Abstract
Interleukin-10 (IL-10) is a promising candidate for the treatment of inflammatory bowel disease. Intragastric administration of Lactococcus lactis genetically modified to secrete IL-10 in situ in the intestine was shown to be effective in healing and preventing chronic colitis in mice. However, its use in humans is hindered by the sensitivity of L. lactis to freeze-drying and its poor survival in the gastrointestinal tract. We expressed the trehalose synthesizing genes from Escherichia coli under control of the nisin-inducible promoter in L. lactis. Induced cells accumulated intracellular trehalose and retained nearly 100% viability after freeze-drying, together with a markedly prolonged shelf life. Remarkably, cells producing trehalose were resistant to bile, and their viability in human gastric juice was enhanced. None of these effects were seen with exogenously added trehalose. Trehalose accumulation did not interfere with IL-10 secretion or with therapeutic efficacy in murine colitis. The newly acquired properties should enable a larger proportion of the administered bacteria to reach the gastrointestinal tract in a bioactive form, providing a means for more effective mucosal delivery of therapeutics.
The growing knowledge of the biochemistry of the human body has led to the development of many new biologicals for the treatment of a variety of diseases. However, in contrast to traditional synthetic compounds, the bioavailability of many of these new drugs following oral administration is too low to induce a clinical response. This is mainly related to their high susceptibility to proteolysis within the intestine. Therefore, there is a need for oral delivery methods that can circumvent these obstacles. Previously, we genetically modified Lactococcus lactis as an effective vehicle for oral delivery of bioactive proteins to treat inflammatory bowel disease (7, 35, 41).
Because impairment of interleukin-10 (IL-10) function is involved in the pathogenesis of inflammatory bowel disease (25, 34), this cytokine is a promising candidate for treatment of Crohn's disease (CD) (42). However, administering IL-10 systemically to CD patients has not been clinically effective (14, 32, 33, 37). Local delivery of IL-10 by L. lactis, which results in high therapeutic concentrations at the site of inflammation, is a promising therapeutic approach (35, 41). For application in humans, a biologically contained L. lactis strain secreting human IL-10 (hIL-10) (36) was developed and evaluated as a treatment for CD patients (7). This phase I, open-label clinical trial demonstrated for the first time that treatment of humans with L. lactis secreting hIL-10 is clinically safe and biologically contained and that it is a realistic therapeutic option. Because reduced viability in the human gastrointestinal (GI) tract is a problem inherent in the use of L. lactis as a delivery vehicle (23, 43), an oral pharmacological formulation was developed for this clinical study (20). Although this formulation, based on freeze-drying (9), protects L. lactis from the detrimental GI environment, it also significantly reduces viability of L. lactis (20, 38). The addition of cryoprotectants and compatible solutes before freeze-drying has been reported to improve viability, but the effect remained marginal (38). Trehalose is a nonreducing disaccharide frequently used as an externally added cryoprotectant. It is commonly produced by fungi, as well as by some bacterial species, plants, and animals (13). Trehalose levels have been shown to correlate very well with cellular stress resistance, e.g., in Saccharomyces cerevisiae (4). Escherichia coli and Pseudomonas putida were shown to fully withstand vacuum drying at 30°C, but only when trehalose was present both inside and outside the cell (16). Also for Lactobacillus acidophilus, the recovery after freeze-drying and storage at 37°C was markedly increased in the presence of 30% trehalose (10). Survival of Lactobacillus acidophilus after several freezing and thawing cycles in the presence of trehalose was shown to depend for the larger part upon its internalization (12). Carvalho et al. reviewed the relevant factors, among which was the presence of trehalose, for the preparation of freeze-dried lactic acid bacteria (LAB) (8). Their main conclusion is that optimum protocols vary widely between species and even between strains. Largely the same conclusion was reached in a recent review also exploring other preservation methods and strains other than LAB (30). Blast analysis of E. coli otsA and otsB genes (22) with the complete genome sequence of L. lactis subsp. lactis IL-1403 (6) revealed no evidence for a trehalose biosynthesis pathway in L. lactis. Here, we demonstrate that trehalose production can be induced in genetically modified L. lactis and that the resulting trehalose accumulation leads to nearly 100% viability following prolonged storage in a freeze-dried form. We further report that trehalose accumulation is responsible for resistance to bile and enhanced viability in human gastric juice and that it does not interfere with the therapeutic efficacy of L. lactis secreting IL-10.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used in the present study are listed in Table 1. Bacteria were routinely grown as standing cultures at 30°C in M17 broth (Difco, Detroit, MI) supplemented with 0.5% glucose and 5 μg/ml chloramphenicol when appropriate (GM17C). Stock suspensions of L. lactis strains were stored at −20°C in 50% glycerol in GM17C.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Reference |
|---|---|---|
| Strains | ||
| E. coli DH5α | K12; source of otsBA genes | 18 |
| L. lactis | ||
| MG1363 | Plasmid-free derivative of SH4109 | 17 |
| NZ9000 | MG1363; pepN::nisRK | 26 |
| Plasmids | ||
| pNZ8048 | PnisA; Cmr | 26 |
| pTre1 | PnisA with otsBA; Cmr | This study |
| pT1hIL10v1 | P1 with usp45-hIL10v1; Ermr | 36 |
| pTre1hIL10v1 | PnisA with otsBA; P1 with usp45-hIL10v1; Cmr | This study |
Cmr, chloramphenicol resistance; Ermr, erythromycin resistance; hIL10v1, modified coding region of mature hIL-10 (see text).
Plasmid pT1hIL10v1 contains the coding region of mature hIL-10 fused to the lactococcal usp45 secretion leader (40), preceded by the coliphage T7 gene 10-ribosome binding site and the lactococcal P1 promoter (44). The sequence of the hIL10v1 coding region is a synthetic one, adapted to the preferred codon usage in L. lactis (15) and with an alanine residue replacing the proline that is in the first position in the mature native hIL-10 (36).
Plasmid constructions.
DNA sequences encoding the trehalose biosynthesis genes in E. coli were retrieved from GenBank (accession no. X69160) (22). E. coli strain DH5α was the source of the trehalose biosynthesis genes otsA and otsB, encoding trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase, respectively. Genomic DNA was purified with a QIAGEN DNeasy kit (Hilden, Germany). The DNA sequence encompassing the otsBA genes, together with primer sequences containing suitable restriction sites for insertion into pNZ8048, were PCR amplified with Vent DNA polymerase (New England Biolabs, Ipswich, MA). The amplified 2,216-bp DNA fragment contained a 5′ NcoI site overlapping the ATG start codon of the otsB cistron. An XbaI site was introduced downstream of the otsA cistron. Insertion of this NcoI-XbaI fragment into pNZ8048 yielded plasmid pTre1, in which the coding sequence of otsB is fused in frame with the initiator ATG of the nisA ribosome binding site. The region encompassing the nisA promoter, the nisA ribosome binding site, and the junction of the initiator ATG with the otsB cistron, was verified by DNA sequencing.
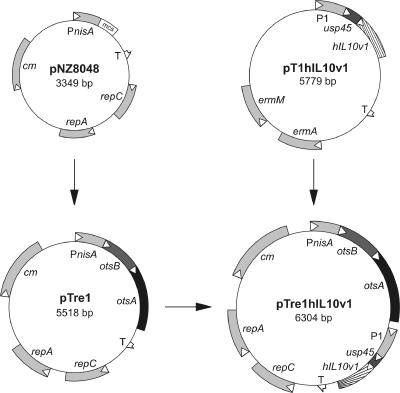
To construct pTre1hIL10v1, we first used Vent DNA polymerase and pT1hIL10v1 as template to amplify the hIL-10 expression cassette with primers containing 5′ and 3′ SpeI restriction sites. The amplified 796-bp DNA fragment was digested with SpeI and ligated into XbaI-opened pTre1. The orientation of the insert was verified by DNA sequencing. Functional maps of the lactococcal expression plasmids are depicted in Fig. 1. L. lactis was transformed by electroporation as previously described (45).
FIG. 1.
Functional maps of the L. lactis expression plasmids. PnisA, inducible nisA promoter; mcs, multiple cloning site; T, transcription terminator; repC and repA, replication genes; cm, chloramphenicol resistance gene; P1, lactococcal constitutive promoter; usp45, lactococcal secretion leader; hIL10v1, human interleukin-10 gene v1; ermA and ermM, erythromycin resistance genes; otsB, trehalose-6-phosphate phosphatase gene; otsA, trehalose-6-phosphate synthase gene.
Intracellular trehalose quantification.
The concentration of trehalose was determined in an enzymatic colorimetric assay by converting trehalose to glucose with trehalase and then measuring the glucose (39). After induction, cells were collected by centrifugation. In order to avoid interference of residual glucose from the medium during the enzymatic trehalose assay, care was taken to completely remove the supernatant from the tubes. The cells were lysed with lysozyme (5 mg/ml) and mutanolysin (100 U/ml) in 0.25 M Na2CO3 for 1 h at 37°C and for 20 min at 95°C. Cell debris was removed by centrifugation. The supernatant was combined with a 0.5 volume of 1 M acetic acid and a 0.5 volume of a buffer consisting of 300 mM sodium acetate and 30 mM CaCl2 (pH 5.5). The mixture was incubated for 2 h at 37°C in the presence of trehalase. Following centrifugation, the supernatant was supplemented with Trinder reagent (glucose oxidase, phenol, and 4-aminophenazone; Dialab, Vienna, Austria) and incubated with shaking for 15 min at 30°C, after which the optical density at 505 nm was automatically recorded in a 96-well VersaMax Tunable Microplate Reader (Molecular Devices, Sunnyvale, CA). Trehalose concentrations were read from a calibration curve obtained with pure trehalose (Sigma, St. Louis, MO); the optical density at 505 nm and trehalose concentration are linearly correlated up to 5 mM trehalose.
Freeze-drying L. lactis cultures.
Bacteria were collected by centrifugation, resuspended in the original volume of 10% (wt/vol) skim milk (Difco), and kept on ice until they were freeze-dried as previously described (20). The vials containing freeze-dried L. lactis cultures were stored under different conditions: low or high temperature (8°C or 25°C) and low or high relative humidity (RH). Ten percent RH was reached by opening the vials and placing them above silica gel for desiccation in a closed container. Sixty percent RH was reached by placing the open vials above a saturated sodium bromide solution.
Viability determination.
Viability of bacteria after freeze-drying and after storage was determined as previously described (20). Bacteria were resuspended in sterile water supplemented with oxgall (Difco) or human gastric juice (postoperative samples with a pH of 1.69 and 2.95), and viability was determined by plating. All dilution series were plated in duplicate on GM17C agar plates and incubated for 24 h at 30°C before colonies were counted (CFU).
hIL-10 quantification.
A sandwich enzyme-linked immunosorbent assay was used to quantify hIL-10 in reconstituted freeze-dried L. lactis powder. hIL-10 was captured from the medium by immobilized polyclonal rat anti-hIL-10 antibody (BD Pharmingen, Franklin Lakes, NJ), quantified by an anti-hIL-10 biotin-coupled rat monoclonal antibody (BD Pharmingen), and revealed with horseradish peroxidase-coupled streptavidin (BD Pharmingen) and TMB (3,3′,5,5′-tetramethylbenzidine) substrate (BD Pharmingen).
Animals.
Female BALB/c mice 11 weeks of age were obtained from Charles River Laboratories Italia S.r.l. (Calco, Italy). They were housed in a specific-pathogen-free animal facility and fed standard laboratory feed and tap water ad libitum. The animal studies were approved by the Ethics Committee of the Department for Molecular Biomedical Research, Ghent University (file no. 04/02).
Induction of chronic colitis by DSS.
Chronic colitis was induced with dextran sodium sulfate (DSS) as previously described (24, 31). Briefly, mice of 15 weeks and weighing approximately 21 g were given 5% DSS (40 kDa; Applichem, Darmstadt, Germany) as drinking water for 7 days, followed by 10 days of normal drinking water. This cycle was repeated four times. On day 21 after the fourth cycle, the daily intragastric administration of 2 × 109 CFU of L. lactis began for 14 days, as previously described (35).
Histological analysis.
The colon was removed, cleaned, and opened longitudinally. A segment of 1 cm was taken from the distal part of the colon, embedded in paraffin, and sectioned longitudinally. Three sections of 4 μm were cut at 200-μm intervals and stained with hematoxylin and eosin. Colon sections were numbered randomly and interpreted semiquantitatively in a blinded manner. The histological score is the sum of the epithelial damage and lymphoid infiltration, each ranging from 0 to 4, as previously described (24).
Statistical analysis.
Data were statistically analyzed with SPSS 12.0 for Windows (SPPS Inc., Chicago, IL). All viability data are expressed as the means ± standard deviations (SDs). Freeze-drying experiments were performed in duplicate unless stated otherwise. The viability of freeze-dried cells of noninduced L. lactis NZ9000(pTre1) and induced NZ9000(pTre1) was normally distributed (Shapiro-Wilk test). Differences were analyzed by a two-sided, unequal variance, independent samples t test where n1 = n2, which renders the independent samples t test insensitive to unequal variances and avoids inflated type I error.
Histological scores, expressed as means ± standard errors of the means, are normally distributed (Shapiro-Wilk test). They were analyzed by one-way analysis of variance followed by a Bonferroni multiple comparisons post hoc test.
RESULTS AND DISCUSSION
Heterologous expression of the otsBA operon in L. lactis NZ9000.
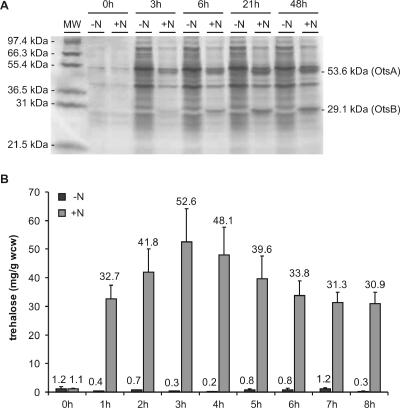
In E. coli the synthesis of trehalose is a two-step process. In the first step, catalyzed by trehalose-6-phosphate synthase (OtsA), trehalose-6-phosphate is synthesized from UDP glucose and glucose-6-phosphate. In the second step, trehalose-6-phosphate is dephosphorylated by trehalose-6-phosphate phosphatase (OtsB). We cloned the E. coli otsBA trehalose biosynthesis operon (22) under control of the nisin-inducible promoter in pNZ8048 (26) to produce plasmid pTre1. To evaluate inducible expression of otsA and otsB, an overnight culture of L. lactis NZ9000(pTre1) was diluted 100-fold in GM17C medium and further incubated at 30°C. After 3 h, when the culture reached the logarithmic phase, the cells were resuspended in the original volume of BM9 medium (35) supplemented with chloramphenicol. Nisin was added to a final concentration of 0.4 μg/ml, and the cultures (induced and noninduced) were further incubated at 30°C for up to 48 h. At several time points, culture samples were taken, and cell fractions were collected, lysed, and subsequently analyzed for protein expression. Following sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining of total cell lysates, two additional protein bands, whose molecular masses of 53.6 kDa and 29.1 kDa are in agreement with those of OtsA and OtsB, respectively, were revealed in the induced culture (Fig. 2A). From the intensities of the protein bands, it is apparent that overall protein synthesis of nisin-treated NZ9000(pTre1) was severely affected soon after induction. Comparative growth curves showed that NZ9000(pTre1) cultures virtually stopped growing as soon as 3 h after the addition of nisin. Noninduced cultures displayed essentially similar growth rates as the controls, i.e., plasmid-free NZ9000 or NZ9000(pNZ8048), with or without nisin (data not shown). In order to remedy this problem, we set up several pregrowth and induction regimes (data not shown), finally resulting in an optimized protocol, in which growth inhibition was minimal. To this end, the bacteria were grown overnight as standing cultures at 30°C in GM17C. Saturated cultures were diluted threefold with fresh medium containing 0.4 μg/ml nisin and further incubated at 30°C with orbital shaking at 200 rpm for 3 h, at which point saturation was reached. The OtsA and OtsB enzymes were functional in L. lactis, as evidenced by intracellular accumulation of trehalose with a tendency to peak at approximately 50 mg/g of wet cell weight after 2 to 3 h of nisin addition (Fig. 2B).
FIG. 2.
Time course of heterologous otsBA expression and trehalose accumulation by L. lactis NZ9000(pTre1). (A) Coomassie blue staining of a 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel of total cell lysates extracted at different time points from noninduced (−N) and nisin induced (+N) NZ9000(pTre1) cultures. Cells collected from an equal volume of culture were loaded onto each lane. MW, molecular weight marker. (B) Trehalose quantification in cell fractions isolated at different time points from noninduced (−N) and nisin induced (+N) NZ9000(pTre1) cultures. wcw, wet cell weight.
Intracellular trehalose accumulation in L. lactis is essential for nearly 100% viability after freeze-drying.
Because trehalose is frequently used as a cryoprotectant to improve viability after freeze-drying, we also evaluated the impact of exogenously supplied trehalose on the viability of L. lactis. Before freeze-drying, cells were collected by centrifugation, resuspended in 10% skim milk, which is the most commonly used freeze-drying matrix for LAB (9), and freeze-dried as previously described (20). After cells were freeze-dried, the viability of noninduced NZ9000(pTre1) was 56.7% ± 9.6% (n = 18), whereas that of induced NZ9000(pTre1) was markedly higher at 94.0% ± 14.2% (n = 18). Moreover, even in the absence of skim milk, the viability of induced NZ9000(pTre1) cells remained at nearly 100%, whereas the viability of noninduced NZ9000(pTre1) cells dropped to 20%. Furthermore, the absence of glucose during the logarithmic phase growth of induced NZ9000(pTre1) resulted in a substantially lower intracellular trehalose concentration (4.2 mg/g ± 0.06 mg/g) and, consequently, in decreased viability after freeze-drying (61.6% ± 1.9%), demonstrating that the intracellular accumulation of trehalose mediates the protective effect. It was shown that the extracellular addition of up to 3.5% trehalose before freeze-drying significantly increased the viability of E. coli (27). Duong et al. recently reported that a 1-h preincubation at 37°C in the presence of either 20% or 10% trehalose allowed Lactobacillus acidophilus to retain full CFU count after 12 and 6 cycles, respectively, of repeated freezing and thawing (12). They further showed that not only the internalization of trehalose via a phosphotransferase system but also its subsequent hydrolysis by a trehalose-6-phosphate hydrolase (treC) contributes to the observed protection. Extracellular addition of trehalose (7%) to noninduced NZ9000(pTre1) cells before freeze-drying or during logarithmic growth (0.5%) did not improve their viability. Andersson et al. characterized a novel metabolic pathway for trehalose utilization in L. lactis subsp. lactis, involving the enzymes trehalose-6-phosphate phosphorylase and β-phosphoglucomutase (1). In a later study including 40 LAB strains, they reported that the trehalose-6-phosphate phosphorylase/β-phosphoglucomutase pathway is crucial for trehalose utilization in all examined L. lactis strains, including the L. lactis subsp. cremoris strain MG1363 (3). According to the genome sequence of L. lactis subsp. lactis IL-1403 (6) both genes are part of a trehalose utilization operon which further encompasses predicted genes for a transcriptional regulator and two trehalose-specific components of a phosphotransferase system (2). There is no evidence for a treC gene as present in Lactobacillus acidophilus. Possibly, the difference between Lactobacillus acidophilus and L. lactis in catabolic pathways of internalized trehalose contributes to the observed difference in cryoprotection offered by external trehalose. Obviously, both strains would have to be compared under identical experimental conditions in order to further substantiate this possibility. When noninduced NZ9000(pTre1) cells were grown to saturation with trehalose as the sole carbon source, we could not detect intracellular trehalose in the cells. Our results suggest that only de novo trehalose synthesis by L. lactis in the presence of glucose can yield internal trehalose levels sufficient to sustain almost full viability after freeze-drying.
Viability of trehalose-accumulating freeze-dried L. lactis after prolonged storage.
A pharmacological formulation should not only maintain high viability after freeze-drying but also have an acceptable shelf life. Induced NZ9000(pTre1) and noninduced NZ9000(pTre1) freeze-dried cells were tested for stability after storage under various conditions of temperature and RH. To compare viability data after storage, relative viability was calculated as a function of viability directly after freeze-drying, since this viability was batch dependent. In all tested conditions, induced NZ9000(pTre1) cells had a longer shelf life than noninduced NZ9000(pTre1) cells. When stored at 8°C and 10% RH, induced NZ9000(pTre1) cells retained almost 100% viability for at least 1 month (Table 2).
TABLE 2.
Influence of storage conditions on viability of freeze-dried L. lactis strains
| Storage conditions (°C/%RH) | Viability (%) in indicated cells after storage for the indicated perioda
|
|||
|---|---|---|---|---|
| 1 wk
|
1 mo
|
|||
| Noninduced NZ9000(pTre1) | Induced NZ9000(pTre1) | Noninduced NZ9000(pTre1) | Induced NZ9000(pTre1) | |
| 8/10 | 100.2 ± 4.1 | 91.3 ± 18.0 | 78.6 ± 0.04b | 104.7 ± 12.1b |
| 8/60 | 33.3 ± 1.3c | 95.6 ± 15.9c | 13.0 ± 0.03d | 81.6 ± 10.3d |
| 25/10 | 45.8 ± 7.4 | 62.3 ± 10.6 | 18.8 ± 4.0d | 45.9 ± 2.4d |
| 25/60 | 1.1 ± 0.03b | 6.8 ± 2.9b | 0.5 ± 0.2 | 1.5 ± 0.6 |
Viability (mean ± SD) after storage was calculated as a percentage of the viability of the culture directly after freeze-drying (relative viability).
Significant difference between viability of noninduced NZ9000(Tre1) and induced NZ9000(Tre1) cells after storage with P values of <0.05 (n = 3).
Significant difference between viability of noninduced NZ9000(Tre1) and induced NZ9000(Tre1) cells after storage with P values of <0.005 (n = 3).
Significant difference between viability of noninduced NZ9000(Tre1) and induced NZ9000(Tre1) cells after storage with P values of <0.0005 (n = 3).
Intracellular trehalose accumulation increases resistance of freeze-dried L. lactis to bile.
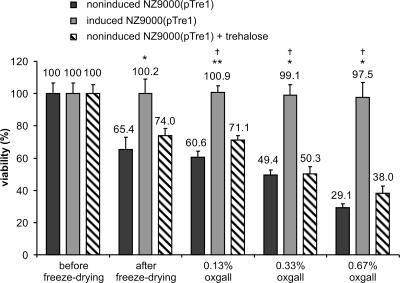
Survival in the human GI tract is one of the more important considerations in L. lactis-mediated delivery of therapeutic proteins (35). Resistance to bile is an important criterion in selecting probiotic strains, and numerous studies have shown that this is highly strain specific (5). L. lactis strains are not only in the very low range of bile resistance, but they are also very sensitive to gastric acidity. Following oral administration of L. lactis subsp. cremoris MG1363, only 1% of the inoculum was recovered alive in the terminal ileum of humans (43). Incorporation of freeze-dried L. lactis in enteric-coated capsules (20) designed to release their contents at near neutral pH in the small intestine can ensure safe transit through the stomach and duodenum, but this does not avoid contact with detrimental bile concentrations in the ileum. In the search for probiotic strains, the influence of GI secretions on survival has been studied in LAB species obtained from the intestine and from other sources (21, 28). None of these studies considered viable recovery of freeze-dried cells upon rehydration in the presence of bile salts or at very low pH. Therefore, we examined the ability of induced NZ9000(pTre1) freeze-dried cells to withstand bile salts. We used oxgall concentrations of 0.13%, 0.33%, and 0.67%, which correspond to physiological bile concentrations in the human terminal ileum, jejunum, and duodenum, respectively (19). Noninduced NZ9000(pTre1) cells with or without 7% extracellular trehalose added before freeze-drying showed a similar, bile concentration-dependent drop in viability that was essentially complete within minutes after resuspension. Exposure of noninduced NZ9000(pTre1) freeze-dried cells to 0.13%, 0.33%, and 0.67% oxgall reduced their viability to 60.6%, 49.4%, and 29.1%, respectively (Fig. 3). On the other hand, induced NZ9000(pTre1) cells maintained 100% viability in all tested oxgall concentrations during the 4-h incubation period. In conclusion, the acquired resistance to bile toxicity was absolutely dependent on intracellular accumulation of trehalose before the freeze-drying step. Because trehalose-accumulating bacteria acquire full resistance to high concentrations of bile, it becomes possible to release bacteria secreting a therapeutic agent in the upper part of the small intestine. This new feature also enables a larger proportion of the administered bacteria to reach their target in a bioactive form, providing a more effective mucosal delivery of therapeutics.
FIG. 3.
Viability of freeze-dried L. lactis strains after bile challenge. Viability (mean ± SD) was calculated as a percentage of the viability of the cultures before freeze-drying. Freeze-dried cells [induced NZ9000(pTre1), noninduced NZ9000(pTre1), and noninduced NZ9000(pTre1) cells supplemented with 7% extracellular trehalose before freeze-drying] were incubated for 4 h at 37°C in 0.13%, 0.33%, or 0.67% oxgall, respectively. Statistically significant differences between the viability of induced NZ9000(pTre1) and noninduced NZ9000(pTre1) cells (*, P < 0.05; **, P < 0.01) and of induced NZ9000(pTre1) and noninduced NZ9000(pTre1) cells supplemented with 7% exogenous trehalose (†, P < 0.05) are indicated.
Intracellular trehalose accumulation enhances gastric acid resistance of freeze-dried L. lactis.
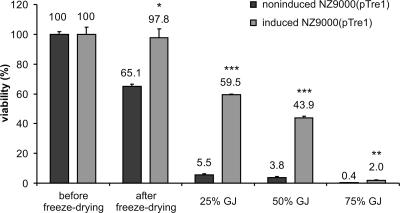
Besides bile salts, gastric acidity also markedly influences the viability of L. lactis during passage through the GI tract. To evaluate acid resistance, we rehydrated freeze-dried cells in the presence of different concentrations of human gastric juice. Not unexpectedly, both noninduced NZ9000(pTre1) and induced NZ9000(pTre1) cells suffered a dramatic reduction in viability in 75% gastric juice (Fig. 4). At intermediate concentrations, the viability of induced NZ9000(pTre1) cells was about 10 times higher than that of the control (59.5% versus 5.5%, respectively, in 25% gastric juice and 43.9% versus 3.8%, respectively, in 50% gastric juice). These data show that internal trehalose accumulation also partially protects freeze-dried L. lactis against the high acidity of human gastric juice. Although accumulation of trehalose provided little protection during a 30-min in vitro incubation in 75% human gastric juice, existing evidence suggests that the in vivo survival in the stomach could possibly be improved by an appropriate administration protocol. For example, administration of L. lactis subsp. lactis IL-1403 together with food was shown to increase the organism's survival in the rat stomach about 15-fold (11). Because trehalose accumulation can ensure safe transit of L. lactis through the stomach and duodenum, this bacterium may be amenable to pharmacological formulations other than enteric-coated capsules.
FIG. 4.
Viability of freeze-dried L. lactis strains after human gastric juice challenge. Viability (mean ± SD) was calculated as a percentage of the viability of the cultures before freeze-drying. Freeze-dried cells [noninduced NZ9000(pTre1)and induced NZ9000(pTre1)] were incubated for 30 min at 37°C in 25%, 50%, or 75% human gastric juice (pH 2.95). Statistically significant differences between the viability of induced NZ9000(pTre1) and noninduced NZ9000(pTre1) cells are indicated as follows: *, P < 0.05; **, P < 0.005; and ***, P < 0.001. These data are representative for the data obtained from the second experiment with gastric juice of pH 1.69.
Intracellular trehalose accumulation in L. lactis does not interfere with IL-10 secretion after freeze-drying and rehydration.
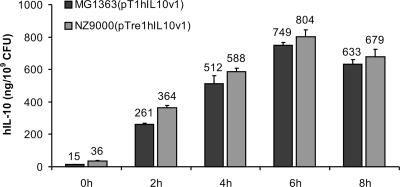
The major goal of the present study is optimization of the L. lactis-based topical delivery of therapeutics to the intestinal mucosa. In order to determine whether induced NZ9000(pTre1) freeze-dried cells retain their capacity to secrete IL-10 following rehydration, we cloned the expression cassette for hIL-10 in plasmid pTre1 and transformed the new plasmid into L. lactis. The resulting strain, L. lactis NZ9000(pTre1hIL10v1), allowed nisin-inducible intracellular accumulation of trehalose and constitutive expression of hIL-10 under control of the lactococcal P1 promoter (44). As expected, NZ9000(pTre1hIL10v1) cells retained nearly 100% viability after freeze-drying (data not shown). Next we compared the hIL-10 secreting capacity of nisin-induced NZ9000(pTre1hIL10v1) cells to that of MG1363(pT1hIL10v1) cells (Fig. 5). Upon rehydration of the freeze-dried cultures, secretion of hIL-10 started immediately and reached a maximum after 6 h of incubation at 37°C in both strains. When expressed as the amount of IL-10 per CFU recovered after freeze-drying and rehydration, both strains secreted almost equal amounts of hIL-10 in the reconstituted medium. Thus, accumulation of trehalose before freeze-drying had no influence on the hIL-10 secretory capacity of L. lactis after freeze-drying.
FIG. 5.
Secretion of hIL-10 by MG1363(pT1hIL10v1) and nisin-induced NZ9000(pTre1hIL10v1) cells after freeze-drying. Freeze-dried cells were reconstituted with a solution consisting of 25 mM Na2CO3, 25 mM NaHCO3, and 0.5% glucose. hIL-10 production (mean ± SD) is expressed as ng/109 CFU.
Therapeutic efficacy of trehalose-accumulating L. lactis secreting IL-10 against murine colitis is maintained.
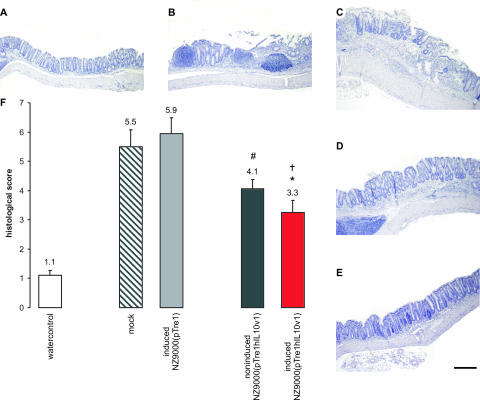
To investigate whether intracellular trehalose accumulation influences the therapeutic effect of IL-10 delivered by L. lactis, we used a model for chronic colitis induced by DSS (24, 31). Mice with chronic DSS-induced colitis were treated daily and examined as previously described (35). Healthy control mice (n = 10) received normal drinking water throughout the experiment and had a histological score of 1.1 ± 0.2 (Fig. 6). Mock-treated animals (n = 9) received BM9 medium daily, which resulted in a histological score of 5.5 ± 0.6. L. lactis-treated groups received daily intragastric inocula of induced NZ9000(pTre1) (n = 9), noninduced NZ9000(pTre1hIL10v1) (n = 9), or induced NZ9000(pTre1hIL10v1) (n = 10) cells resuspended in BM9 medium. The induced NZ9000(pTre1) control group had a histological score of 5.9 ± 0.5. The histological scores of the groups treated with induced or noninduced NZ9000(pTre1hIL10v1) cells were 3.3 ± 0.4 and 4.1 ± 0.3, representing a reduction in inflammation to, respectively, 56% and 69% of the control group treated with induced NZ9000(pTre1). Therefore, IL-10-producing L. lactis cells that had accumulated trehalose maintained essentially the same curative effect on DSS-induced colitis as found after treatment with trehalose-free bacteria.
FIG. 6.
Analysis of morbidity in chronic DSS-induced colitis. (A to E) Representative histology of the distal colon (hematoxylin and eosin staining) in healthy control mice (A) and in mice with chronic DSS-induced colitis either mock-treated (B) or treated with induced NZ9000(pTre1) (C), noninduced NZ9000(pTre1hIL10v1) (D), or induced NZ9000(pTre1hIL10v1) (E). Human IL-10 acts on both human and murine cells (29). Scale bar, 200 μm. (F) Statistical analysis of the histological score (mean ± standard error of the mean) of the distal colon in chronic DSS-induced colitis. Statistically significant differences are indicated as follows: #, P < 0.05 in comparison with the induced NZ9000(pTre1)-treated group; * and †, P < 0.005 and P < 0.0005, respectively, in comparison with the mock-treated and induced NZ9000(pTre1)-treated groups.
Concluding remarks.
Intracellular trehalose accumulation enabled 100% recovery of freeze-dried viable L. lactis cells, even in the absence of skim milk, a commonly used but bulky cryoprotectant. Remarkably, the cells acquired full resistance to physiological concentrations of bile as well as a 10-fold stronger protection against gastric juice. Trehalose accumulation did not interfere with IL-10 secretion or with the therapeutic efficacy of L. lactis secreting IL-10 as a treatment for murine colitis. The work presented here paves the way for improvement and diversification of pharmacological formulations for human therapy with live genetically modified L. lactis. It should in principle also be applicable to other bacterial species with health-promoting properties.
Acknowledgments
We thank I. Bruggeman, H. Devlies, and K. Van Laer for technical assistance. We also thank P. De Bleser for BLAST analysis, J. P. Remon for use of the freeze-drying facilities, M. De Vos for kindly providing human gastric juice, J. Thevelein for trehalase enzyme, NIZO (Ede, The Netherlands) for L. lactis NZ9000 and plasmid pNZ8048, and A. Bredan for editorial assistance.
This research was supported by the Research Fund of Ghent University (GOA project no. 12050700) and Flanders Interuniversity Institute for Biotechnology. K.V. is supported by the Broad Medical Research Program.
Footnotes
Published ahead of print on 6 October 2006.
REFERENCES
- 1.Andersson, U., F. Levander, and P. Radstrom. 2001. Trehalose-6-phosphate phosphorylase is part of a novel metabolic pathway for trehalose utilization in Lactococcus lactis. J. Biol. Chem. 276:42707-42713. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, U., D. Molenaar, P. Radstrom, and W. M. de Vos. 2005. Unity in organisation and regulation of catabolic operons in Lactobacillus plantarum, Lactococcus lactis, and Listeria monocytogenes. Syst. Appl. Microbiol. 28:187-195. [DOI] [PubMed] [Google Scholar]
- 3.Andersson, U., and P. Radstrom. 2002. Beta-glucose 1-phosphate-interconverting enzymes in maltose- and trehalose-fermenting lactic acid bacteria. Environ. Microbiol. 4:81-88. [DOI] [PubMed] [Google Scholar]
- 4.Arguelles, J. C. 2000. Physiological roles of trehalose in bacteria and yeasts: a comparative analysis. Arch. Microbiol. 174:217-224. [DOI] [PubMed] [Google Scholar]
- 5.Begley, M., C. G. Gahan, and C. Hill. 2005. The interaction between bacteria and bile. FEMS Microbiol. Rev. 29:625-651. [DOI] [PubMed] [Google Scholar]
- 6.Bolotin, A., P. Wincker, S. Mauger, O. Jaillon, K. Malarme, J. Weissenbach, S. D. Ehrlich, and A. Sorokin. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11:731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braat, H., P. Rottiers, D. W. Hommes, N. Huyghebaert, E. Remaut, J. P. Remon, S. J. van Deventer, S. Neirynck, M. P. Peppelenbosch, and L. Steidler. 2006. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn's disease. Clin. Gastroenterol. Hepatol. 4:754-759. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho, A. S., J. Silva, P. Ho, P. Teixeira, F. X. Malcata, and P. Gibbs. 2004. Effects of various sugars added to growth and drying media upon thermotolerance and survival throughout storage of freeze-dried Lactobacillus delbrueckii ssp. bulgaricus. Biotechnol. Prog. 20:248-254. [DOI] [PubMed] [Google Scholar]
- 9.Champagne, C. P., N. Gardner, E. Brochu, and Y. Beaulieu. 1991. The freeze-drying of lactic acid bacteria: a review. Can. Inst. Food Sci. Technol. J. 24:118-128. [Google Scholar]
- 10.Conrad, P. B., D. P. Miller, P. R. Cielenski, and J. J. de Pablo. 2000. Stabilization and preservation of Lactobacillus acidophilus in saccharide matrices. Cryobiology 41:17-24. [DOI] [PubMed] [Google Scholar]
- 11.Drouault, S., G. Corthier, S. D. Ehrlich, and P. Renault. 1999. Survival, physiology, and lysis of Lactococcus lactis in the digestive tract. Appl. Environ. Microbiol. 65:4881-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duong, T., R. Barrangou, W. M. Russell, and T. R. Klaenhammer. 2006. Characterization of the tre locus and analysis of trehalose cryoprotection in Lactobacillus acidophilus NCFM. Appl. Environ. Microbiol. 72:1218-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elbein, A. D., Y. T. Pan, I. Pastuszak, and D. Carroll. 2003. New insights on trehalose: a multifunctional molecule. Glycobiology 13:17R-27R. [DOI] [PubMed] [Google Scholar]
- 14.Fedorak, R. N., A. Gangl, C. O. Elson, P. Rutgeerts, S. Schreiber, G. Wild, S. B. Hanauer, A. Kilian, M. Cohard, A. LeBeaut, B. Feagan, et al. 2000. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn's disease. Gastroenterology 119:1473-1482. [DOI] [PubMed] [Google Scholar]
- 15.Fuglsang, A. 2003. Lactic acid bacteria as prime candidates for codon optimization. Biochem. Biophys. Res. Commun. 312:285-291. [DOI] [PubMed] [Google Scholar]
- 16.Garcia De Castro, A., H. Bredholt, A. R. Strom, and A. Tunnacliffe. 2000. Anhydrobiotic engineering of gram-negative bacteria. Appl. Environ. Microbiol. 66:4142-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant, S. G., J. Jessee, F. R. Bloom, and D. Hanahan. 1990. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc. Natl. Acad. Sci. USA 87:4645-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenberger, N., and G. Paumgartner. 2001. Diseases of the gallbladder and bile ducts, p. 1776-1777. In E. Braunwald, L. Hauser, A. Fauci, D. Kasper, D. Longo, and L. Jameson (ed.), Harrison's principles of internal medicine. McGraw-Hill, New York, N.Y.
- 20.Huyghebaert, N., A. Vermeire, S. Neirynck, L. Steidler, E. Remaut, and J. P. Remon. 2005. Development of an enteric-coated formulation containing freeze-dried, viable recombinant Lactococcus lactis for the ileal mucosal delivery of human interleukin-10. Eur. J. Pharm. Biopharm. 60:349-359. [DOI] [PubMed] [Google Scholar]
- 21.Jacobsen, C. N., V. Rosenfeldt Nielsen, A. E. Hayford, P. L. Moller, K. F. Michaelsen, A. Paerregaard, B. Sandstrom, M. Tvede, and M. Jakobsen. 1999. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 65:4949-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaasen, I., J. McDougall, and A. R. Strom. 1994. Analysis of the otsBA operon for osmoregulatory trehalose synthesis in Escherichia coli and homology of the OtsA and OtsB proteins to the yeast trehalose-6-phosphate synthase/phosphatase complex. Gene 145:9-15. [DOI] [PubMed] [Google Scholar]
- 23.Klijn, N., A. H. Weerkamp, and W. M. de Vos. 1995. Genetic marking of Lactococcus lactis shows its survival in the human gastrointestinal tract. Appl. Environ. Microbiol. 61:2771-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kojouharoff, G., W. Hans, F. Obermeier, D. N. Mannel, T. Andus, J. Scholmerich, V. Gross, and W. Falk. 1997. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin. Exp. Immunol. 107:353-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhn, R., J. Lohler, D. Rennick, K. Rajewsky, and W. Muller. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263-274. [DOI] [PubMed] [Google Scholar]
- 26.Kuipers, O. P., P. G. G. A. de Ruyter, M. Kleerebezem, and W. M. de Vos. 1998. Quorum sensing-controlled gene expression in lactic acid bacteria. J. Biotechnol. 64:15-21. [Google Scholar]
- 27.Leslie, S. B., E. Israeli, B. Lighthart, J. H. Crowe, and L. M. Crowe. 1995. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl. Environ. Microbiol. 61:3592-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marteau, P., M. Minekus, R. Havenaar, and J. H. Huis in't Veld. 1997. Survival of lactic acid bacteria in a dynamic model of the stomach and small intestine: validation and the effects of bile. J. Dairy Sci. 80:1031-1037. [DOI] [PubMed] [Google Scholar]
- 29.Moore, K. W., R. de Waal Malefytqq, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 30.Morgan, C. A., N. Herman, P. A. White, and G. Vesey. 2006. Preservation of micro-organisms by drying: a review. J. Microbiol. Methods 66:183-193. [DOI] [PubMed] [Google Scholar]
- 31.Okayasu, I., S. Hatakeyama, M. Yamada, T. Ohkusa, Y. Inagaki, and R. Nakaya. 1990. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98:694-702. [DOI] [PubMed] [Google Scholar]
- 32.Sands, B. E. 2000. Therapy of inflammatory bowel disease. Gastroenterology 118:S68-S82. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber, S., R. N. Fedorak, O. H. Nielsen, G. Wild, C. N. Williams, S. Nikolaus, M. Jacyna, B. A. Lashner, A. Gangl, P. Rutgeerts, K. Isaacs, S. J. van Deventer, J. C. Koningsberger, M. Cohard, A. LeBeaut, S. B. Hanauer, et al. 2000. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn's disease. Gastroenterology 119:1461-1472. [DOI] [PubMed] [Google Scholar]
- 34.Schreiber, S., T. Heinig, H. G. Thiele, and A. Raedler. 1995. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology 108:1434-1444. [DOI] [PubMed] [Google Scholar]
- 35.Steidler, L., W. Hans, L. Schotte, S. Neirynck, F. Obermeier, W. Falk, W. Fiers, and E. Remaut. 2000. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 289:1352-1355. [DOI] [PubMed] [Google Scholar]
- 36.Steidler, L., S. Neirynck, N. Huyghebaert, V. Snoeck, A. Vermeire, B. Goddeeris, E. Cox, J. P. Remon, and E. Remaut. 2003. Biological containment of genetically modified Lactococcus lactis for intestinal delivery of human interleukin 10. Nat. Biotechnol. 21:785-789. [DOI] [PubMed] [Google Scholar]
- 37.Tilg, H., C. van Montfrans, A. van den Ende, A. Kaser, S. J. van Deventer, S. Schreiber, M. Gregor, O. Ludwiczek, P. Rutgeerts, C. Gasche, J. C. Koningsberger, L. Abreu, I. Kuhn, M. Cohard, A. LeBeaut, P. Grint, and G. Weiss. 2002. Treatment of Crohn's disease with recombinant human interleukin 10 induces the proinflammatory cytokine interferon gamma. Gut 50:191-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.To, B. C. S., and M. R. Etzel. 1997. Spray-drying, freeze-drying, or freezing of three different lactic acid bacteria species. J. Food Sci. 62:576-585. [Google Scholar]
- 39.Trinder, P. 1969. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J. Clin. Pathol. 22:158-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Asseldonk, M., G. Rutten, M. Oteman, R. J. Siezen, W. M. de Vos, and G. Simons. 1990. Cloning of usp45, a gene encoding a secreted protein from Lactococcus lactis subsp. lactis MG1363. Gene 95:155-160. [DOI] [PubMed] [Google Scholar]
- 41.Vandenbroucke, K., W. Hans, J. Van Huysse, S. Neirynck, P. Demetter, E. Remaut, P. Rottiers, and L. Steidler. 2004. Active delivery of trefoil factors by genetically modified Lactococcus lactis prevents and heals acute colitis in mice. Gastroenterology 127:502-513. [DOI] [PubMed] [Google Scholar]
- 42.van Deventer, S. J., C. O. Elson, R. N. Fedorak, et al. 1997. Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn's disease. Gastroenterology 113:383-389. [DOI] [PubMed] [Google Scholar]
- 43.Vesa, T., P. Pochart, and P. Marteau. 2000. Pharmacokinetics of Lactobacillus plantarum NCIMB 8826, Lactobacillus fermentum KLD, and Lactococcus lactis MG 1363 in the human gastrointestinal tract. Aliment. Pharmacol. Ther. 14:823-828. [DOI] [PubMed] [Google Scholar]
- 44.Waterfield, N. R., R. W. Le Page, P. W. Wilson, and J. M. Wells. 1995. The isolation of lactococcal promoters and their use in investigating bacterial luciferase synthesis in Lactococcus lactis. Gene 165:9-15. [DOI] [PubMed] [Google Scholar]
- 45.Wells, J. M., P. W. Wilson, and R. W. Le Page. 1993. Improved cloning vectors and transformation procedure for Lactococcus lactis. J. Appl. Bacteriol. 74:629-636. [DOI] [PubMed] [Google Scholar]