrane (Hybond N+; Amersham Pharmacia Biotech). The probe specific for the qnrA gene consisted of a 661-bp PCR internal fragment generated from whole-cell DNA of E. coli Lo (13), and that specific for the qnrS gene consisted of a 550-bp PCR fragment generated from whole-cell DNA of E. coli 287 (19). Labeling of the probe and signal detection were carried out using an ECL nonradioactive labeling and detection kit according to the manufacturer's instructions (Amersham Pharmacia Biotech).
Conjugation, electroporation, and plasmid DNA content analysis.
Conjugation experiments were performed with qnrA- and qnrS-positive clinical isolates from the E. coli J53 recipient strain as reported previously (13), with selection on agar plates containing 100 μg of azide per ml and 8 μg of nalidixic acid per ml. Plasmid DNA extraction was performed by using the Kieser method (12).
RESULTS
Epidemiology of Qnr determinants.
Two collections of enterobacterial isolates were studied. Since ESBLs and plasmid-mediated Qnr determinants have been reported to be associated frequently, the first collection was made of 186 ESBL-positive isolates recovered from January 2002 to June 2005. A second collection, made of 185 nalidixic acid-resistant and ESBL-negative isolates recovered from January to June 2005, was included to analyze the recent distribution of Qnr determinants. These 185 isolates were recovered from a total of 1,156 enterobacterial isolates obtained during that period of time, thus corresponding to 16% of the nalidixic acid-resistant isolates.
The first collection of clinical isolates consisted of E. coli (n = 93), K. pneumoniae (n = 32), Enterobacter aerogenes (n = 18), Enterobacter cloacae (n = 16), Klebsiella oxytoca (n = 8), Citrobacter freundii (n = 6), Proteus mirabilis (n = 4), Citrobacter koseri (n = 3), Providencia stuartii (n = 3), Proteus vulgaris (n = 1), Salmonella enterica serovar Typhimurium (n = 1), and Serratia marcescens (n = 1) isolates. Those isolates were distributed among 28 strains recovered in 2002, 36 in 2003, 76 in 2004, and 46 in the first 6 months of 2005. Four qnrA-positive isolates (2.2%) were identified in that collection, namely, an E. cloacae strain A1 isolate producing the ESBL SHV-12 and recovered in 2004, an E. aerogenes strain A2 isolate recovered in 2005 and producing ESBLs SHV-12 and CTX-M-1 (Table 1), and E. coli Lo and E. cloacae GOC isolates recovered in 2003 and producing VEB-1, as previously reported (13, 21). Three isolates (1.6%) were qnrS positive (all from 2005), namely, an E. cloacae S1 isolate producing SHV-12, an E. cloacae S2 isolate producing TEM-52, and an E. coli S7 isolate producing CTX-M-1 (Table 1).
TABLE 1.
Features of the QnrA- and QnrS-positive enterobacterial isolates
| Strain | Qnr determinant | Date of isolation | Specimen | Hospitalization unita | ESBL phenotype | Genetic structure surrounding the qnr genesb | Source or reference |
|---|---|---|---|---|---|---|---|
| E. cloacae GOC | QnrA1 | 01/09/03 | Urine | Urology | Yes | A | 20 |
| E. coli Lo | QnrA1 | 12/07/03 | Urine | ICU | Yes | A | 12 |
| E. cloacae A1 | QnrA1 | 07/03/04 | Urine | ICU | Yes | A | This study |
| E. aerogenes A2 | QnrA1 | 03/27/05 | Urine | Internal Medicine | Yes | B | This study |
| K. pneumoniae A3 | QnrA1 | 03/12/05 | Urine | Urology | No | C | This study |
| E. cloacae S1 | QnrS1 | 05/31/05 | Peritoneal fluid | PICU | Yes | E | This study |
| E. cloacae S2 | QnrS1 | 06/30/05 | Blood | PICU | Yes | E | This study |
| E. cloacae S3 | QnrS1 | 03/17/05 | Blood | Nephrology | No | D | This study |
| E. cloacae S4 | QnrS1 | 01/15/05 | Urine | Urology | No | D | This study |
| E. cloacae S5 | QnrS1 | 04/16/05 | Urine | ICU | No | D | This study |
| E. cloacae S6 | QnrS1 | 05/31/05 | Skin | Geriatric dept. | No | D | This study |
| E. coli S7 | QnrS1 | 02/07/05 | Urine | Nephrology | Yes | E | This study |
| S. marcescens S8 | QnrS1 | 01/31/05 | Urine | Internal medicine | No | E | This study |
ICU, intensive care unit, PICU, pediatric intensive care unit.
See the figures for corresponding data.
The second collection of nalidixic acid-resistant enterobacterial isolates consisted of E. coli (n = 117), P. mirabilis (n = 17), K. pneumoniae (n = 15), E. cloacae (n = 11), C. freundii (n = 8), E. aerogenes (n = 7), P. stuartii (n = 6), S. marcescens (n = 2), K. oxytoca (n = 1), and Morganella morganii (n = 1) isolates. The qnrA gene that expressed a narrow-spectrum TEM-1 β-lactamase in addition to naturally occurring β-lactamase SHV-1 was identified in a single K. pneumoniae isolate (0.5% of that collection), whereas the qnrS gene was identified in five isolates (2.7%), i.e., four E. cloacae isolates (strains S4 to S6; see below) and a single S. marcescens isolate (strain S7), the last strain expressing penicillinase TEM-1 in addition to its naturally encoded AmpC β-lactamase (Table 1).
The qnrA-like genes were qnrA1 in all cases, whereas the qnrS-like genes were always qnrS1.
Detailed analysis of the QnrS1-positive E. cloacae isolates.
Six out of the eight qnrS1-positive isolates were E. cloacae. PFGE analysis indicated that isolates S1 and S4, on the one hand, and isolates S5 and S6, on the other hand, were clonally related, whereas isolates S2 and S3 were different (data not shown). These strains expressed a β-lactam resistance profile likely related to the expression of a clavulanic acid-inhibited narrow-spectrum penicillinase. None of those strains possessed a known β-lactamase gene according to PCR experiment results. Clonings identified a novel β-lactamase gene, blaLAP-1, encoding a narrow-spectrum Ambler class A β-lactamase that has only 62% and 61% amino acid identity with TEM-1 and SHV-1, respectively (L. Poirel and P. Nordmann, unpublished data).
For evaluation of additional chromosome-encoded mechanisms of resistance to quinolones, sequencing of the gyrA and parC subunits identified nucleotide changes in the quinolone resistance determinant regions of those genes, leading to amino acid substitutions (Table 2) .
TABLE 2.
Chromosomal substitutions identified in the genes coding for GyrA and ParC in the QnrA- and QnrS-positive E. cloacae isolates
| Isolate | Amino acid (corresponding nucleotides) for indicated gene product and position
|
|||
|---|---|---|---|---|
| GyrA
|
ParC
|
|||
| 83 | 87 | 80 | 84 | |
| Wild-type sequence | Ser (TCC) | Asp (GAG) | Ser (AGC) | Glu (ACC) |
| S1 | Phe (TTC) | |||
| S2 | Ile (ATC) | Ile (ATC) | ||
| S3 | Val (GTC) | |||
| S4 | Phe (TTC) | |||
| S5 | Tyr (TAC) | |||
| S6 | Tyr (TAC) | |||
| A1 | Phe (TTC) | Arg (AGA) | ||
Susceptibility testing and genetic vehicles of the qnrA1 and qnrS1 genes.
Transconjugants were obtained for two out of three qnrA1-positive donors (isolates A2 and A3) and for three out of eight QnrS-positive donors (isolates S1, S3, and S4) (Table 3). MICs of β-lactams and quinolones for the QnrA1- and QnrS1-positive isolates and their transconjugants are shown in Table 3. MICs of nalidixic acid for all transconjugants were increased 8-fold and those of fluoroquinolones 30-fold (ofloxacin) to 60-fold (ciprofloxacin). Interestingly, using the same E. coli background as that of the recipient strain, QnrS1-positive transconjugants showed only a twofold MIC increase for ciprofloxacin compared to those for QnrA1-positive transconjugants.
TABLE 3.
MICs of quinolones, fluoroquinolones, and β-lactams for clinical strains, their transconjugants (Tc strains), and the E. coli J53 recipient strain
| Isolate | Qnr determinant | β-Lactamase(s)a | MIC (μg/ml)
|
Plasmid sizeb (kb) | Associated non-β-lactam antibiotic resistance markersc | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| NAL | OFX | CIP | AMX | CAZ | CTX | |||||
| E. cloacae A1 | QnrA1 | TEM-1 + SHV-12 | >256 | >32 | 12 | >256 | >256 | >256 | 75 | CHL KAN RIF SUL TET TOB |
| E. aerogenes A2 | QnrA1 | TEM-1 + SHV-12 + CTX-M-1 | >256 | >32 | >32 | >256 | >256 | >256 | 150 | CHL KAN RIF SUL TET TOB |
| TcA2 | QnrA1 | TEM-1 + SHV-12 | 32 | 1 | 0.25 | 0.5 | 1 | 0.12 | 150 | KAN TET TOB |
| K. pneumoniae A3 | QnrA1 | TEM-1 + SHV-1 | >256 | >32 | >32 | >256 | >256 | >256 | 40 | CHL KAN SUL TET TOB |
| TcA3 | QnrA1 | TEM-1 | 32 | 1 | 0.25 | 0.5 | 1 | 0.12 | 40 | CHL KAN TET TOB |
| E. cloacae S1 | QnrS1 | TEM-1 + LAP-1 + SHV-12 | >256 | >32 | >32 | >256 | >256 | >256 | 100 | CHL KAN RIF SUL TET TOB |
| TcS1 | QnrS1 | LAP-1 | 32 | 2 | 0.5 | 128 | 0.25 | 0.25 | 100 | None |
| E. cloacae S2 | QnrS1 | TEM-2 + TEM-52 | >32 | >32 | >32 | >256 | >256 | >256 | 65 | KAN SUL TET TOB |
| E. cloacae S3 | QnrS1 | LAP-1 | >256 | >32 | >32 | >256 | 2 | 1 | 100 | KAN RIF SUL TET TOB |
| TcS3 | QnrS1 | LAP-1 | 32 | 2 | 0.5 | 256 | 0.25 | 0.25 | 100 | None |
| E. cloacae S4 | QnrS1 | TEM-1 + LAP-1 | >256 | >32 | 4 | >256 | 2 | 0.5 | 100 | CHL KAN RIF SUL TET TOB |
| TcS4 | QnrS1 | LAP-1 | 32 | 2 | 0.5 | 256 | 0.25 | 0.25 | 100 | None |
| E. cloacae S5 | QnrS1 | TEM-1 + LAP-1 | >256 | >32 | 12 | >256 | 1 | 0.5 | 100 | CHL RIF SUL TET |
| E. cloacae S6 | QnrS1 | TEM-1 + LAP-1 | >256 | >32 | 12 | >256 | 1 | 1 | 100 | CHL RIF SUL TET |
| E. coli S7 | QnrS1 | TEM-1 + CTX-M-1 | >256 | >32 | >32 | >256 | 8 | >256 | 35 | CHL KAN SUL TET TOB |
| TcS7 | QnrS1 | TEM-1 + CTX-M-1 | 32 | 2 | 0.5 | >256 | 4 | 256 | 35 | None |
| S. marcescens S8 | QnrS1 | TEM-1 | >256 | >32 | >32 | >256 | 2 | 0.5 | 65 | CHL KAN RIF TET TOB |
| E. coli J53 | 4 | 0.12 | 0.01 | 0.06 | 0.01 | 0.01 | ||||
Associated non-β-lactam resistance markers. LAP-1 is a novel narrow-spectrum β-lactamase which has been identified in the present study.
The size of the plasmid for which a hybridization signal has been obtained with the corresponding qnrA1- or qnrS1-specific probe.
Antibiotic resistance markers: CAZ, ceftazidime; CHL, chloramphenicol; CIP, ciprofloxacin; CTX, cefotaxime; KAN, kanamycin; MXF, moxifloxacin; NAL, nalidixic acid; OFX, ofloxacin, RIF, rifampin; SUL, sulfonamides; TET, tetracycline; TOB, tobramycin.
The qnrA1- and qnrS1-positive plasmids varied in size and structure (data not shown) (Table 3).
Genetic structures surrounding the qnrA1 genes.
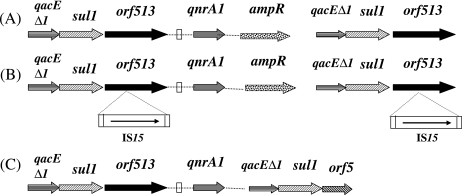
PCR mapping identified the genetic structures surrounding the qnrA1 genes in E. cloacae A1, E. aerogenes A2, and K. pneumoniae A3. In those strains, the orf513 recombinase gene was located upstream of the qnrA1 gene. The ampR gene of M. morganii was located just downstream of qnrA1, as already reported for In36 and In37 from Shanghai and Australian isolates (23, 30). The qacEΔ1-sul1 gene tandem was found downstream of the ampR gene, as observed in most sul1 type integrons. Downstream of those genes, the orf5 gene usually identified in class 1 integrons was found in K. pneumoniae A3, whereas it was absent in isolates E. cloacae A1 and A2, being replaced by a second copy of orf513, as observed in K. pneumoniae (pMG252) and E. coli (pQR1) (Fig. 1) (12, 23). In addition, an insertion of the IS15 element was identified in both orf513 genes bracketing the qnrA1 gene in isolate A2 (Fig. 1). These insertions were identified at the exact same location in each orf513 gene, likely indicating duplication of that truncated orf513 gene.
FIG. 1.
Schematic representation of the sequences surrounding the qnrA1 gene in the isolates studied. Genes are shown as arrows, with their orientations of transcription indicated by the arrowheads. Insertion sequence elements are indicated by horizontal rectangles. The white vertical rectangles indicate the right-hand boundary of the CR1 element. Panel A corresponds to the structure identified in E. cloacae A1 and E. aerogenes A2, panel B to that identified in K. pneumoniae A3, and panel C to that identified in E. coli Lo and E. aerogenes GOC (13, 21).
Genetic structures surrounding the qnrS1 genes.
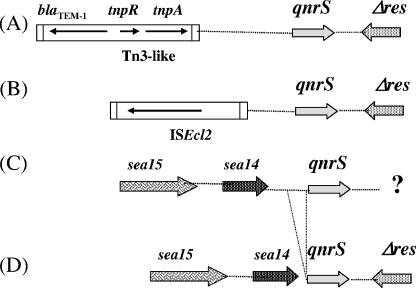
By using PCR mapping, the sequences surrounding the qnrS1 genes in strains S1 to S7 were determined and compared to those reported from the S. flexneri 2b isolate from Japan (7) or the E. cloacae 287 isolate from Vietnam (19). Compared to the structure identified in plasmid p287HN2 from E. cloacae 287, the truncated resolvase-like sequences (Δres) located at the 3′ end extremity of the qnrS1 gene were identified in all the isolates of our study, except in E. cloacae S4 and E. coli S7 (Fig. 2).
FIG. 2.
Schematic representation of the sequences surrounding the qnrS1 gene in the isolates studied. Genes are shown as arrows, with their orientations of transcription indicated by the arrowheads. Insertion sequence elements are indicated by horizontal rectangles. Panel A corresponds to the structure identified in S. flexneri 2b (Japan); panel B to that identified in E. cloacae S3, S4, S5, S6, and 287 (France and Vietnam) (19); panel C to the structure identified in E. cloacae S1, E. coli S7, and S. marcescens S8 (France); and panel D to that identified in E. cloacae S2 (France). Δres is for the truncated resolvase gene, and sea14 and sea15 are for the genes encoding proteins of unknown functions identified on a plasmid from Serratia entomophila (8). The question mark indicates an unknown sequence.
No Tn3-like transposon carrying the blaTEM-1 gene was identified here in the 5′ vicinity of the qnrS1 gene, whereas it was next to the qnrS1 gene of S. flexneri 2b (Fig. 2, panel A). However, an IS2-like element was identified in several isolates. PCR mapping revealed that this structure identified in E. cloacae 287 from Vietnam was also present in isolates S3, S4, S5, and S6 from France (Fig. 2B). A detailed analysis of the boundaries of that novel insertion element also identified in E. cloacae 287 from Vietnam (19) allowed the identification of a novel insertion element, ISEcl2, belonging to the IS3 family. It possessed imperfect inverted repeats, and its insertion had likely generated a 4-bp duplication as its insertion target site. Its presence upstream of qnrS1 had likely occurred as a separate event and suggested no role for qnrS1 mobilization. PCR mapping revealed that ISEcl2 was absent upstream of the qnrS1 gene in isolates S1, S2, S7, and S8 (Fig. 1C). However, in those strains, two open reading frames were identified upstream of qnrS1, sharing 89 and 82% amino acid identities with Sea14 (184 amino acids long) and Sea15 (277 amino acids long), respectively, which are proteins of unknown functions, identified on a plasmid harboring pathogenicity determinants from a Serratia entomophila isolate (Fig. 2) (8). Interestingly, the DNA sequence separating the sea14-like gene from the qnrS gene in E. cloacae S2 was shorter than that identified in isolates S1, S7, and S8. Indeed, a 108-bp fragment was absent in isolate S2 (Fig. 2).
DISCUSSION
Here, we report on the distribution of the QnrA and QnrS determinants among a collection of 371 enterobacterial isolates recovered in the same hospital during a 3.5-year-period, half of these isolates being ESBL producers and the other half being very recently identified nalidixic acid-resistant strains. We show that the spread of the QnrA determinant is quite limited and did not seem to have increased significantly, at least during those last 3 years.
This study constitutes the first epidemiological survey of the QnrS determinant among European isolates and identified the QnrS1 determinant mostly in E. cloacae and not in E. coli or K. pneumoniae, as observed for the QnrA-like determinants. Interestingly, the qnrS1 gene was mostly identified in non-ESBL-positive strains, whereas it was the opposite for the QnrA1-positive strains. Although based on a limited number of positive isolates, our results likely indicate that the spread of the QnrS determinant could be more recent than that of QnrA, at least in our hospital. Indeed, whereas no positive strain was identified in 2003 or 2004, eight QnrS1-positive isolates were identified during a 6-month period in 2005. The prevalence of the QnrA determinant was low in our study, consistent with a recent Spanish study conducted from 2004 to 2005 showing that only 2 out of 100 nalidixic acid-resistant enterobacterial isolates were QnrA1 positive, these two isolates being ESBL-positive C. freundii and E. cloacae recovered from a single patient (M. E. Cano, L. Martinez-Martinez, J. M. Garcia-Lobo, J. Calvo, and J. Aguero, Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C1-1043/69, 2005). In contrast, a surprisingly high rate (32%) of QnrA-positive enterobacterial isolates was reported for 47 ciprofloxacin-resistant and ESBL-positive strains recovered during the 2003 to 2005 period in a hospital in the United Kingdom (4).
It is noteworthy that several qnrS-positive isolates have been reported recently in nalidixic acid-susceptible enterobacterial isolates (M. E. Cano, J. M. Rodriguez-Martinez, J. Aguero, A. Pascual, J. M. Garcia-Lobo, C. Velasco, and L. Martinez-Martinez, Abstr. 16th Eur. Congr. Clin. Microbiol. Infect. Dis., abstr. O52, 2006). Thus, the true prevalence of the QnrS determinant may be underestimated in our study, which focused mostly on nalidixic acid-resistant isolates. Such isolates could be an additional reservoir for undetected spread of plasmid-mediated quinolone resistance (16).
In our study, the qnrA1 gene was identified in E. coli, E. cloacae, E. aerogenes, and K. pneumoniae isolates whereas the qnrS1 gene was found in S. marcescens and in E. cloacae. The qnrA1 gene was identified associated with the orf513 recombinase gene, as previously observed (13, 16, 32; Cano et al., 45th ICAAC abstr. C1-1043/69). The qnrA1 gene was found in association with a gene for ESBL SHV-12, as recently identified in United Kingdom isolates (4).
Genotyping performed on five E. cloacae isolates demonstrated that the occurrence of QnrS-positive strains was not the result of the dissemination of a single isolate. The rather nosocomial enterobacterial species E. cloacae and E. coli may be an important reservoir for Qnr determinants, which parallels the recent identification of outbreaks due to a QnrA-positive E. cloacae strain in The Netherlands (17) and in Korea (J. Jun, Y. Kwak, S. Kim, J. Lee, S. Choi, J. Jeong, Y. Kim, and J. Woo, Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C2-787, 2005) and the identification of a high prevalence of QnrA and QnrB determinants in Enterobacter sp. isolates from the United States (24). However, in the study conducted in the United States, no QnrS determinant was identified.
The genetic backgrounds of the qnrA1 and qnrS1 genes were variable, ruling out the hypothesis for the spread of identical plasmids. Whereas the association of the qnrA gene with sul1 type integrons was confirmed, our study identified insertion sequence structures associated with the qnrS1 gene. The latter result indicates that the processes of acquisition and plasmid integration for these qnr genes in Enterobacteriaceae may be different.
Acknowledgments
This work was funded by a grant from the Ministère de l'Education Nationale et de la Recherche (UPRES-EA3539), Université Paris XI, France, and mostly by grants from the European Community (6th PCRD, LSHM-CT-2003-503335 and LSHM-CT-2005-018705). L.P. is a researcher from the INSERM (Paris, France).
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Ambler, R. P., A. F. W. Coulson, J.-M. Frère, J. M. Ghuysen, B. Joris, M. Forsman, R. C. Levesque, G. Tiraby, and S. G. Waley. 1991. A standard numbering scheme for the class A β-lactamases. Biochem. J. 276:269-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung, T. K., Y. W. Chu, M. Y. Chu, C. H. Ma, R. W. Yung, and K. M. Kam. 2005. Plasmid-mediated resistance to ciprofloxacin and cefotaxime in clinical isolates of Salmonella enterica serotype Enteritidis in Hong Kong. J. Antimicrob. Chemother. 56:586-589. [DOI] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing; fifteenth informational supplement. M100-S15. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 4.Corkill J. E., J. J. Anson, and C. A. Hart. 2005. High prevalence of the plasmid mediated quinolone resistance determinant QnrA in multidrug resistant Enterobacteriaceae from blood cultures in Liverpool, UK. J. Antimicrob. Chemother. 56:1115-1117. [DOI] [PubMed] [Google Scholar]
- 5.Gay, K., A. Robicsek, J. Strahilevitz, C. H. Park, G. Jacoby, T. J. Barrett, F. Medalla, T. M. Chiller, and D. C. Hooper. 2006. Plasmid-mediated quinolone resistance in non-Typhi serotypes of Salmonella enterica. Clin. Infect. Dis. 43:297-304. [DOI] [PubMed] [Google Scholar]
- 6.Girlich, D., L. Poirel, A. Leelaporn, A. Karim, C. Tribuddharat, M. Fennewald, and P. Nordmann. 2001. Molecular epidemiology of the integron-located VEB-1 extended-spectrum β-lactamase in nosocomial enterobacterial isolates in Bangkok, Thailand. J. Clin. Microbiol. 39:175-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hata, M., M. Suzuki, M. Matsumoto, M. Takahashi, K. Sato, S. Ibe, and K. Sakae. 2005. Cloning of a novel gene for quinolone resistance from a transferable plasmid in Shigella flexneri 2b. Antimicrob. Agents Chemother. 49:801-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurst, M. R., T. R. Glare, T. A. Jackson, and C. W. Ronson. 2000. Plasmid-located pathogenicity determinants of Serratia entomophila, the causal agent of amber disease of grass grub, show similarity to the insecticidal toxins of Photorhabdus luminescens. J. Bacteriol. 182:5127-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacoby, G. A., K. E. Walsh, D. M. Mills, V. J. Walker, H. Oh, A. Robicsek, and D. C. Hooper. 2006. qnrB, another plasmid-mediated gene for quinolone resistance. Antimicrob. Agents Chemother. 50:1178-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarlier, V., M.-H. Nicolas, G. Fournier, and A. Philippon. 1988. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev. Infect. Dis. 10:867-878. [DOI] [PubMed] [Google Scholar]
- 11.Jeong, J. Y., H. J. Yoon, E. S. Kim, Y. Lee, S. H. Choi, N. J. Kim, J. H. Woo, and Y. S. Kim. 2005. Detection of qnr in clinical isolates of Escherichia coli from Korea. Antimicrob. Agents Chemother. 49:2522-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kieser, T. 1984. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid 12:19-36. [DOI] [PubMed] [Google Scholar]
- 13.Mammeri, H., M. Van De Loo, L. Poirel, L. Martinez-Martinez, and P. Nordmann. 2005. Emergence of plasmid-mediated quinolone resistance in Escherichia coli in Europe. Antimicrob. Agents Chemother. 49:71-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Martinez, L., A. Pascual, and G. A. Jacoby. 1998. Quinolone resistance from a transferable plasmid. Lancet 351:797-799. [DOI] [PubMed] [Google Scholar]
- 15.Nazic, H., L. Poirel, and P. Nordmann. 2005. Further identification of plasmid-mediated quinolone resistance determinant in Enterobacteriaceae in Turkey. Antimicrob. Agents Chemother. 49:2146-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nordmann, P., and L. Poirel. 2005. Emergence of plasmid-mediated resistance to quinolones in Enterobacteriaceae. J. Antimicrob. Chemother. 56:463-469. [DOI] [PubMed] [Google Scholar]
- 17.Paauw, A., A. C. Fluit, J. Verhoef, and M. A. Leverstein-van Hall. 2006. Enterobacter cloacae outbreak and emergence of quinolone resistance gene in Dutch hospital. Emerg. Infect. Dis. 12:807-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poirel, L., O. Menuteau, N. Agoli, C. Cattoen, and P. Nordmann. 2003. Outbreak of extended-spectrum β-lactamase VEB-1-producing isolates of Acinetobacter baumannii in a French hospital. J. Clin. Microbiol. 41:3542-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poirel, L., T. Vu N′Guyen, A. Weintraub, C. Leviandier, and P. Nordmann. 2006. Plasmid-mediated quinolone resistance determinant qnrS in Enterobacter cloacae. Clin. Microbiol. Infect. 12:1021-1023. [DOI] [PubMed] [Google Scholar]
- 20.Poirel, L., J. D. D. Pitout, L. Calvo, J.-M. Rodriguez-Martinez, D. Church, and P. Nordmann. 2006. In vivo selection of fluoroquinolone-resistant Escherichia coli isolates expressing plasmid-mediated quinolone resistance and expanded-spectrum β-lactamase. Antimicrob. Agents Chemother. 50:1525-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poirel, L., M. Van De Loo, H. Mammeri, and P. Nordmann. 2005. Association of plasmid-mediated quinolone resistance with extended-spectrum β-lactamase VEB-1. Antimicrob. Agents Chemother. 49:3091-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robicsek, A., D. F. Sahm, J. Strahilevitz, G. A. Jacoby, and D. C. Hooper. 2005. Broader distribution of plasmid-mediated quinolone resistance in the United States. Antimicrob. Agents Chemother. 49:3001-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robicsek, A., J. Strahilevitz, G. A. Jacoby, M. Macielag, D. Abbanat, C. H. Park, K. Bush, and D. C. Hooper. 2006. Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nat. Med. 12:83-88. [DOI] [PubMed] [Google Scholar]
- 24.Robicsek, A., J. Strahilevitz, D. F. Sahm, G. A. Jacoby, and D. C. Hooper. 2006. qnr prevalence in ceftazidime-resistant Enterobacteriaceae isolates from the United States. Antimicrob. Agents Chemother. 50:2872-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Martinez, J. M., L. Poirel, A. Pascual, and P. Nordmann. 2006. Plasmid-mediated quinolone resistance in Australia. Microb. Drug Res. 12:99-102. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz, J. 2003. Mechanisms of resistance to quinolones: target alterations, decreased accumulation and DNA gyrase protection. J. Antimicrob. Chemother. 51:1109-1117. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 28.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran, J. H., G. A. Jacoby, and D. C. Hooper. 2005. Interaction of the plasmid-encoded quinolone resistance protein Qnr with Escherichia coli DNA gyrase. Antimicrob. Agents Chemother. 49:118-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran, J. H., G. A. Jacoby, and D. C. Hooper. 2005. Interaction of the plasmid-encoded quinolone resistance protein QnrA with Escherichia coli topoisomerase IV. Antimicrob. Agents Chemother. 49:3050-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, M., D. F. Sahm, G. A. Jacoby, and D. C. Hooper. 2004. Emerging plasmid-mediated quinolone resistance associated with the qnr gene in Klebsiella pneumoniae clinical isolates in the United States. Antimicrob. Agents Chemother. 48:1295-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, M., J. H. Tran, G. A. Jacoby, Y. Zhang, F. Wang, and D. C. Hooper. 2003. Plasmid-mediated quinolone resistance in clinical isolates of Escherichia coli from Shangai, China. Antimicrob. Agents Chemother. 47:2242-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]