Abstract
Since N-acyl homoserine lactones (AHLs) are key mediators of cell density-dependent regulation of traits involved in virulence and epiphytic fitness in gram-negative bacteria such as Pseudomonas syringae, a variety of plant species were examined to determine their production of leaf surface compounds that could interact with these signaling systems. Leaf washings of 17 of 52 plant species tested stimulated or inhibited AHL-dependent traits in at least one of the bacterial reporter strains used. The active compounds from most plants could be distinguished from known AHLs due to different patterns of mobility during C8 and C18 reverse-phase thin-layer chromatography (TLC) and normal-phase TLC compared to the patterns for authentic bacterial AHLs. All plant extracts were also tested to determine their abilities to sequester iron and trigger bacterial siderophore synthesis on a medium containing abundant iron. Leaf washings from 16 of the 52 plant species, as well as tannic acid solutions, stimulated pyoverdine synthesis in P. syringae in a high-iron medium. These preparations also inhibited the growth of a P. syringae mutant unable to produce pyoverdine siderophores but not the growth of the wild-type bacterium. The stimulation of siderophore production and the growth inhibition by plant extracts and purified tannins were both reversed by addition of ferric chloride to culture media, indicating that iron was made unavailable by the compounds released onto the leaf surface.
Leaf surfaces support the development of large populations of bacteria, called epiphytes, that can have both beneficial and detrimental effects on plant productivity. Most plant-pathogenic bacteria, such as Pseudomonas syringae, multiply on the surface of healthy plants before they initiate disease (3, 16, 19). The probability of foliar bacterial diseases is strongly correlated with the size of the epiphytic population of phytopathogenic bacteria on healthy plants (17). Thus, the epiphytic phase of these pathogens can be considered the first step in the infection process. Other deleterious bacteria, such as ice-nucleation-active (Ice+) strains of P. syringae, Erwinia herbicola, Pseudomonas fluorescens, and Xanthomonas campestris that can catalyze damaging ice formation on frost-sensitive plants at temperatures as high as −2°C, are also common on plants (18). Conversely, non-ice-nucleation-active bacteria can exclude Ice+ strains by a preemptive competitive exclusion process (18, 19). Auxin-producing bacteria are also common epiphytes and can alter the normal development of the plants on which they live (20). Thus, factors that affect the sizes of the populations of such epiphytes are important in dictating plant health.
The leaf surface is the first point of contact between immigrant microorganisms and plants, and it might be expected that plants and bacteria have evolved a variety of adaptations that enable them to coexist in a continuously changing environment (1). P. syringae cells apparently use chemical and physical signals on the plant surface to coordinate their responses to the plant, such as induction of virulence gene expression and expression of a variety of genes involved in epiphytic fitness (26, 31). Likewise, plants might be expected to produce a variety of compounds to defend against microbial colonization. Such processes have been addressed primarily after invasion of the plant, and little is known about preexisting defenses that plants might mount on the leaf surface. However, there have been a few reports of antimicrobial compounds and proteins secreted onto the surfaces of plants (17, 45).
P. syringae exhibits strong cell density-dependent behaviors on plants that affect both its epiphytic fitness and virulence (37, 38). Coordinated expression of bacterial traits in a cell density-dependent fashion, often referred to as quorum sensing (QS), is now recognized as a common process in bacteria in a variety of habitats, including on and in plants (36, 50, 53). Bacterial traits such as bioluminescence, antibiotic production, siderophore production, pigment formation, motility, extracellular polysaccharide production, and virulence, as well as other traits, are controlled when bacteria reach a sufficiently high population density via similar quorum-sensing mechanisms involving the production of small diffusible signal molecules (36, 41, 50, 53). The most common chemical signals used by bacterial cells are N-acyl homoserine lactones (AHLs) (36, 51, 53). The quantity of AHLs increases as the bacterial population density increases, and AHLs serve as coinducers along with cognate transcriptional regulators to coordinate the expression of specialized sets of genes. In different bacterial species the length of the N-acyl side chain of AHLs (4 to 14 carbon atoms), the presence of an acyl C3 substituent (oxo- or hydroxy), and the degree of saturation are different. Cha et al. (4) found that 60 of 106 strains representing seven genera of gram-negative plant-associated bacteria that were tested produced AHLs. Quorum sensing contributes to the virulence of plant-pathogenic bacteria (9, 36, 51), as well as to the epiphytic phase of bacteria on healthy plants (37, 38) and interactions between bacteria on plants (32, 35, 36), suggesting that a wide range of bacterial traits mediating plant-bacterium interactions are regulated by cell density. The finding that QS can be disrupted by other organisms, such as bacteria (29, 32, 35), fungi (40), and algae (2, 14, 48), raised the question of whether higher plants use such a strategy to defend themselves against bacteria. In an initial study, Teplitski et al. (49) found that roots of seedling pea, soybean, rice, tomato, crown vetch, and Medicago truncatula plants secrete compounds that stimulate specific AHL reporters, while roots of pea and crown vetch plants secrete compounds that inhibit AHL-regulated traits. This work was expanded in a subsequent study that showed that Medicago produces a wide variety of compounds, apparently unrelated to AHLs, that can affect bacterial gene expression (13), although the chemical identity of these compounds has not been reported yet. More recently, it was reported that extracts of garlic also could alter autoinduction in Pseudomonas aeruginosa (39). In a study that revealed additional complexity of plant-microbe interactions, exogenous bacterial AHLs were shown to cause numerous changes in the patterns of gene expression in M. truncatula (27), suggesting that plants have evolved mechanisms to recognize bacterial AHLs as signature molecules and mount defensive responses. It seems likely that it is common for plants to interfere with the coordinated expression of virulence or other deleterious traits by either blocking or inappropriately stimulating QS (5, 6). Indeed, transgenic plants that express bacterial AHLs have altered responses to pathogenic bacteria. In most but not all cases such plants apparently prematurely induce the expression of bacterial virulence traits that otherwise would be expressed only when cell densities are high at later stages of the infection process in plants (12, 25, 50). Apparently, most plants mount a defense against bacterial pathogens only after they recognize AHLs directly, or they mount a defense indirectly after they recognize virulence factors that are expressed only upon AHL production in bacteria (2, 42). It therefore could be expected that plants defend themselves against deleterious bacteria by producing compounds that interfere with the QS system of bacteria. There has been much interest in developing new strategies for plant disease control based on altering the quorum-sensing process in plants (2, 5, 12, 42). While compounds that do this have been found in roots, there has been no description of such a phenomenon on the foliar parts of plants apart from a limited survey performed by McLean et al. (29), who tested leaf fragments from 50 terrestrial or aquatic plants directly using an agar overlay of the biosensor organisms. This assay was not designed to detect compounds found principally on the surface rather than the interior of leaves and did not reveal the presence of compounds that interfered with quorum sensing in any plant species (29). Given that the initial interaction between microbes and plants is on the plant surface, we focused on materials that could be washed off leaves of a variety of plants with a gentle extraction technique and examined their capacity to interact with the QS signaling systems in different bacteria in this study.
In addition to organic compounds that could affect the behavior of bacteria upon migration to a leaf, inorganic compounds, such as iron, play an important role in microbial behavior. Iron availability profoundly affects gene expression in prokaryotes and thus can regulate antagonistic interactions among different microorganisms on plant surfaces and in the rhizosphere due to its control of secondary metabolite biosynthesis (22, 33). For example, iron availability influences the abundance of antibiotics and other toxic compounds made by Pseudomonas species (7, 8, 15, 33). Iron is also essential for expression of the phytopathogenicity of a variety of plant-pathogenic bacteria, such as Erwinia chrysanthemi and Erwinia amylovora (10). Under iron-limiting conditions most microbes produce siderophores and corresponding membrane receptors for iron acquisition (22, 33). Pseudomonas species typically produce yellow-green fluorescent siderophores, (pyoverdines or pseudobactins) having a high affinity for iron in order to acquire it in environments in which the abundance of this element low (22). Iron levels are apparently rather low on many plants (24), and pyoverdines therefore can inhibit the growth of neighboring cells by sequestering iron as ferric-pyoverdine complexes (8, 22). Since limiting iron availability with strong iron chelators is a general mechanism of bacterial disease control in animals (21, 52), it might be expected that at least some plants could also limit the size of the population of deleterious microbes by a process that makes iron unavailable. While it has been reported that defense of some plants may be enhanced by polyphenols that keep iron away from the microbes (30), iron sequestration by plants has received very little attention.
In this study we explored plant production of compounds that interfere with QS on leaf surfaces. In this study we also discovered that some plant species produce compounds that are powerful chelators of iron. We thus also describe the iron sequestration capabilities of leaf surface compounds from a variety of plant species and their effects on the growth of isogenic strains of P. syringae differing in the ability to produce siderophores.
MATERIALS AND METHODS
Plant material.
Plant samples were collected from the University of California, Berkeley, Botanical Garden and from the UC Berkeley campus. Some plants were also grown from seed in a greenhouse. Fifty-two plant species representing major plant families, as well as a few plant species that are hosts of bacteria in which quorum sensing is known to occur (9), were selected. To ensure that compounds isolated from leaf surfaces were of plant origin and not of leaf-associated bacterial origin, six of the plant species tested that exhibited AHL mimic activity when field-sampled tissues were examined were retested after they were grown aseptically in a greenhouse. Ipomoea purpurea, Passiflora incarnata, Salvia mexicana, Alyssum maritimum, Oxalis pes-caprae, and Brassica napus were cultivated either using surface-sterilized seeds (soaked in 10% chlorine bleach for 10 min) or using surface-sterilized cuttings grown in MS medium or sterilized soil.
Leaf surface compounds were recovered immediately after sampling as follows. Five grams of intact leaves was washed with a 1:1 mixture of methanol and water for 30 min with gentle agitation. No obvious plant damage or solvent infiltration occurred during this process. The plant material was then removed, and samples were filter sterilized (pore size, 0.2 μm) and tested for bacterial contamination. Uncontaminated samples were condensed to dryness in a rotary evaporator, resuspended in 1 ml of 50% methanol in water, and again concentrated to a final volume of 300 μl using a rotary vacuum evaporator. Samples were then kept at 4°C until they were used.
Bacterial strains and culture conditions.
The following four AHL indicator organisms were used: Chromobacterium violaceum strain CV0blu, which is a derivative of wild-type strain ATCC 31532, which is deficient for production of N-hexanoyl-l-homoserine lactone (N-hexanoyl-l-HSL) (47); Agrobacterium tumefaciens strain NT1(pSVB33, pJM749), which lacks the Ti plasmid and thus is deficient for production of AHL and which harbors a traG::lacZ fusion (44); P. syringae strain B728a, which produces 3-oxo-hexanolyl homoserine lactone; and P. syringae strain BHSL, which is an isogenic mutant of strain B728a which is deficient for AHL production (37). Both P. syringae strains harbored an ahlI::gfp fusion on plasmid pBQ9 (37). All strains were stored at −80°C in 15% glycerol. C. violaceum CV0blu was grown on LΑ agar containing chloramphenicol (10 μg/ml) and tetracycline (15 μg/ml). A. tumefaciens NT1(pSVB33, pJM749) was grown at 28°C on LA agar with kanamycin (50 μg/ml) and carbenicillin (100 μg/ml). The P. syringae indicator strains were grown on KB containing rifampin (100 μg/ml) and spectinomycin (20 μg/ml). P. syringae strain B728a I-1 was a nonfluorescent mutant of strain B728a (23) and was cultured on KB containing rifampin and kanamycin at 28°C.
Bioassays for detection of compounds that interfere with AHL from plants.
C. violaceum CV0blu was used to detect both stimulation and inhibition of AHL-regulated violacein biosynthesis. Stock cultures were grown in LB overnight at 28°C. Five hundred microliters of a culture containing about 1 × 109 to 2 × 109 bacteria was pelleted by centrifugation and resuspended in 5 ml of warm LA medium containing 0.5% agar. To test whether plant extracts stimulated violacein synthesis, the inoculated soft agar overlay was poured directly on the surface of regular LA agar plates, and sterile paper disks treated with 10 μl of the plant extract were then placed on the agar. The plates were incubated for 18 to 24 h at 28°C. Violacein production, easily visualized as a purple pigment around the disks, was considered evidence of AHL-like activity in plant extracts. To test whether the extracts inhibited violacein production, 50 nmol N-hexanoyl-l-HSL was added to the soft agar overlay before it was poured onto the surface of the bioassay plate; a colorless halo surrounding disks on an otherwise purple plate was considered evidence of inhibition of AHL signaling.
A. tumefaciens NT1(pSVB33, pJM749) was used to detect both stimulation and inhibition of AHL-regulated β-galactosidase activity. This strain was cultured in M9 minimal broth until early stationary phase was reached (1 × 109 to 2 × 109 cells/ml) and then was inoculated into M9 minimal medium soft agar supplemented with 60 μg/ml 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside (X-Gal). Five milliliters of the warm overlay was poured on top of 20 ml of regular M9 agar in each plate. Paper disks to which the plant extracts were applied were placed on top of the solidified overlay as described above. The plates were then incubated for 24 to 48 h at 28°C, and β-galactosidase activity, visualized as a blue pigment around disks, was considered evidence of AHL-like activity in plant extracts. To test whether plant extracts inhibited AHL-mediated β-galactosidase activity, 50 nmol N-octanoyl-l-HSL was added to the soft agar overlay; a colorless halo surrounding disks on an otherwise blue plate was considered evidence of inhibition of AHL signaling.
P. syringae BHSL(pBQ9) was used to detect N-oxohexanoyl-l-HSL-induced production of green fluorescent protein (GFP), and strain B728a(pBQ9) was used to detect plant inhibition of AHL-dependent GFP fluorescence in this strain. A plant extract was tested with both indicator strains on the same LA agar plate. One sterile paper disk treated with 10 μl of the extract was placed on the agar at the center of the plate, and 10-μl portions of the two P. syringae indicator strains grown in LB broth to densities of about 109 cells/ml were streaked in lines perpendicular to each other on opposite sides of the paper disk. The plates were incubated for 18 to 24 h at 28°C, and GFP fluorescence was visualized using a Zeiss SV11 stereoscope equipped with a Kramer epifluorescence/Optronix Color DEI450 system. Green fluorescence limited to cells of P. syringae BHSL(pBQ9) was considered evidence of the presence of AHL-like molecules in plant extracts, while a lack of green fluorescence in otherwise green fluorescent cells of B728a(pBQ9) was considered evidence of inhibition of AHL-mediated signaling in P. syringae. A diffusible yellow-green fluorescence around streaks of either strain on LA agar was considered evidence of the presence of plant factors that induced siderophore production in P. syringae.
Thin-layer chromatography.
Plant extracts that showed stimulation or inhibition of the bioreporters were analyzed further by thin-layer chromatography (TLC). Both C18 and C8 reverse-phase TLC plates, as well as silica gel plates, were used for chromatographic analysis.
Extracts (1 to 10 μl) were applied to C18 or C8 reverse-phase TLC plates, which were then developed with methanol-water (60:40) as previously described (44). Normal-phase TLC analysis was performed with samples applied to silica gel plates and developed with a mixture containing dichloromethane, methanol, and water (70:30:3). After the solvent was evaporated, the plates were overlaid with fresh cultures of the appropriate indicator strain [C. violaceum CV0blu or A. tumefaciens NT1(pSVB33, pJM749)] in soft agar at 40°C with and without appropriate AHLs and X-Gal as described above. As a control, a mixture containing 7.5 nmol of C12-HSL, 6 nmol of C10-HSL, 2 nmol of C8-HSL, 0.01 nmol of oxo-C8-HSL, and 0.2 nmol of oxo-C6-HSL was spotted onto TLC plates that were overlaid with A. tumefaciens NT1(pSVB33, pJM749), and a mixture containing 2 nmol of C8-HSL, 0.1 nmol of oxo-C6-HSL, 0.05 nmol of C6-HSL, and 4 nmol of C4-HSL was spotted on plates that were overlaid with C. violaceum CV0blu. Pea seedling root extract, recovered as described by Teplitski et al. (49), was also chromatographed on each TLC plate as a positive control. After the agar overlays were solidified, the TLC plates were incubated for 18 to 36 h at 28°C in a sealed container to prevent evaporation and contamination. β-Galactosidase and violacein were visualized as described above.
Siderophore biosynthesis.
The growth of and diffusible pigment production by P. syringae strain B728a and isogenic siderophore mutant strain I-1 were compared by streaking 106 to 107 cells/ml of the two strains perpendicular to each other on LA agar on opposite sides of a paper disk treated with 10 μl of a plant extract as described above. The plates were incubated for 24 to 48 h at 28°C, and siderophore production by B728a was quantified by determining the distance from the disk to the distal extent of yellow-green fluorescence visualized under UV light. Iron-dependent inhibition of growth of P. syringae by plant extracts was determined by a lack of growth of strain I-1 but not of strain B728a in the vicinity of the disk. As a positive control, paper disks with different concentrations of tannin (10−3 to10−2 Μ) were used, while paper disks impregnated with both plant extract and different concentrations of FeCl3 (10−5 to10−3 M) were used to verify the iron dependence of growth inhibition.
RESULTS
Stimulation and inhibition of bacterial QS by leaf surface compounds.
We used a variety of bacterial reporter strains to detect compounds obtained from leaf surfaces that interfered with AHLs based on their high levels of sensitivity, as well as their different responses to various N-acylated derivatives of HSL. To detect a wide range of different molecules, we used (i) A. tumefaciens NT1(pSVB33, pJM749), which responds to C6- to C12-alkanoyl, 3-oxo-alkanoyl, and 3-hydroxy-alkanoyl side chain HSLs by expressing β-galactosidase activity; (ii) C. violaceum CV0blu, which responds positively to the presence of short (C4 to C8) alkanoyl or 3-oxo-alkanoyl side chain HSLs by producing violacein (the QS of this strain is also inhibited by the presence of long N-acyl side chain [C10 to C14] HSLs, thus blocking violacein production in the presence of a stimulator [N-hexanoyl-l-HSL in our studies]); (iii) P. syringae BHSL(pBQ9), which responds to N-(3-oxo-hexanoyl)-l-HSL by expressing GFP fluorescence; and (iv) P. syringae B728a(pBQ9), which exhibits GFP fluorescence at high cell densities unless it is inhibited by another compound.
The β-galactosidase activity of A. tumefaciens NT1(pSVB33, pJM749) was stimulated by 11 of the 52 plant species tested. Leaf surface extracts from I. purpurea, P. incarnata, Bougainvillea sp., Agapanthus africanus, Vicia faba, S. mexicana, Angelica archangelica, Romneya trichocalyx, Erigeron glaucus, A. maritimum, and O. pes-caprae all strongly stimulated QS in A. tumefaciens (Table 1). In contrast, when N-(octanoyl)-l-HSL was added to test plates, leaf surface extracts from Clematis vitalba, Geranium molle, Pisum sativum (seedling roots), and Tropaeolum majus inhibited the β-galactosidase activity of this indicator strain.
TABLE 1.
Stimulation and inhibition of AHL-regulated phenotypes of the bioreporters A. tumefaciens NT1(pSVB33, pJM749), C. violaceum CV0blu, P. syringae B728a(pBQ9), and P. syringae BHSL(pBQ9) by extracts from 52 plant species
| Plant | Family | Bioreporter strains
|
|||||
|---|---|---|---|---|---|---|---|
| NT1
|
CV0blu
|
B728a inhibition | BHSL stimulation | ||||
| Stimulation | Inhibition | Stimulation | Inhibition | ||||
| Prunus laurocerasus | Rosaceae | − | − | − | − | − | − |
| Heteromeles arbutifolia | Rosaceae | − | − | − | − | − | − |
| Raphyolepis indica | Rosaceae | − | − | − | − | − | − |
| Nerium oleander | Apocynaceae | − | − | − | − | − | − |
| Trachelospermum jasminoides | Apocynaceae | − | − | − | − | − | − |
| Capsicum annuum | Solanaceae | − | − | − | − | − | − |
| Nicotiana tabacum | Solanaceae | − | − | − | − | − | − |
| Ipomoea purpurea | Convolvulaceae | + | − | − | − | − | − |
| Clematis vitalba | Ranunculaceae | − | + | − | − | − | − |
| Pelargonium odoratissimum | Geraniaceae | − | − | − | − | − | − |
| Geranium molle | Geraniaceae | − | + | − | − | − | − |
| Hedera helix | Araliaceae | − | − | − | − | − | − |
| Passiflora incarnata | Passifloraceae | + | − | − | + | − | − |
| Bougainvillea sp. | Nyctaginaceae | + | − | − | − | − | − |
| Rhus laurina | Anacardinaceae | −a | −a | −a | −a | − | − |
| Cynoglossum amabile | Boraginaceae | − | − | − | − | − | − |
| Agapanthus africanus | Liliaceae | + | − | + | − | − | − |
| Amorpha fruticosa | Fabaceae | − | − | − | − | − | − |
| Pisum sativumb | Fabaceae | − | +/− | − | +/+ | − | − |
| Acacia sp. | Fabaceae | − | − | − | − | − | − |
| Phaseolus vulgaris | Fabaceae | − | − | − | − | − | − |
| Vicia faba | Fabaceae | + | − | − | − | − | − |
| Salvia karwinskii | Lamiaceae | − | − | − | − | − | − |
| Salvia mexicana | Lamiaceae | + | − | + | − | − | − |
| Sedum spectabile | Crassulaceae | −a | −a | −a | −a | − | − |
| Angelica archangelica | Apiaceae | + | − | − | − | − | − |
| Foeniculum vulgare | Apiaceae | − | − | − | − | − | − |
| Romneya trichocalyx | Papaveraceae | + | − | − | + | − | − |
| Camellia sp. | Theaceae | − | − | − | − | − | − |
| Erigeron glaucus | Asteraceae | + | − | − | − | − | − |
| Heterotheca mucronata | Asteraceae | − | − | − | − | − | − |
| Ajania pacifica | Asteraceae | − | − | − | − | − | − |
| Unknown | Labiatae | − | − | − | − | + | − |
| Picea morrisonicola | Pinaceae | − | − | − | − | − | − |
| Phillyrea angustifolia | Oleaceae | − | − | − | − | − | − |
| Olea europaea | Oleaceae | − | − | − | − | − | − |
| Comarostaphylis glaucescens | Ericaceae | −a | −a | − | − | − | − |
| Arbutus unedo | Ericaceae | −a | +a | −a | +a | − | − |
| Cistus incanus | Cistaceae | −a | −a | −a | −a | − | − |
| Hibiscus schizopetalus | Malvaceae | − | − | − | − | − | − |
| Eucalyptus resinifera | Myrtaceae | −a | −a | −a | −a | − | − |
| Myrica rubra | Myricaceae | − | − | − | − | − | − |
| Brassica napus | Brassicaceae | − | − | − | + | − | − |
| Alyssum maritimum | Brassicaceae | + | − | − | + | − | − |
| Barbarea vulgaris | Brassicaceae | − | − | − | − | − | − |
| Ruta graveolens | Rutaceae | − | − | − | − | − | − |
| Festuca californica | Poaceae | − | − | − | − | − | − |
| Zauschneria epilobium | Onagraceae | −a | −a | −a | −a | − | − |
| Blechnum spicant | Blechnaceae | − | − | − | − | − | − |
| Oxalis pes-caprae | Oxalidaceae | + | − | − | − | − | − |
| Monnina xalapensis | Polygonaceae | −a | −a | −a | −a | −a | −a |
| Tropaeolum majus | Tropaeolaceae | − | + | − | − | − | − |
Plant extract inhibited the growth of the bioreporter.
P. sativum seedling root extracts and leaves of a mature plant exhibited different activities.
Leaf surface extracts from only A. africanus and S. mexicana stimulated violacein production in C. violaceum CV0blu. However, when N-hexanoyl-l-HSL was added to test plates, extracts from seven plant species inhibited violacein production. A leaf surface extract from P. incarnata, as well as extracts from seedling roots of P. sativum, strongly inhibited violacein production, while R. trichocalyx, A. maritimum, Ruta graveolens, B. napus, and P. sativum leaf surface extracts caused slight inhibition of QS (Table 1 and Fig. 1).
FIG. 1.
Slight inhibition of AHL-regulated violacein synthesis in C. violaceum CV0blu by an extract from A. maritimum (left panel) and strong inhibition by an extract from P. incarnata (right panel) in LA agar plates supplied with oxo-C6-HSL.
None of the leaf surface extracts stimulated GFP fluorescence in P. syringae BHSL(pBQ9), while a plant species belonging to the family Labiatae strongly inhibited GFP fluorescence in P. syringae B728a(pBQ9).
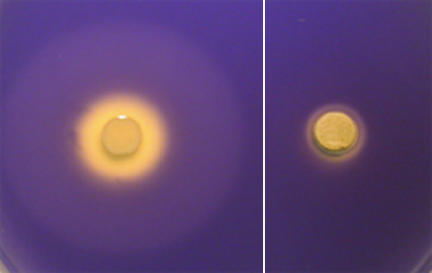
Compounds on the leaves of some plant species both inhibited growth and interfered with QS in bioreporter strains (Table 1). Growth inhibition caused by substances in plant extracts was apparent as a clear zone around a paper disk where no bacteria could be recovered. This zone was usually much smaller than the zones where there was altered β-galactosidase activity or violacein production, indicative of interference of QS, suggesting that the antimicrobial activities did not obscure detection of activities that interfered with QS in the extracts. In fact, both activities were detected in extracts from Arbutus unedo (Table 1). In this case, growth inhibition was evident as a small clear zone, while inhibition of both violacein production and β-galactosidase activity was apparent because of a much larger secondary zone around the paper disk (Fig. 2).
FIG. 2.
Growth inhibition (internal zone) and inhibition of AHL-regulated violacein synthesis in C. tumefaciens CV0blu (external zone) by an extract from A. unedo (left panel) and growth inhibition by an extract from C. incanus (right panel) in LA agar plates supplied with oxo-C6-HSL.
To verify that the AHL mimics in these plants were of plant origin and not associated with the small number of epiphytic bacteria present on the leaves of the field-grown plants at the time of assay, we propagated six plants of the 11 plant species that exhibited such activity under axenic conditions in a greenhouse and retested them for AHL mimic activity. Leaf surface extracts from axenically grown I. purpurea, P. incarnata, S. mexicana, A. maritimum, O. pes-caprae, and B. napus all had effects on the A. tumefaciens NT1 bioreporter similar to the effects of the extracts from field-grown plants, indicating that the AHL mimics were of plant origin.
TLC analysis of plant extracts.
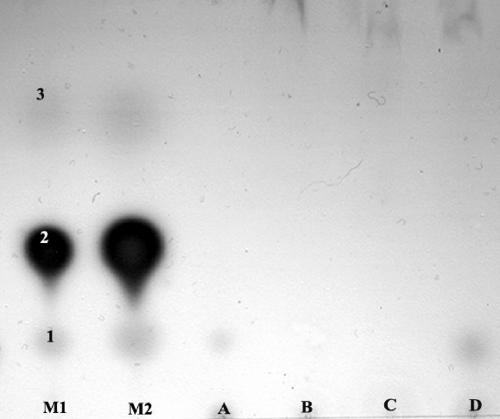
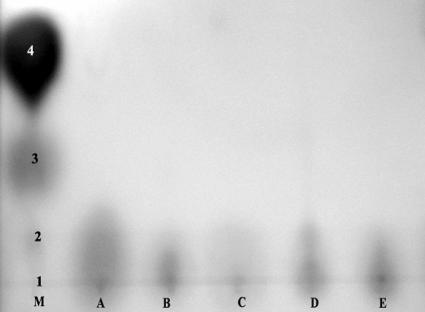
In order to gain some insight into the nature of the leaf surface compounds that stimulated or inhibited bacterial QS, extracts were fractionated using a variety of thin-layer chromatography conditions before bioassays were performed in an attempt to distinguish the compounds from known bacterial AHLs. Extracts were subjected to both C18 and C8 reverse-phase TLC, and the TLC plates were overlaid with either A. tumefaciens NT1(pSVB33, pJM749) or C. violaceum CV0blu in bioassays similar to the bioassay used in the original agar plate screening analysis. A compound in extracts from leaf surfaces of S. mexicana and A. africanus that stimulated QS in A. tumefaciens migrated in a manner similar to that of N-octanoyl-l-HSL in both C18 and C8 reverse-phase TLC (Fig. 3). A compound in leaf surface extracts from these two plant species that also stimulated QS in C. violaceum also migrated in a manner similar to that of N-octanoyl-l-HSL. Compounds that stimulated QS in A. tumefaciens in extracts from R. trichocalyx, I. purpurea, A. archangelica, P. incarnata, A. maritimum, E. glaucus, and O. pes-caprae migrated only slightly from the origin, unlike all of the AHLs except those with very long acyl side chains (Fig. 4). Compounds that stimulated QS in A. tumefaciens in extracts from V. faba and Bougainvillea sp. did not migrate under our TLC conditions. While extracts from five plant species inhibited QS in A. tumefaciens in the agar plate assay, these compounds did not migrate on reverse-phase TLC plates. While extracts from seven plant species inhibited violacein production by C. violaceum in the agar plate assay, no compounds with such activity migrated on TLC plates. As a control, we detected a compound in extracts from seedling pea roots that strongly inhibited QS in A. tumefaciens as determined by this TLC assay.
FIG. 3.
AHL mimic compounds in extracts from A. africanus (lane A) and S. mexicana (lane D) developed on a C18 reverse-phase thin-layer plate inoculated with the bioreporter A. tumefaciens NT1(pSVB33, pJM729). No AHL mimic compounds were observed in extracts from V. faba (lane B) and Bougainvillea sp. (lane C). Mixtures of the standard AHLs C8-HSL (spot 1), oxo-C8-HSL (spot 2), and oxo-C6-HSL (spot 3) were also included (lanes M1 and M2).
FIG. 4.
AHL mimic compounds extracted from P. incarnata (lane A), Ipomoea (lane B), A. maritimum (lane C), A. archangelica (lane D), and R. trichocalyx (lane E), developed on a C18 reverse-phase thin-layer plate, and then inoculated with the bioreporter A. tumefaciens NT1(pSVB33, pJM729). Mixtures of the standard AHLs C12-HSL (spot 1), C10-HSL (spot 2), C8-HSL (spot 3), and oxo-C8-HSL (spot 4) were also included (lane M).
While compounds from both S. mexicana and A. africanus migrated like N-octanoyl-l-HSL in reverse-phase TLC, these compounds were easily distinguished from this AHL in normal-phase TLC. The known AHLs, but not the compounds in plant extracts, moved from the origin. Since none of the plant extracts moved from the origin, they were distinct from the bacterial AHLs tested.
Iron sequestration by plant extracts.
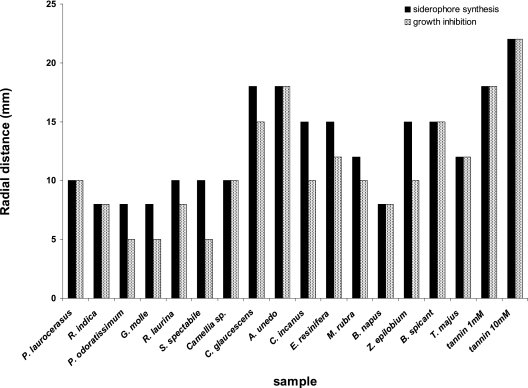
Siderophore synthesis in P. syringae B728a was stimulated in culture media containing abundant iron by leaf surface extracts from 16 of the 52 plant species tested. This strain did not produce visible siderophore fluorescence when it was grown on LA medium, while strong diffusible fluorescence was observed when cells were grown in the vicinity of many leaf surface extracts. The extracts from G. molle, Pelargonium odoratissimum, Sedum spectabile, Rhus laurina, Myrica rubra, Camellia sp., Cistus incanus, Zauschneria epilobium, Prunus laurocerasus, Raphyolepis indica, B. napus, T. majus, Comarostaphylis glaucescens, A. unedo, Blechnum spicant, and Eucalyptus resinifera each stimulated siderophore production in P. syringae, although the magnitude of the effect was different for different plant species (Fig. 5). In all cases the stimulation of siderophore production in P. syringae B728a was inhibited when FeCl3 was added at a concentration of 10−4 M to the media (data not shown). These results suggest that one or more compounds in plant extracts made the iron that was present in LA medium unavailable to P. syringae, thus stimulating siderophore biosynthesis in this species.
FIG. 5.
Radial distances from the site of application of plant extracts that siderophore production by P. syringae wild-strain B728a was stimulated on LA medium (black bars) and growth of the isogenic siderophore-deficient mutant strain I-1 was inhibited (gray bars) by extracts of 16 plant species, as well as by two concentrations of tannin.
To determine the relative binding efficiencies of the apparent iron sequestration compounds present in the plant extracts, we compared the growth of wild-type P. syringae strain B728a with the growth of isogenic mutant strain I-1, which is deficient in pyoverdine production. Since the growth of pseudomonads that are deficient in siderophore production is inhibited in the presence of a strong iron chelator (34), we wanted to determine if the plant iron-sequestering compounds were sufficiently strong iron chelators to inhibit the growth of strains lacking their own siderophore. All 16 plant surface extracts inhibited the growth of P. syringae strain I-1 on LA medium. Importantly, in no case did the plant extract inhibit the growth of P. syringae B728a on LA medium. The extracts from seven plants stimulated siderophore production in the wild-type strain over a distance from the disk on which plant extracts were applied similar to the distance over which the same extracts inhibited the growth of strain I-1 (Fig. 5). This suggests that the iron sequestration capabilities of compounds from these plants are very high and that the presence of the compounds is sufficient to make iron limiting for the P. syringae strain lacking a siderophore. It is important that the extracts from the rest of the plant species induced siderophore production in P. syringae wild-type strain B728a over a distance that was substantially greater than the distance over which they inhibited growth of strain I-1. This result suggests that while the compounds in these extracts were sufficient to sequester iron, their affinities for iron were not strong enough to deplete the available iron to a level that could limit bacterial growth at the low concentrations present after diffusion from the source on the assay disks.
DISCUSSION
The secretion of compounds that could alter bacterial quorum sensing was quite common among the plants surveyed in this study. In contrast to other workers (29), we tested leaf surface extracts and not leaf fragments since compounds important in initial interactions between bacteria and plants should be found prominently on the leaf. In fact, little evidence of AHL mimics in leaves was obtained using a leaf disk assay (29). Total plant extracts from 7 of 21 plant species tested were also recently found to contain quorum-sensing inhibitors (39); such extracts presumably included leaf surface compounds, but the compounds were not concentrated. It is noteworthy that in our study leaf surface extracts from 17 of the 52 plant species tested yielded a positive or negative QS reaction with at least one bacterial bioreporter. It is also important to note that leaf surface extracts from all six plants (of the 17 plants producing AHL mimics) that were grown under aseptic conditions contained QS interference activity similar to that of plants harvested from the field, strongly suggesting that the compounds were of plant origin and not associated with the small number of epiphytic bacteria present on the leaves of the field-grown plants at the time of the initial assay. We intentionally studied only the compounds that could be readily washed off leaves with a gentle extraction technique in order to assess the chemical defenses that a microbe might first encounter upon contact with a plant. It seems likely that there were other compounds that interfered with QS that were more internalized in the plants, which could alter normal microbial behavior only after they invaded the plant. Given that a wide variety of QS signal molecules have been described (53), our use of only three bacterial species as bioreporters undoubtedly resulted in overlooking some molecules that were not readily detected by these strains. However, our use of C. violaceum, A. tumefaciens, and P. syringae, which recognize AHLs with different side chain lengths and substitutions, should have enabled us to detect a wide variety of different AHLs. It is noteworthy that the number of plants (11 species) that were found to produce compounds that stimulated QS in A. tumefaciens was greater than the number of plants that were found to produce compounds that stimulated QS in the C. violaceum (2 species) and P. syringae (no species) bioreporters. This result was not unexpected as the QS system in A. tumefaciens is rather promiscuous, responding to far more AHLs than the other two bioreporter species respond to (4). The different bioreporters generally detected different compounds. Only extracts from A. africanus and S. mexicana stimulated QS in both C. violaceum and A. tumefaciens. Likewise, leaf surface extracts from nine plant species inhibited QS in either A. tumefaciens or C. violaceum, while leaf surface extracts from only one species inhibited QS in P. syringae; however, none of the extracts except that from P. sativum inhibited QS in more than one bioreporter species. Teplitski et al. (49) also found that the effects of P. sativum root exudates on QS varied; these exudates inhibited one bacterial bioreporter but stimulated other bacterial bioreporters. It is noteworthy that leaf surface extracts from A. maritimum, P. incarnata, and R. trichocalyx caused both stimulation of QS in A. tumefaciens and inhibition of violacein production in C. violaceum. The apparently contradictory activity conferred by these three plant extracts is not inconsistent, since some molecules, such as long-chain AHLs, including C12-HSL, stimulate QS in A. tumefaciens while they inhibit QS in C. violaceum (9, 28). The leaf surface compounds with QS-stimulating activity had features that were distinct from the features of common AHLs. Compounds in leaf surface extracts from I. purpurea, A. archangelica, and O. pes-caprae that stimulated QS in A. tumefaciens did not migrate substantially under the reverse-phase TLC conditions used here. Such molecules clearly are not very hydrophilic and may represent AHLs with very long side chains or other very hydrophobic compounds. Additionally, their inability to inhibit violacein production by C. violaceum suggests either that the concentration was not high enough or that the chemical structure differs substantially from that of more common AHLs. Analysis of these compounds by silica gel TLC with different solvent systems revealed that they clearly were not bacterial AHLs. Further chemical analysis is needed to determine the identities of these compounds.
The release of compounds that interfere with QS onto leaves may differ from the release onto other plant parts. Compounds in P. sativum that interfered with QS differed with both tissue type and plant age. Extracts of seedling roots inhibited violacein synthesis in C. violaceum, as reported previously (49), and also inhibited β-galactosidase production in A. tumefaciens (Table 1). In contrast, extracts from leaves of mature P. sativum plants only slightly inhibited violacein production and did not interfere with QS in A. tumefaciens (Table 1). The differences may have been due either to the different tissues that were examined or to the different ages of the plants. Roots are known to have a higher rate of exudation of many compounds than leaves have (11), and it is possible that the higher levels of compounds that interfere with QS seen in root extracts were due to greater availability in this plant part. Alternatively, plants may alter bacterial quorum sensing only in early stages of growth when the plants are more vulnerable to bacterial attack or need to attract appropriate symbiotic partners, as suggested for the ability of Lotus corniculatus seedlings to inactivate AHLs (5).
Iron sequestration appears to be a very common phenomenon on leaf surfaces. Leaf surface extracts from nearly 30% of the plant species tested triggered siderophore production in P. syringae in LA medium, a culture medium with sufficient iron to repress siderophore biosynthesis in this species. The apparent iron-sequestering compounds also had a relatively high affinity for iron, inhibiting the growth of a P. syringae mutant unable to make its own siderophore for iron acquisition (Fig. 5). These results suggest that epiphytic bacteria must have an effective iron acquisition system in order to grow on plants. Plants might thus influence their microflora by withholding iron and might permit growth on their surfaces of only strains that are able to compete with the plant for iron chelation. Iron-dependent bacterial traits that might be inhibitory to plants, such as phytotoxin production (15) and expression of other virulence factors required for pathogenicity (10), might also be inhibited by such a strategy of iron limitation mediated by such plant compounds.
It is tempting to suggest that the iron-sequestering activities observed in this study were associated with tannins that were released onto the surfaces of the plants. It is noteworthy that purified tannins stimulated siderophore production in wild-type P. syringae and inhibited growth of the siderophore mutant over equal distances from the site of application and to similar extents as many leaf surface extracts (Fig. 5). The iron sequestration capacity of these compounds, which has been recognized previously (30, 43, 46), suggests that these common plant constituents may play a larger role in influencing microbial behavior on plants than previously recognized. Plant-mediated iron deficiency on leaves thus appears to be an important preexisting growth-limiting factor that affects bacterial colonization of plants immediately after the bacteria arrive at a leaf, along with other physical defenses and preexisting plant chemicals.
The release of compounds that interfere with bacterial QS as well as iron sequestration on leaves appears to be quite widespread among plant species. These two phenomena are not highly phylogenetically conserved, since not all plants belonging to a family exhibit the same behavior. Both phenomena are rather important to bacterial existence on leaves since they can govern bacterial growth by iron depravation and expression of coordinated bacterial traits by altering cell density-dependent cell signaling that is essential to epiphytic fitness (37, 38). Given that a linkage between quorum sensing and siderophore production has been shown in species such as Pseudomonas putida and P. aeruginosa (41), it is tempting to speculate that plant compounds that interfere with bacterial quorum sensing might also interfere with the ability of epiphytic bacteria to acquire needed iron on plants with restricted iron levels on their leaves. Clearly, more work with additional bacterial reporter strains and further chemical analysis are needed to better define the nature and function of compounds that interfere with QS. Further examination of the identities and abundance of iron-sequestering compounds on plants should clarify their role in altering bacterial colonization of plants and reveal whether such compounds might provide useful traits to select for in efforts to breed plants that are resistant to bacterial pests.
Acknowledgments
We thank Barbara Keller of the UC Botanical Garden for providing most of the plants tested and A. Molassiotis for assisting in axenic production of some of the plants used in this study.
Footnotes
Published ahead of print on 22 September 2006.
REFERENCES
- 1.Andrews, J. H., and R. F. Harris. 2000. The ecology and biogeography of microorganisms on plant surfaces. Annu. Rev. Phytopathol. 38:145-180. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, W. D., and J. B. Robinson. 2002. Disruption of bacterial quorum sensing by other organisms. Curr. Opin. Biotechnol. 13:234-237. [DOI] [PubMed] [Google Scholar]
- 3.Beattie, G. A., and S. E. Lindow. 1995. The secret life of foliar bacterial pathogens on leaves. Annu. Rev. Phytopathol. 33:145-172. [DOI] [PubMed] [Google Scholar]
- 4.Cha, C., P. Gao, Y.-C. Chen, P. D. Shaw, and S. K. Farrand. 1998. Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. Mol. Plant-Microbe Interact. 11:1119-1129. [DOI] [PubMed] [Google Scholar]
- 5.Cui, X., and R. Harling. 2005. N-Acyl-homoserine lactone-mediated quorum sensing blockage, a novel strategy for attenuating pathogenicity of gram-negative bacterial plant pathogens. Eur. J. Plant Pathol. 111:327-339. [Google Scholar]
- 6.Delalande, L., D. Faure, A. Raffux, S. Uroz, C. D'Angelo-Picard, M. Elasri, A. Carlier, R. Berruyer, A. Petit, P. Williams, and Y. Dessaux. 2005. N-Acyl-l-homoserine lactone, a mediator of bacterial quorum-sensing regulation, exhibits plant-dependent stability and may be inactivated by germinating Lotus corniculatus seedlings. FEMS Microbiol. Ecol. 52:13-20. [DOI] [PubMed] [Google Scholar]
- 7.Duffy, B. K., and G. Défago. 1999. Environmental factors modulating antibiotic and siderophore biosynthesis by Pseudomonas fluorescens biocontrol strains. Appl. Environ. Microbiol. 65:2429-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duffy, B. K., and G. Défago. 1997. Zinc improves biocontrol of Fusarium crown and root rot of tomato by Pseudomonas fluorescens and represses the production of pathogen metabolites inhibitory to bacterial antibiotic biosynthesis. Phytopathology 87:1250-1257. [DOI] [PubMed] [Google Scholar]
- 9.Elasri, M., S. Delorme, P. Lemanceau, G. Stewart, B. Laue, E. Glickman, P. M. Oger, and Y. Dessaux. 2001. Acyl-homoserine lactone production is more common among plant-associated Pseudomonas spp. than among soilborne Pseudomonas spp. Appl. Environ. Microbiol. 67:1198-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Expert, D. 1999. Withholding and exchanging iron: interactions between Erwinia spp. and their plant hosts. Annu. Rev. Phytopathol. 37:307-334. [DOI] [PubMed] [Google Scholar]
- 11.Farrar, J., M. Hawes, D. Jones, and S. Lindow. 2003. How roots control the flux of carbon to the rhizosphere. Ecology 84:827-837. [Google Scholar]
- 12.Fray, R. G. 2002. Altering plant-microbe interaction through artificially manipulating bacterial quorum sensing. Ann. Bot. 89:245-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao, M., M. Teplitski, J. B. Robinson, and W. D. Bauer. 2003. Production of substances by Medicago truncatula that affect bacterial quorum sensing. Mol. Plant-Microbe Interact. 16:827-834. [DOI] [PubMed] [Google Scholar]
- 14.Givskov, M., R. de Nys, M. Manefield, L. Gram, R. Maximilien, L. Eberl, S. Molin, P. D. Steinberg, and S. Kjelleberg. 1996. Eukaryotic interference with homoserine lactone-mediated prokaryotic signaling. J. Bacteriol. 178:6618-6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross, D. C. 1985. Regulation of syringomycin synthesis in Pseudomonas syringae pathovar syringae and defined conditions for its production. J. Appl. Bacteriol. 58:167-174. [DOI] [PubMed] [Google Scholar]
- 16.Hirano, S. S., and C. D. Upper. 2000. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae: a pathogen, ice nucleus, and epiphyte. Microbiol. Mol. Biol. Rev. 64:624-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy, B. S., M. T. Nielsen, R. F. Severson, V. A. Sisson, M. K. Stephenson, and D. M. Jackson. 1992. Leaf surface chemicals from Nicotiana affecting germination of Peronospora tabacina (Adam) sporangia. J. Chem. Ecol. 18:1467-1479. [DOI] [PubMed] [Google Scholar]
- 18.Lindow, S. E. 1995. Control of epiphytic ice nucleation-active bacteria for management of plant frost injury, p. 239-256. In R. E. Lee, G. J. Warren, and L. V. Gusta (ed.), Biological ice nucleation and its applications. APS Press, St. Paul, MN.
- 19.Lindow, S. E., and M. T. Brandl. 2003. Microbiology of the phyllosphere. Appl. Environ. Microbiol. 69:1875-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindow, S. E., C. Desurmont, R. Elkins, G. McGourty, E. Clark, and M. T. Brandl. 1998. Occurrence of indole-3-acetic acid-producing bacteria on pear trees and their association with fruit russet. Phytopathology 88:1149-1157. [DOI] [PubMed] [Google Scholar]
- 21.Litwin, C. M., and S. B. Calderwood. 1993. Role of iron in regulation of virulence genes. Clin. Microbiol. Rev. 6:137-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loper, J. E., and J. S. Buyer. 1991. Siderophores in microbial interactions on plant surfaces. Mol. Plant-Microbe Interact. 4:5-13. [Google Scholar]
- 23.Loper, J. E., and S. E. Lindow. 1987. Lack of evidence for in situ fluorescent pigment production by Pseudomonas syringae pv. syringae on bean leaf surface. Phytopathology 77:1449-1454. [Google Scholar]
- 24.Loper, J. E., and S. E. Lindow. 1994. A biological sensor for iron available to bacteria in their habitats on plant surfaces. Appl. Environ. Microbiol. 60:1934-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mäe, A., M. Montesano, V. Koiv, and E. T. Palva. 2001. Transgenic plants producing the bacterial pheromone N-acyl-homoserine lactone exhibit enhanced resistance to the bacterial phytopathogen Erwinia carotovora. Mol. Plant-Microbe Interact. 14:1035-1042. [DOI] [PubMed] [Google Scholar]
- 26.Marco, M. L., J. Legac, and S. E. Lindow. 2005. Pseudomonas syringae genes induced during colonization of leaf surfaces. Environ. Microbiol. 7:1379-1391. [DOI] [PubMed] [Google Scholar]
- 27.Mathesius, U., S. Mulders, M. Gao, M. Teplitski, G. C. Anollés, B. G. Rolfe, and W. D. Bauer. 2003. Extensive and specific responses of a eukaryote to bacterial quorum-sensing signals. Proc. Natl. Acad. Sci. USA 100:1444-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClean, K. H., M. K. Winson, L. Fish, A. Taylor, S. R. Chhabra, M. Camara, M. Daykin, J. H. Lamb, S. Swift, B. W. Bycroft, G. S. A. B. Stewart, and P. Williams. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703-3711. [DOI] [PubMed] [Google Scholar]
- 29.McLean, R. J. C., L. S. Pierson III, and C. Fuqua. 2004. A simple protocol for the identification of quorum sensing signal antagonists. J. Microbiol. Methods 58:351-360. [DOI] [PubMed] [Google Scholar]
- 30.Mila, I., A. Scalbert, and D. Expert. 1996. Iron withholding by plants polyphenols and resistance to pathogens and rots. Phytochemistry 42:1551-1555. [Google Scholar]
- 31.Mo, Y. Y., and D. C. Gross. 1991. Plant signal molecules activate the syrB gene, which is required for syringomycin production by Pseudomonas syringae pv. syringae. J. Bacteriol. 173:5784-5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morello, J. E., E. A. Pierson, and L. S. Pierson. 2004. Negative cross-communication among wheat rhizosphere bacteria: effect on antibiotic production by the biological control bacterium Pseudomonas aureofaciens 30-84. Appl. Environ. Microbiol. 70:3103-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neilands, J. B., and S. A. Leong. 1986. Siderophores in relation to plant growth and disease. Annu. Rev. Plant Physiol. 37:187-208. [Google Scholar]
- 34.Paulitz, T. C., and J. E. Loper. 1991. Lack of a role for fluorescent siderophore production in the biological control of pythium damping-off of cucumber by a strain of Pseudomonas putida. Phytopathology 81:930-935. [Google Scholar]
- 35.Pierson, E. A., D. W. Wood, J. A. Cannon, and F. M. Blachere III. 1998. LSP: interpopulation signaling via N-acyl-homoserine lactones among bacteria in the wheat rhizosphere. Mol. Plant-Microbe Interact. 11:1078-1084. [Google Scholar]
- 36.Pierson, L. S., III, W. W. Derek, and A. Pierson. 1998. Homoserine lactone-mediated gene regulation in plant-associated bacteria. Annu. Rev. Phytopathol. 36:207-225. [DOI] [PubMed] [Google Scholar]
- 37.Quiñones, B., C. J. Pujol, and S. E. Lindow. 2004. Regulation of AHL production and its contribution to epiphytic fitness in Pseudomonas syringae. Mol. Plant-Microbe Interact. 17:521-531. [DOI] [PubMed] [Google Scholar]
- 38.Quiñones, B., G. Dulla, and S. E. Lindow. 2005. Quorum sensing regulates exopolysaccharide production, motility, and virulence in Pseudomonas syringae. Mol. Plant-Microbe Interact. 18:682-693. [DOI] [PubMed] [Google Scholar]
- 39.Rasmussen, T. B., T. Bjarnsholt, M. E. Skindersoe, M. Hentzer, P. Kristoffersen, M. Köte, J. Nielsen, L. Eberl, and M. Givskov. 2005. Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J. Bacteriol. 187:1799-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasmussen, T. B., M. E. Skindersoe, T. Bjarnsholt, R. K. Phipps, K. B. Christensen, P. O. Jensen, J. B. Andersen, B. Koch, T. O. Larsen, M. Hentzer, L. Eberl, N. Hoiby, and M. Givskov. 2005. Identity and effects of quorum-sensing inhibitors produced by Penicillium species. Microbiol. 151:1325-1340. [DOI] [PubMed] [Google Scholar]
- 41.Ren, D., R. Zuo, and T. K. Wood. 2005. Quorum-sensing antagonist (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone influences siderophore biosynthesis in Pseudomonas putida and Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 66:689-695. [DOI] [PubMed] [Google Scholar]
- 42.Savka, M. A., Y. Dessaux, P. Oger, and S. Rossbach. 2002. Engineering bacterial competitiveness and persistence in the phyllosphere. Mol. Plant-Microbe Interact. 15:866-874. [DOI] [PubMed] [Google Scholar]
- 43.Scalbert, A. 1991. Antimicrobial properties of tannins. Biochemistry 30:3875-3883. [Google Scholar]
- 44.Shaw, P. D., G. Ping, S. L. Daly, C. Cha, J. E. Cronan, Jr., K. L. Rinehart, and S. K. Farrand. 1997. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA 94:6036-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shepherd, R. W., W. T. Bass, R. L. Houtz, and G. J. Wagner. 2005. Phylloplanins of tobacco are defensive proteins deployed on aerial surfaces by short glandular trichomes. Plant Cell 17:1851-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.South, P. K., and D. D. Miler. 1998. Iron binding by tannic acid: effects of selected ligands. Food Chem. 63:167-172. [Google Scholar]
- 47.Swift, S., A. Karlyshev, L. Fish, E. L. Durant, M. K. Winson, S. R. Chhabra, P. Williams, S. MacIntyre, and G. S. A. B. Stewart. 1997. Quorum sensing in Aeromonas hydrophila and Aeromonas salmonicida: identification of the LuxRI homologs AhyRI and AsaRI and their cognate N-acylhomoserine lactone signal molecules. J. Bacteriol. 179:5271-5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teplitski, M., H. Chen, S. Rajamani, M. Gao, M. Merighi, R. T. Sayre, J. B. Robinson, B. G. Rolfe, and W. D. Bauer. 2004. Chlamydomonas reinhardtii secretes compounds that mimic bacterial signals and interfere with quorum sensing regulation in bacteria. Plant Physiol. 134:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teplitski, M., J. B. Robinson, and W. D. Bauer. 2000. Plants secrete substances that mimic bacterial N-acyl-homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol. Plant-Microbe Interact. 13:637-646. [DOI] [PubMed] [Google Scholar]
- 50.Toth, I. K., J. A. Newton, L. J. Hyman, A. K. Lees, M. Daykin, C. Ortori, P. Williams, and R. G. Fray. 2004. Potato plants genetically modified to produce N-acylhomoserine lactones increase susceptibility to soft rot erwiniae. Mol. Plant-Microbe Interact. 17:880-887. [DOI] [PubMed] [Google Scholar]
- 51.Von Bodman, S. B., W. D. Bauer, and D. L. Coplin. 2003. Quorum sensing in plant-pathogenic bacteria. Annu. Rev. Phytopathol. 41:455-482. [DOI] [PubMed] [Google Scholar]
- 52.Weinberg, E. D. 1984. Iron withholding: a defense against infection and neoplasia. Physiol. Rev. 64:65-102. [DOI] [PubMed] [Google Scholar]
- 53.Whitehead, N. A., A. M. L. Barnard, H. Slater, N. J. L. Simpson, and G. P. C. Salmond. 2001. Quorum-sensing in gram-negative bacteria. FEMS Microbiol. Rev. 25:365-404. [DOI] [PubMed] [Google Scholar]