Abstract
In addition to the previously characterized pyruvate oxidase PoxB, the Lactobacillus plantarum genome encodes four predicted pyruvate oxidases (PoxC, PoxD, PoxE, and PoxF). Each pyruvate oxidase gene was individually inactivated, and only the knockout of poxF resulted in a decrease in pyruvate oxidase activity under the tested conditions. We show here that L. plantarum has two major pyruvate oxidases: PoxB and PoxF. Both are involved in lactate-to-acetate conversion in the early stationary phase of aerobic growth and are regulated by carbon catabolite repression. A strain devoid of pyruvate oxidase activity was constructed by knocking out the poxB and poxF genes. In this mutant, acetate production was strongly affected, with lactate remaining the major end product of either glucose or maltose fermentation. Notably, survival during the stationary phase appeared to be dramatically improved in the poxB poxF double mutant.
Acetate is the major fermentation end product of the lactic acid bacterium Lactobacillus plantarum when cultivated under aerobic conditions and sugar limitation. It is produced at the expense of lactate as glucose becomes depleted and cells enter the stationary phase of growth. The pathway for lactate-to-acetate conversion under these conditions has been shown to involve three enzymatic steps (2, 6, 12, 20): oxidation of lactate to pyruvate by the NAD-dependent d- and l-lactate dehydrogenases (LDH), oxidative decarboxylation of pyruvate to acetyl-phosphate (acetyl∼P) by pyruvate oxidase (POX), and dephosphorylation of acetyl∼P to acetate by acetate kinase (ACK). This last step produces ATP, which is believed to provide the cells with the additional energy needed for survival in the stationary phase. Acetate itself could also be involved in increased survival by maintaining the pH homeostasis (12, 20). Concerning applications, the maintenance of a high viability in the stationary phase under aerobic conditions could be relevant in the development of long-shelf-life probiotic dairy products containing L. plantarum (14, 29). Besides its implication in cell survival, acetate is also an important flavor compound of fermented products (e.g., sourdoughs) in which L. plantarum plays a major role (4, 5). Therefore, a better understanding of the pathways involved in acetate production in this species could contribute to the improvement of fermentation processes and products.
Previously, it has been established that the oxidative decarboxylation of pyruvate catalyzed by POX is a key step in the lactate-to-acetate conversion pathway (12, 27). A null mutant for the gene encoding PoxB, the major POX of L. plantarum, shows a decrease in acetate production up to 80% compared to the parent strain, depending on the growth conditions (12).
This LDH-POX-ACK pathway is under control of two environmental factors: sugar and oxygen availability. Regulation takes place essentially at the level of POX activity, which is induced by oxygen or hydrogen peroxide and repressed by glucose (12, 19, 20, 27). In the presence of excess glucose, POX activity is strongly repressed as a result of the carbon catabolite repressor protein CcpA binding to the cre sequence located in the poxB promoter (12). The repression is relieved when glucose concentration becomes limiting for growth, explaining the peak levels of poxB mRNA and the corresponding POX activity in the early stationary phase of growth. This CcpA/cre-dependent repression of poxB expression is not observed when cells are grown with a non-PTS sugar such as maltose (12). Oxygen regulation takes place at two levels: first, it is required as a substrate of the POX enzyme and, second, it strongly induces transcription from the poxB promoter by an unknown mechanism (12).
Previous work suggested the presence of multiple POX-encoding genes in L. plantarum since disruption of the poxB gene alone did not completely abolish POX activity (12). Indeed, the genome sequence of L. plantarum WCFS1 revealed the presence of four other putative POX-encoding genes (lp_3587 [poxC], lp_0849 [poxD], lp_0852 [poxE], and lp_2629 [poxF]) (9, 12). This high redundancy of putative POX genes in L. plantarum is unique among lactic acid bacteria since similarity searches for orthologous genes in all publicly available genome sequences revealed the presence of not more than two pox genes per genome.
The present study focuses on the contribution of each additional pox gene to the global POX activity and evaluates the physiological importance of POX activity and acetate production by L. plantarum cells during the stationary phase of aerobic growth.
Conservation and sequence analysis of pox genes in L. plantarum strain Lp80.
The poxD, poxE, and poxF genes were amplified from Lp80 chromosomal DNA by using primers derived from the WCFS1 genome sequence. These PCR products were then sequenced (accession no. DQ315396, DQ315397, and DQ315398, respectively). The Lp80 poxC sequence was obtained from plasmid pGIF009 containing the Lp80 poxB-poxC locus (accession no. DQ315399) (12). The encoded PoxC, PoxD, PoxE, and PoxF proteins of strain Lp80 display between 99.5 and 99.8% identity with their counterparts encoded in the WCFS1 genome sequence. Globally, the five POX enzymes of L. plantarum Lp80 display a percentage of identity comprised of between 37% (between PoxE and PoxF) and 49% (between PoxC and PoxE) (see Fig. S1A in the supplemental material). Analysis of the predicted protein sequences revealed that most of the residues potentially involved in catalysis and substrate and cofactor binding in L. plantarum PoxB (15-17, 31) are conserved in the four other Pox proteins (see Fig. S2 in the supplemental material). Analysis of their promoter regions showed the presence of potential cre-like boxes, suggesting that they are regulated by CcpA-mediated carbon catabolite control (Fig. 1D and data not shown).
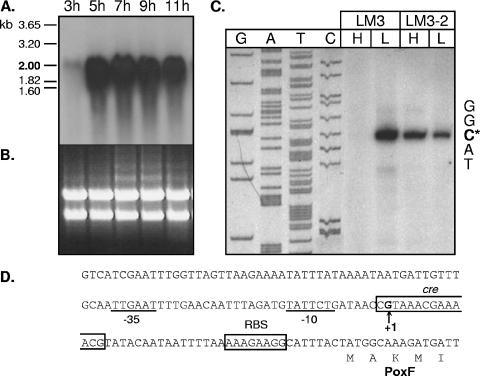
FIG. 1.
Transcriptional analysis of poxF in L. plantarum. (A) Northern blot analysis of poxF expression in the wild-type Lp80 strain. Cells were grown in aerobiosis with glucose 0.2% and harvested at different times during growth (indicated at the top). Escherichia coli rRNA 16S (1.60 kb) and 23S (3.20 kb) and Caenorhabditis elegans rRNA 18S (1.82 kb) and 28S (3.65 kb) were used as molecular markers (left). (B) RNA electrophoresis gel used in the Northern blots presented in panel A. (C) Primer extension analysis of poxF mRNA. Primer extension products were obtained by using oligonucleotide poxF1 and the total RNA extracted from L. plantarum LM3 or LM3-2 (ΔccpA) grown with glucose 2% (H) or 0.2% (L). The poxF transcription start nucleotide is indicated by an asterisk. As a reference, a sequencing reaction was performed on poxF using the same primer. (D) Nucleotide sequence of the poxF promoter region in L. plantarum Lp80. Putative −35 and −10 boxes of a vegetative promoter are underlined; the ribosome-binding site (RBS) and cre sequence are boxed. The transcription start nucleotide (+1) is in boldface. The N-terminal PoxF deduced amino acid sequence is shown.
Transcriptional analysis of the pox genes of L. plantarum Lp80.
In order to demonstrate the carbon catabolite repression of poxC, poxD, poxE, and poxF, Northern blot analyses were performed on RNA extracted from the wild-type Lp80 strain at different times during aerobic growth in a modified MRS broth containing no acetate and no citrate (MRS-CA) and supplemented with glucose 0.2% (wt/vol) (6, 12). Total RNA was hybridized with PCR-generated probes specific to the poxC (primers poxC1-poxC2), poxD (poxD1-poxD2), poxE, (poxE2-poxE3), or poxF (poxF2-poxF3) genes as previously described (12). The probes (0.6 to 0.8 kb) were designed in regions that do not display more than 50% identity with the corresponding fragments of the four other pox genes. Northern blot experiments were performed in high-stringency conditions in order to avoid any cross-hybridization with other pox mRNAs.
Since it was found that only PoxF contributed significantly to POX activity (see below), only the transcription analysis for the poxF gene will be presented here. The poxF mRNA was detected as a single band with a size of approximately 2 kb, indicating that poxF is transcribed as a monocistronic mRNA (Fig. 1A). The abundance of the poxF transcript displayed a profile similar to that of the poxB mRNA (12): it was barely detectable during exponential growth (3 h) and became strongly expressed upon glucose exhaustion (5 h). Unlike the poxB mRNA level, which was decreased strongly in stationary phase (11 h of growth) (12), the poxF transcript was stably maintained until 11 h of growth (Fig. 1A).
The transcription start of the poxF transcript was mapped by primer extension (12) (Fig. 1C and D). This analysis was carried out by using RNA obtained from L. plantarum strain LM3 since a ccpA mutant derivative of this strain was already available (LM3-2) (21). No extension product could be observed in cells grown in the presence of 2% glucose (Fig. 1C), supporting the regulation of poxF expression by carbon catabolite repression. As a control, the same analysis was carried out on total RNA extracted from L. plantarum Lp80 grown on 0.2 and 2% glucose. As expected, the transcription start was found to be identical in strains LM3 and Lp80, in agreement with the fact that the LM3 poxF promoter region is 100% identical to the corresponding region of L. plantarum Lp80. Similar to what was observed in L. plantarum LM3, expression of the poxF gene in L. plantarum Lp80 was repressed by high glucose concentrations (data not shown). Derepression of poxF expression in the ccpA mutant strain (LM3-2) when grown in excess glucose conditions further confirmed the role of CcpA in poxF transcription control (Fig. 1C).
These data established ccpA-mediated carbon catabolite control of poxF expression. Similar transcriptional patterns with an absence of extension products in the presence of 2% glucose were observed for the poxC and poxE genes (data not shown), while no transcript of poxD could be detected in strains Lp80 and LM3 under any condition tested. These results support a role of CcpA in the global control of pox-like gene expression in L. plantarum.
Single knockout of the poxC, poxD, poxE, and poxF genes.
Each individual pox gene of L. plantarum Lp80 was inactivated by using a single crossover knockout strategy (7). PCR-generated internal fragments of the poxC (primers poxC3-poxC4), poxD (poxD1-poxD2), poxE (poxE2-poxE3), and poxF (poxF4-poxF5) genes of L. plantarum Lp80 were cloned into pUC18Ery (poxC) or pGIM008 (poxD, poxE, and poxF) using the primer derived cloning sites, yielding plasmids pGIF020, pGIF029, pGIF030, and pGIF031, respectively (Table 1) . These knockout vectors were transformed separately into L. plantarum Lp80, and selection of the mutants, as well as confirmation of the anticipated genetic organization of the mutated loci, was performed as previously described (7). The poxC, poxD, poxE, and poxF mutant strains were designated FL105, FL107, FL108, and FL111, respectively.
TABLE 1.
Bacterial strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Characteristic(s)a | Source or reference |
|---|---|---|
| Strains | ||
| L. plantarum | ||
| Lp80 | Wild-type, silage strain | 8 |
| FL104 | Lp80 derivative; ΔpoxB | 12 |
| FL105 | Lp80 derivative; poxC::pGIF020; PoxC, truncation from aa 369 to 584 | This study |
| FL107 | Lp80 derivative; poxD::pGIF029; PoxD, truncation from aa 378 to 579 | This study |
| FL108 | Lp80 derivative; poxE::pGIF030; PoxE, truncation from aa 400 to 586 | This study |
| FL111 | Lp80 derivative; poxF::pGIF031; PoxF, truncation from aa 430 to 585 | This study |
| FL112 | FL104 derivative; ΔpoxB poxF::pGIF031 | This study |
| LM3 | Wild type | 21 |
| LM3-2 | LM3 derivative; ccpA1 | 21 |
| E. coli | ||
| TG1 | supE hsdΔ5 thi Δ(lac-proAB) F′(traD36 proAB+lacIq lacZΔM15) | 26 |
| Plasmids | ||
| pUC18Ery | Emr Apr; pUC18 derivative with a 1.1-kb insert containing the erm gene | 30 |
| pGIM008 | Cmr Tcr; pACYC184 derivative replicating exclusively in gram-negative strains | M. Deghorain laboratory collection |
| pGIF020 | Apr Emr; pUC18Ery derivative with a 0.6-kb central fragment of poxC | This study |
| pGIF029 | Cmr Tcr; pGIM008 derivative with a 0.6-kb central fragment of poxD | This study |
| pGIF030 | Cmr Tcr; pGIM008 derivative with a 0.6-kb central fragment of poxE | This study |
| pGIF031 | Cmr Tcr; pGIM008 derivative with a 0.65-kb central fragment of poxF | This study |
| Primers | ||
| poxC1 | 5′-GCGCCGTTCATCTCTTGAACGG-3′ | This study |
| poxC2 | 5′-GCTTCTAGCACCCCTTCAGCC-3′ | This study |
| poxC3 | 5′-TCAGATGATGTTCAGCAGAC-3′ | This study |
| poxC4 | 5′-TGCCCATTCCGTATTATTCC-3′ | This study |
| poxD1 | 5′-CATGCCATGGTGCACACTGTTAACTATCC-3′ | This study |
| poxD2 | 5′-GGGGTACCAAACAGCTTTGTCACTAC-3′ | This study |
| poxE2 | 5′-CTAGCCATGGCTAAAAGCAGCTAAGCATCC-3′ | This study |
| poxE3 | 5′-GGGGTACCTTGCTCCTGATCCATTGGGAG-3′ | This study |
| poxF1 | 5′-GCATCCGCTGCCGCAGCGAGGGCC-3′ | This study |
| poxF2 | 5′-CGCGTTGGATGTTGAGCAAGAACG-3′ | This study |
| poxF3 | 5′-GCCGACCAAACTTGCCAGGATCC-3′ | This study |
| poxF4 | 5′-GCTCTAGAAACCGAGGACATGATGGCG-3′ | This study |
| poxF5 | 5′-GGGGTACCCAGGATAACTCATCTTCGCCG-3′ | This study |
Emr, Apr, Cmr, and Tcr indicate resistance to erythromycin, ampicillin, chloramphenicol, and tetracycline, respectively. Underlined nucleotides in the primer sequences correspond to extensions for cloning purposes. aa, amino acid.
The POX activity and acetate production were determined for each individual pox mutant and compared to the wild-type strain (Lp80) and the previously characterized poxB mutant (12). The strains were grown under aeration in MRS-CA supplemented with glucose 0.2%. Cells were collected at the entry of stationary phase, where the POX activity reaches its highest level (12, 27) and where the lactate-to-acetate conversion takes place (2, 12, 20, 27). The acetate and lactate concentrations present in the supernatant of these cultures in late stationary phase (30 h) were measured by high-pressure liquid chromatography as previously described (12). Compared to the wild-type strain, no measurable effect of the poxC, poxD, or poxE mutations was observed with regard to growth, total POX activity, or lactate-to-acetate conversion under these conditions (data not shown and Table 2). Only the poxF mutant strain (FL111) displayed an ca. 40% decrease in POX activity level compared to the parent strain (Table 2). However, acetate production after 30 h of growth was not affected in the poxF mutant (Table 2). Similarly, the previously characterized poxB mutant displayed an ca. 30% decrease in POX activity level compared to the wild type, which had a marginal effect on acetate production (12) (Table 2).
TABLE 2.
POX activity and acetate production of L. plantarum wild-type and pox mutants
| Carbon sourcea | Strain | Genotype | μmax (h−1)b | Mean POX activity (U/mg of total protein [10−2]) ± SDc | Acetate (%)d |
|---|---|---|---|---|---|
| 0.2% glucose | Lp80 | Wild type | 0.20 | 14.4 ± 1.7 | 83 |
| FL104 | ΔpoxB | 0.20 | 10.0 ± 1.2 | 59 | |
| FL105 | poxC::pGIF020 | 0.19 | 13.5 ± 0.9 | 85 | |
| FL107 | poxD::pGIF029 | 0.20 | 15.0 ± 1.6 | 84 | |
| FL108 | poxE::pGIF030 | 0.20 | 13.5 ± 1.1 | 84 | |
| FL111 | poxF::pGIF031 | 0.20 | 8.7 ± 2.0 | 84 | |
| FL112 | ΔpoxB poxF::pGIF031 | 0.19 | ND | 4 | |
| 0.2% maltose | Lp80 | Wild type | 0.22 | 22.4 ± 2.9 | 100 |
| FL104 | ΔpoxB | 0.21 | 1.5 ± 0.8 | 46 | |
| FL111 | poxF::pGIF031 | 0.22 | 19.9 ± 3.4 | 100 | |
| FL112 | ΔpoxB poxF::pGIF031 | 0.19 | ND | 3 |
The cells were grown at 28°C under aerobic conditions in MRS-CA (12) supplemented with 0.2% glucose or 0.2% maltose.
μmax is the maximum specific growth rate, expressed as the variation in the optical density at 600 nm per hour. The mutant strains FL105, FL107, FL108, FL111, and FL112 were cultivated in the presence of antibiotics that were shown to have a marginal effect on growth parameters. These data represent average values from at least two independent experiments.
POX activity was measured at the entry into stationary phase, when the sugar was completely exhausted. POX activity was assayed by oxidative coupling of the reaction product H2O2 with 4-aminoantipyrine in the presence of horseradish peroxidase and 2-hydroxy-3,5-dichlorobenzene sulfonate (12, 24). One unit corresponds to 1 μmol of pyruvate consumed min−1 mg of total protein−1. The total protein concentration was measured by using the Bradford method (1). These data represent average values (from at least four independent measures). ND, not detected.
The percentage of acetate in the fermentation end products was measured after 30 h of growth. These data represent average values from at least four independent experiments.
The same experiment was repeated using maltose 0.2% (wt/vol) instead of glucose as a carbon source in order to relieve the carbon catabolite repression of POX activity. POX activity levels did not appear to be significantly affected in maltose-grown cells by the poxF mutation (Table 2), which is in good agreement with the observation that PoxB accounts for most of the POX activity under these conditions (12) (Table 2). In analogy, acetate represented 100% of the fermentation end products from maltose in the wild type and its poxF mutant derivative (Table 2), whereas a reduction of acetate production by more than 50% was observed in the poxB mutant (12) (Table 2).
These results show that PoxF is involved in POX activity in L. plantarum grown on glucose, whereas PoxC, PoxD, and PoxE do not seem to participate in the POX activity. In addition, PoxB and PoxF appear to be differentially regulated when maltose is used as a carbon source: PoxF activity seems to be repressed, whereas PoxB activity is not affected, confirming that PoxB is the major POX under these conditions (12). Although the negative effect of maltose on POX activity has been previously reported in the L. plantarum poxB mutant (12), we are not aware of other observations of such a maltose repression effect, and there is no obvious hypothesis on its mechanism.
Construction and characterization of a poxB poxF double mutant.
The poxF mutation was introduced in the ΔpoxB mutant background (FL104) using pGIF031 as described above, generating the double poxB poxF mutant strain FL112.
No POX activity could be detected in the poxB poxF mutant background in cells grown on either glucose or maltose (Table 2), demonstrating that PoxB and PoxF are the two major POXs in L. plantarum under the conditions tested. As expected, lactate-to-acetate conversion in the POX-deficient FL112 strain was dramatically affected: this metabolite only accounted for 4 and 3% of the fermentation end products after 30 h of growth on glucose and maltose, respectively (Table 2).
To evaluate the effects of the reduced acetate production on the physiology of L. plantarum, the optical density (OD) at 600 nm and cell viability (measured as CFU/ml) of the wild-type and its single and double poxB poxF mutant derivatives were monitored throughout aerobic growth in MRS-CA supplemented with 0.2% glucose. Moreover, lactate and acetate concentrations in the supernatant of these cultures were measured by high-pressure liquid chromatography, while cell lysis in stationary phase was monitored by assaying the NAD-dependent LDH activity in the supernatant as described previously (12).
No significant difference could be observed between the four strains during the exponential phase of growth (Fig. 2). Glucose was completely exhausted and converted to lactate after 6 h (data not shown). Lactate concentrations peaked between 6 and 7 h of growth and were equivalent in all strains (Fig. 2C). As previously reported (12), the wild-type strain rapidly converted lactate to acetate during the stationary phase, whereas this process was slowed down in the poxB mutant strain (Fig. 2C). Conversion of lactate to acetate also occurred in the poxF mutant strain but was only slightly delayed compared to the wild-type strain (Fig. 2C). In these three strains, it appeared that more acetate was produced than lactate consumed, resulting in an apparent carbon balance greater than 1 (Fig. 2C) (12). This suggests that the additional acetate was produced at the expense of one or more compounds present in the growth medium, as previously reported (6, 12, 20). Recently, Liu et al. showed that the catabolism of amino acids was responsible for acetate production by growing and nongrowing cells of L. plantarum (10). In addition, l-serine catabolism was shown to play a key role for acetate production in this species (10). The authors of that study suggested that l-serine was deaminated via a serine dehydratase into pyruvate, which was subsequently converted into acetate by the POX enzyme (11).
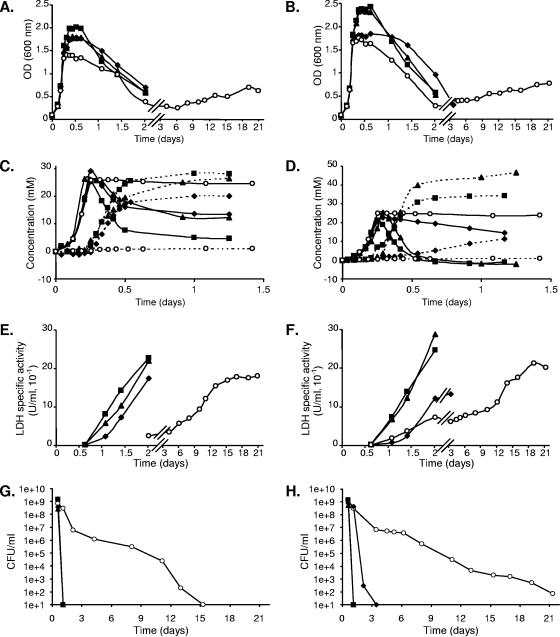
FIG. 2.
Growth and acetate production by L. plantarum strains affected in POX activity. The wild-type Lp80 (▪), poxB mutant (⧫), poxF mutant (▴), and poxB poxF double-mutant (○) strains of L. plantarum were grown in aerobic conditions at 28°C in MRS-CA supplemented with 0.2% glucose (A, C, E, and G) or 0.2% maltose (B, D, F, and H). Growth was monitored as the OD at 600 nm (A and B). Lactate (solid lines) and acetate (dashed lines) concentrations were measured in the culture supernatants (C and D). Concentrations are given as the difference between the measured concentration and the initial concentration in the culture medium. (E and F) The release of LDH in the supernatant was assayed. The viability (CFU/ml) of the different strains is presented in panels G and H. The data presented are from one of at least three independent experiments that gave essentially the same results.
The wild-type strain and its poxB and poxF single-mutant derivatives displayed similar growth (Fig. 2A) and viability (Fig. 2G) characteristics when grown on glucose. A drastic loss of cell viability was observed for these three strains after 24 h of growth, which was accompanied by high levels of extracellular LDH activity in the supernatant of these cultures, indicating that the cells had lysed (Fig. 2E). In contrast, growth halted abruptly when all glucose was consumed in the poxB poxF double mutant (Fig. 2A), and the lactate concentration remained stable up to 6 days after inoculation, whereas only very small amounts of acetate could be detected in the supernatant (Fig. 2C and data not shown). The abrupt termination of growth and the lower OD reached by the poxB poxF double mutant is in agreement with the suggestion that the energy generated by the conversion of lactate to acetate can be used for growth (20). Importantly, although the intial OD decrease of the double mutant appeared to be similar to that observed for the wild type (Fig. 2A), prolonged stationary-phase incubation revealed a drastic improvement of cell viability for the double-mutant strains relative to the single-mutant or wild-type strains (Fig. 2G). In analogy, significantly lower levels of LDH activity could be detected in the culture supernatant of the double mutant compared to the other strains (Fig. 2E). Therefore, lactate and acetate concentrations were measured in the supernatant of the poxB poxF double-mutant cultures in the late stationary phase (up to 15 days), revealing a very slow lactate-to-acetate conversion in this strain: 7.5 mM acetate produced and 4.3 mM lactate consumed after 15 days of incubation. However, acetate only accounted for 25% of the end products from glucose when no remaining viable cells could be detected (after 2 weeks of growth). The same experiment was carried out using the non-PTS sugar maltose (0.2%) as a carbon source. Identical conclusions could be drawn for the poxF mutant grown on glucose or maltose (Fig. 2B, D, F, and H). The wild-type and poxF mutant strains grown on maltose did not survive longer than 24 h (data not shown). Lactate-to-acetate conversion by the poxB mutant was slower on maltose than on glucose (Fig. 2D), which is in agreement with the much lower POX activity of this mutant on maltose (Table 2) (12). This resulted in an abrupt stop of growth after maltose exhaustion, and the OD was maintained for longer than in the wild-type and poxF mutant strains (Fig. 2B), confirming the previously reported data (12). In the present study, we were able to demonstrate that cell survival was significantly prolonged in the poxB mutant (up to 50 h, Fig. 2H) as previously suggested from the lower LDH activities detected in the culture supernatants (Fig. 2F) (12). The growth of the poxB poxF double mutant with maltose as a substrate was similar to that of the same mutant grown on glucose (Fig. 2B, D, and F). Its phenotype was even more striking under these conditions, the strain retaining viability up to 3 weeks after inoculation (Fig. 2G), while acetate represented only 30% of the fermentation end products from maltose after this period (Fig. 2D).
Concluding remarks.
Among the five predicted POXs encoded in the L. plantarum genome (9), only PoxB and PoxF appear to be involved in the generation of acetate from lactate during the stationary phase of aerobic growth. No expression of poxD could be observed in the present study, but it cannot be excluded that PoxD acts as a POX under different conditions. In order to detect PoxD-associated POX activity in vivo, the poxD gene was overexpressed under the control of a constitutive promoter in L. plantarum Lp80. The cells were grown in anaerobic conditions with excess glucose (2%), and their fermentation pattern was examined in aerated cell suspensions with glucose as a substrate. However, no acetate production could be detected under these conditions (data not shown), in contrast to what had been observed in a similar experiment with the functional PoxB enzyme (12). The poxC and poxE genes were found to be transcribed, but the corresponding enzymes do not seem to participate in POX activity. It is possible that PoxC and PoxE are not functional or that their preferred substrate is not pyruvate but rather another compound, since it was previously shown that a purified preparation of POX enzymes from L. plantarum displayed a significant activity (up to 20% of the activity with pyruvate) on alternative substrates (e.g., methylglyoxal, acetaldehyde, and α-ketobutyrate) (28). It is interesting that the functionality of the POX enzymes could not be inferred from the in silico analysis of their primary sequence. Indeed, among all residues suggested to be required for the POX activity of PoxB (15-17, 31), PoxF did not show a higher level of conservation than PoxC, PoxD, or PoxE (see additional comments, Fig. S1, and Fig. S2 in the supplemental material). This emphasizes the need for in vitro and in vivo analyses, which is of particular importance for the construction of metabolic models based on automated annotation of a genome sequence.
Acetate production was strongly reduced in the poxB poxF double mutant, showing that the LDH-POX-ACK enzyme combination is by far the major pathway for lactate-to-acetate conversion in L. plantarum. However, although no POX activity could be detected, acetate production was not completely abolished. Acetate production in this strain was very slow, and it cannot be excluded that this could be due to undetectable levels of POX activity. In addition, it should be noted that the assay used for POX activity determination is specific for H2O2-producing POX (24), possibly suggesting that acetate production in the poxB poxF double mutant is dependent on the presence of an H2O-producing POX such as PoxB of E. coli (25). Nevertheless, this H2O-producing POX activity should still be extremely low to account for the very low rates of acetate production observed in the poxB poxF double mutant. Finally, acetate could also be produced by a POX-independent pathway such as a combination of pyruvate dehydrogenase, phosphotransacetylase, and ACK. However, all attempts to detect pyruvate dehydrogenase activity in L. plantarum have been unsuccessful (3, 12, 19).
A strong correlation between acetate production rate and cell viability was revealed, i.e., the slower lactate was converted to acetate, the longer cells retained viability. The improved survival of the POX-deficient L. plantarum strain is reminiscent of the phenotype of a Staphylococcus aureus mutant defective for its cidC-encoded POX (22). However, improved cell viability of the S. aureus cidC mutant was correlated with the ability to utilize acetate, whereas in the POX-deficient L. plantarum strain, it is correlated with the inability to produce acetate.
This situation was unexpected since previous hypotheses from the literature in which acetate production from lactate during the stationary growth phase of L. plantarum cell cultures was associated with the maintenance of ATP production and pH homeostasis and thus believed to improve survival (12, 20). In our experimental setup with a low glucose concentration, the pH homeostasis hypothesis could not be invoked since the conversion of lactate to acetate has a marginal impact on the final pH of the culture medium. For instance, wild-type cells displayed a pH of 4.9 at the onset of the stationary phase when lactate production is maximal and a pH of 5.2 after 30 h when most of the lactate was converted into acetate. Similarly, all constructed pox mutants did not display external pH variations of more than 0.3 U during the stationary phase (data not shown).
An alternative hypothesis could be linked to the production of H2O2, a major product of the reaction catalyzed by POX, which is known to have toxic effects on many species of bacteria (13). In a strain with reduced POX activity, the production of this compound could be lowered (or slowed down), leading to better survival. However, we do not favor this hypothesis since L. plantarum is known to resist to high levels of H2O2 (18, 19). Moreover, studies on the POX (SpxB) of Streptococcus pneumoniae have shown that POX activity itself contributes to hydrogen peroxide resistance in this species by providing acetyl∼P, which can serve as an alternative source of ATP during the oxidative stress generated by H2O2 (23).
Acetate itself does not seem to be responsible for cell death in the stationary phase since the addition of acetate (30 mM, corresponding to the maximal concentration observed for the wild-type strain after complete lactate consumption) to a glucose-grown culture of the poxB poxF double-mutant strain at the onset of the stationary phase did not revert the observed survival phenotype. Another possible explanation is that a product of the LDH-POX-ACK pathway acts as a messenger to regulate cell death and lysis in L. plantarum. As previously suggested (12), this could be achieved through ATP, i.e., the slow production of acetate observed in the poxB poxF double mutant would delay ATP depletion, and hence the proton motive force would not be dissipated as fast as in the wild-type strain, resulting in delayed autolysis.
Supplementary Material
Acknowledgments
This research was carried out with the financial support of FNRS and MIUR-PRIN 2002.
We are grateful to K. Schanck, B. Delplace, and E. Viaene for technical assistance. We warmly thank J. Delcour for fruitful discussions and scientific advice. F.L. and P.G. hold doctoral fellowships from FRIA. P.H. is a research associate at FNRS.
Footnotes
Published ahead of print on 29 September 2006.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 2.Condon, S. 1987. Responses of lactic-acid bacteria to oxygen. FEMS Microbiol. Rev. 46:269-280. [Google Scholar]
- 3.Dirar, H., and E. B. Collins. 1973. Aerobic utilization of low concentrations of galactose by Lactobacillus plantarum. J. Gen. Microbiol. 78:211-215. [DOI] [PubMed] [Google Scholar]
- 4.Gilliland, S. E. 1985. Bacterial starters cultures in food. CRC Press, Inc., Boca Raton, Fla.
- 5.Gobbetti, M., P. Lavermicocca, F. Minervini, M. De Angelis, and A. Corsetti. 2000. Arabinose fermentation by Lactobacillus plantarum in sourdough with added pentosans and α-l-arabinofuranosidase: a tool to increase the production of acetic acid. J. Appl. Microbiol. 88:317-324. [DOI] [PubMed] [Google Scholar]
- 6.Goffin, P., F. Lorquet, M. Kleerebezem, and P. Hols. 2004. Major role of NAD-dependent lactate dehydrogenases in aerobic lactate utilization in Lactobacillus plantarum during early stationary phase. J. Bacteriol. 186:6661-6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hols, P., C. Defrenne, T. Ferain, S. Derzelle, B. Delplace, and J. Delcour. 1997. The alanine racemase gene is essential for growth of Lactobacillus plantarum. J. Bacteriol. 179:3804-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Josson, K., T. Scheirlinck, F. Michiels, C. Platteeuw, P. Stanssens, H. Joos, P. Dhaese, M. Zabeau, and J. Mahillon. 1989. Characterization of a gram-positive broad-host-range plasmid isolated from Lactobacillus hilgardii. Plasmid 21:9-20. [DOI] [PubMed] [Google Scholar]
- 9.Kleerebezem, M., J. Boekhorst, R. van Kranenburg, D. Molenaar, O. P. Kuipers, R. Leer, R. Tarchini, S. A. Peters, H. M. Sandbrink, M. W. Fiers, W. Stiekema, R. M. Lankhorst, P. A. Bron, S. M. Hoffer, M. N. Groot, R. Kerkhoven, M. de Vries, B. Ursing, W. M. de Vos, and R. J. Siezen. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 100:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu, S. Q., R. Holland, and V. L. Crow. 2003. The potential of dairy lactic acid bacteria to metabolize amino acids via non-transaminating reactions and endogenous transamination. Int. J. Food Microbiol. 86:257-269. [DOI] [PubMed] [Google Scholar]
- 11.Liu, S. Q., R. Holland, P. McJarrow, and V. L. Crow. 2003. Serine metabolism in Lactobacillus plantarum. Int. J. Food Microbiol. 89:265-273. [DOI] [PubMed] [Google Scholar]
- 12.Lorquet, F., P. Goffin, L. Muscariello, J. B. Baudry, V. Ladero, M. Sacco, M. Kleerebezem, and P. Hols. 2004. Characterization and functional analysis of the poxB gene, which encodes pyruvate oxidase in Lactobacillus plantarum. J. Bacteriol. 186:3749-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller, R. A., and B. E. Britigan. 1997. Role of oxidants in microbial pathophysiology. Clin. Microbiol. Rev. 10:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molin, G. 2001. Probiotics in foods not containing milk or milk constituents, with special reference to Lactobacillus plantarum 299v. Am. J. Clin. Nutr. 73:380S-385S. [DOI] [PubMed] [Google Scholar]
- 15.Muller, Y. A., Y. Lindqvist, W. Furey, G. E. Schulz, F. Jordan, and G. Schneider. 1993. A thiamin diphosphate binding fold revealed by comparison of the crystal structures of transketolase, pyruvate oxidase, and pyruvate decarboxylase. Structure 1:95-103. [DOI] [PubMed] [Google Scholar]
- 16.Muller, Y. A., and G. E. Schulz. 1993. Structure of the thiamine- and flavin-dependent enzyme pyruvate oxidase. Science 259:965-967. [DOI] [PubMed] [Google Scholar]
- 17.Muller, Y. A., G. Schumacher, R. Rudolph, and G. E. Schulz. 1994. The refined structures of a stabilized mutant and of wild-type pyruvate oxidase from Lactobacillus plantarum. J. Mol. Biol. 237:315-335. [DOI] [PubMed] [Google Scholar]
- 18.Murphy, M. G., and S. Condon. 1984. Comparison of aerobic and anaerobic growth of Lactobacillus plantarum in a glucose medium. Arch. Microbiol. 138:49-53. [Google Scholar]
- 19.Murphy, M. G., and S. Condon. 1984. Correlation of oxygen utilization and hydrogen peroxide accumulation with oxygen induced enzymes in Lactobacillus plantarum cultures. Arch. Microbiol. 138:44-48. [DOI] [PubMed] [Google Scholar]
- 20.Murphy, M. G., L. O'Connor, D. Walsh, and S. Condon. 1985. Oxygen-dependent lactate utilization by Lactobacillus plantarum. Arch. Microbiol. 141:75-79. [DOI] [PubMed] [Google Scholar]
- 21.Muscariello, L., R. Marasco, M. De Felice, and M. Sacco. 2001. The functional ccpA gene is required for carbon catabolite repression in Lactobacillus plantarum. Appl. Environ. Microbiol. 67:2903-2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patton, T. G., K. C. Rice, M. K. Foster, and K. W. Bayles. 2005. The Staphylococcus aureus cidC gene encodes a pyruvate oxidase that affects acetate metabolism and cell death in stationary phase. Mol. Microbiol. 56:1664-1674. [DOI] [PubMed] [Google Scholar]
- 23.Pericone, C. D., S. Park, J. A. Imlay, and J. N. Weiser. 2003. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the Fenton reaction. J. Bacteriol. 185:6815-6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Risse, B., G. Stempfer, R. Rudolph, H. Mollering, and R. Jaenicke. 1992. Stability and reconstitution of pyruvate oxidase from Lactobacillus plantarum: dissection of the stabilizing effects of coenzyme binding and subunit interaction. Protein Sci. 1:1699-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell, P., L. P. Hager, and R. B. Gennis. 1977. Characterization of the proteolytic activation of pyruvate oxidase. Control by specific ligands and by the flavin oxidation-reduction state. J. Biol. Chem. 252:7877-7882. [PubMed] [Google Scholar]
- 26.Sambrook, J., E. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, New York, N.Y.
- 27.Sedewitz, B., K. H. Schleifer, and F. Gotz. 1984. Physiological role of pyruvate oxidase in the aerobic metabolism of Lactobacillus plantarum. J. Bacteriol. 160:462-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sedewitz, B., K. H. Schleifer, and F. Gotz. 1984. Purification and biochemical characterization of pyruvate oxidase from Lactobacillus plantarum. J. Bacteriol. 160:273-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah, N. P. 2000. Probiotic bacteria: selective enumeration and survival in dairy foods. J. Dairy Sci. 83:894-907. [DOI] [PubMed] [Google Scholar]
- 30.van Kranenburg, R., J. D. Marugg, I. I. van Swam, N. J. Willem, and W. M. de Vos. 1997. Molecular characterization of the plasmid-encoded eps gene cluster essential for exopolysaccharide biosynthesis in Lactococcus lactis. Mol. Microbiol. 24:387-397. [DOI] [PubMed] [Google Scholar]
- 31.Wille, G., M. Ritter, M. S. Weiss, S. Konig, W. Mantele, and G. Hubner. 2005. The role of Val-265 for flavin adenine dinucleotide (FAD) binding in pyruvate oxidase: FTIR, kinetic, and crystallographic studies on the enzyme variant V265A. Biochemistry 44:5086-5094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.