Abstract
PmxaF is a strong methanol-inducible promoter in Methylobacterium extorquens. When this promoter is cloned in expression vectors and used to drive heterologous gene expression, methanol inducibility is either greatly reduced or entirely lost. In order to bestow inducibility upon the cloned PmxaF promoter in expression vectors, we adopted combinational methods (regulatory elements of the Pseudomonas putida F1 cym and cmt operons and Tn7 transposon system) to control reporter gene expression at the transcriptional level in M. extorquens. An operator fragment (26 nucleotides) of the cmt operon was inserted downstream of the cloned PmxaF promoter in the broad-host-range expression vector (pCHOI3). The repressor gene (cymR) located upstream of the cym operon in P. putida F1 was amplified by PCR. To avoid cellular toxicity for M. extorquens caused by the overexpression of CymR, single and/or double copies of cymR were integrated into the chromosome of M. extorquens using the mini-Tn7 transposon system. Cultures containing the chromosomally integrated cymR gene were subsequently transformed with pCHOI3 containing modified PmxaF (i.e., PmxaF plus operator). In this construct, inducibility is afforded by cumate (p-isopropylbenzoate). In this report, we describe the inducible and tightly regulated expression of heterologous genes (bgl [for β-galactosidase], est [for esterase], and gfp [for green fluorescent protein]) in M. extorquens. This is the first documented example of an inducible/regulated heterologous gene expression system in M. extorquens.
Methylotrophic bacteria are a diverse group of microorganisms with the ability to utilize single-carbon (C1) substrates more reduced than carbon dioxide as their sole source of carbon and energy. Among the methylotrophs, members of the genus Methylobacterium have been described as being ubiquitous, participating in a myriad of favorable interactions with nature (24, 26, 27). Furthermore, Methylobacterium spp. naturally produce several substances of commercial importance, including poly-β-hydroxybutyrate (3, 4), vitamin B12 (28), pyrroloquinoline quinone (1, 10), and carotenoids (29). Over the past decade, Methylobacterium extorquens AM1 has been extensively studied and characterized both genetically and physiologically (6, 18, 20). The wealth and depth of understanding of M. extorquens and closely related strains suggest the potential of M. extorquens as a source of industrially pertinent natural products and recombinant proteins. The salient aspects for this potential have been described elsewhere (2, 8, 9), and they include (i) simple and inexpensive cultivation requirements, (ii) optimized high-cell-density fermentation protocols, (iii) available genome sequence for M. extorquens AM1, and (iv) availability of suitable genetic tools for M. extorquens (8, 13, 19, 21, 22). Application of these tools has made it possible to overexpress a variety of recombinant proteins in the range of 3 to 6 g/liter under high-cell-density growth conditions (8, 9, 13). However, inducible/regulated expression of recombinant genes in M. extorquens or in any other methylotroph, to our knowledge, has not yet been attained. M. extorquens possesses native methanol-inducible promoters, notably promoters which are located upstream of genes that encode methanol dehydrogenase and other proteins required for its activity and enzymes required for the synthesis of the methanol dehydrogenase prosthetic group, pyrroloquinoline quinone. Of these, the promoter PmxaF has been thoroughly scrutinized both biochemically and in expression studies (8, 9, 19, 21, 30). In its native form in the chromosome, this strong promoter is methanol inducible. However, when this promoter is cloned in expression vectors, it acts essentially in a constitutive mode. The mechanism by which PmxaF is regulated in the chromosome is still not fully known. Therefore, the reason the recombinant PmxaF reverts from inducible to constitutive remains speculative. Expression of the green fluorescent protein (GFP)-encoding gene (gfp) under the control of cloned PmxaF in the plasmid pCM110 was observed even after the culture was grown repeatedly on succinate as the sole source of carbon (unpublished results). This fact makes PmxaF unsuitable in applications where regulated expression is paramount, such as in expression of recombinant proteins potentially toxic to the host or in metabolic flux and pathway engineering applications where the effect of expression of specific genes on metabolism is required. Several other heterologous inducible promoters, such as Plac, λPL, and PR, have been tested with M. extorquens ATCC 55366; however, gene expression under the control of these promoters was leaky and weak (unpublished results). To develop a regulated and inducible expression system, we adapted the regulatory element of Pseudomonas putida F1. P. putida F1 degrades p-cymene (p-isopropyltoluene) through p-cumate (p-isopropylbenzoate) to isobutyrate, pyruvate, and acetyl coenzyme A (11, 12). The genes encoding the enzymes required for this degradation are grouped in two distinct operons, called cym and cmt. The cym operon encodes the conversion of p-cymene to p-cumate. Located downstream of the cym operon is the cmt operon, which encodes the catabolism of p-cumate. A regulatory protein known as CymR, encoded upstream of the cym operon, has been shown to bind to specific operator-promoter regions in both operons and controls expression of both operons. Induction is afforded by p-cumate, the end product of the first operon, but not by p-cymene (11, 12).
We decided to apply the regulatory elements, the cymR gene and the operator fragment of the cmt operon from P. putida F1, to M. extorquens ATCC 33566 in the hope of bestowing inducibility and regulation on the existing constitutive expression vectors for Methylobacterium strains. If successful, we would obtain an inducible expression system able to operate during growth of Methylobacterium on a preferred growth substrate, methanol, while controlling the expression of heterologous genes from the strong PmxaF promoter using the nontoxic and inexpensive inducer cumate.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. M. extorquens (ATCC 55366) was used for the preparation of the heterologous gene expression host. Escherichia coli strain Top10 was used for cloning and propagation of recombinant plasmid DNA, and S17-1 λ pir (recA thi pro hsdR− M+ RP4:2-Tc:Mu:Km Tn7, λ pir lysogen; Smr Tpr) was used for propagation of the helper plasmid (17). E. coli was cultured in Luria-Bertani broth at 37°C. M. extorquens was grown in CHOI medium, as previously described (9), and 1% (vol/vol) methanol was used as the sole carbon source. Media were solidified by 1.8% agar (Difco) when appropriate. Antibiotics were used for E. coli and M. extorquens at the following concentrations (in μg/ml): ampicillin, 100; kanamycin (Km), 40; tetracycline (Tc), 35.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference(s) or source |
|---|---|---|
| M. extorquens | ||
| ATCC 55366 | Wild type | ATCC |
| Transformants | ||
| CymR1 | Modified host strain containing single copy of cymR expression cassette in the chromosome | This study |
| CymR2 | Modified host strain containing double copy of cymR expression cassette in the chromosome | This study |
| GFP | M. extorquens (CymR2) containing pCUM-gfp | This study |
| BGL | M. extorquens (CymR2) containing pCUM-bgl | This study |
| EST | M. extorquens (CymR2) containing pCUM-est | This study |
| Pseudomonas putida F1 | Source of cymR gene in the cym operon | 11, 12 |
| E. coli strains | ||
| Top10 | Strain for cloning and propagating plasmid DNA | Invitrogen, Inc. |
| S17-1/λ (pir) | Host strain for pUX-BF13 | 17 |
| Plasmids | ||
| pBK-miniTn7-ΩSm2 | pUC19-based delivery plasmid for a miniTn7-Km transposon; Kmr Smr | 17 |
| pBRI-tet | pUC19-based delivery plasmid for a mini-Tn7 transposon; Tetr | This study |
| pBRI80 | pUC19-based delivery plasmid for a mini-Tn7 transposon containing PmxaF and RBS; Tetr | This study |
| pBRI-cymR1 | pBRI80 plasmid containing one copy of cymR expression cassette | This study |
| pBRI-cymR2 | pBRI80 plasmid containing two copies of cymR expression cassettes | This study |
| pUX-BF13 | R6K replicon-based helper plasmid | 17 |
| pCR2.1-TOPO | PCR cloning vector | Invitrogen, Inc. |
| pCR-cymR | pCR2.1-TOPO plasmid containing cymR | This study |
| PCR-MDHOP | pCR2.1-TOPO plasmid containing PmxaF+operator | This study |
| pCR-bgl | pCR2.1-TOPO plasmid containing bgl | This study |
| pCR-est | pCR2.1-TOPO plasmid containing estI | This study |
| pCR-gfp | pCR2.1-TOPO plasmid containing gfp | This study |
| pCM110 | Wide-host-range cloning vector containing PmxaF; Tetr | 19 |
| pCHOI3 | Wide-host-range cloning vector containing PmxaF; Kmr | This study |
| pCUM50 | Newly constructed regulative expression vector for M. extorquens (CymR2) | This study |
| pCUM-bgl | pCUM50 plasmid containing β-galactosidase expression cassette | This study |
| pCUM-est | pCUM50 plasmid containing esterase expression cassette | This study |
| pCUM-gfp | pCUM50 plasmid containing GFP expression cassette | This study |
| pUC19 | Multipurpose cloning vector | Invitrogen, Inc. |
| pCESTa | estI gene source | 9 |
| pEBIG4 | bgl gene source | 15 |
| pQBI63 | gfp gene source | Qbiogene, Inc. |
Construction of expression hosts.
The M. extorquens expression hosts (CymR1 and CymR2) were constructed by insertion of the cymR gene of P. putida F1 using the mini-Tn7 integration system with the mini-Tn7 vector pBRI80 with a helper plasmid (12, 17). Primers used in the cymR amplification were designed on the basis of the nucleotide sequence of the P. putida F1 cym operon (11, 12). In order to achieve tightly regulated induction, two copies of the cymR gene were integrated into the M. extorquens chromosome. Briefly, the mini-Tn7 base vector pBRI80 was constructed as follows: a 1,955-bp PstI fragment containing tetA and tetR was amplified from pCM110 (19) by PCR using the primers Tet-F-Pst (5′-GCTGCAGTCAATCGTCACCCTTTCTCGGTC-3′) (PstI site is underlined) and Tet-R-Pst (5′-GCTGCAGTCAGCGATCGGCTCGTTGCCCTG-3′) (PstI site is underlined). This fragment was then replaced with a kanamycin-resistant-protein-encoding gene in pBK-miniTn7-ΩSm2 to form pBRI-tet.
The PmxaF promoter plus the ribosomal binding site (PmxaF-RBS) was amplified from pCESTc using the primers MDH-F-Pst (5′-GGCTGCAGGTTGACGACAACGGTGCGATG-3′) (PstI site is underlined) and MDH-R-Mlu (5′-CCGACGCGTATGTATATCTCCTTCTTAAAG-3′) (MluI site is underlined). The PCR fragment containing PmxaF-RBS was cloned into pBRI-tet, which was partially digested with PstI/MluI to delete the Smr/Spr cassette to generate pBRI80.
To generate pBRI-cymR1, cymR was amplified from chromosomal DNA of P. putida F1 using the primers CYM-F-Afl (5′-GCTTAAGAAGATGGTGATCATGAGTCCAAAGAGAAGAAC3′) (AflII site is underlined) and CYM-R-Not (5′-CAGCGGCCGCCTAGCGCTTGAATTTCGCGTACCGCTCTCGCG-3′) (NotI site is underlined). A 612-bp AflII-NotI fragment from pCR-cymR was ligated into the AflII-NotI site of pBRI80 to form pBRI-cymR1. To obtain pBRI-cymR2, containing two copies of cymR expression cassettes, a second copy of cymR was amplified from pBRI-cymR using the primers MDH-CYM-F-Not (5′-CAGCGGCCGCGTTGACGACAACGGTGCGATGGGTC-3′) (NotI site is underlined) and CYM-R-Apa (5′-CAGGGCCCCTAGCGCTTGAATTTCGCGTACCGCTCTCGCG-3′) (ApaI site is underlined). The amplified fragment containing PmxaF-RBS-cymR was then ligated into the NotI-ApaI site of pBRI-cymR1 to generate pBRI-cymR2.
The genotypes of the cymR-integrated host strains were confirmed by Southern hybridization using the 612-bp cymR fragment as a probe. Since we have identified the specific Tn7 insertion site (attTn7) for M. extorquens in a previous study (8), the integration of the target gene into the chromosome of M. extorquens was also determined by colony PCR using designed primers which include common Tn7 primers (PTn7RF, 5′-ATTAGCTTACGACGCTACACCC-3′; PTn7LR, 5′-CACAGCATAACTGGACTGATTTC-3′) and gene-specific primers (PdhaTF, 5′-CATCGCGATTGTCGATTCGG-3′; and PglmSR, 5′-CTGAAGGAAATCAGCTACATC-3′). The cymR-positive strains were finally confirmed by Western blotting. Then, the electrocompetent cells were prepared using cymR-positive M. extorquens, essentially as described previously (9, 13).
Inducible expression vector construction.
Manipulations and sequencing of DNA were carried out using standard procedures. The operator sequence of the cmt operon from P. putida F1 was introduced downstream of the methanol dehydrogenase promoter, PmxaF, by PCR. The pCUM50 regulative expression vector was obtained in several steps: first, the PmxaF plus synthetic operator sequence (PmxaF+operator) was amplified by PCR from pCM110 using primers MDH-F-PST (5′-GCTGCAGGTCGACTCTAGATCACCTCCTTAAGC-3′) (the PstI site is underlined) and MDH-CUM-R (5′-CGAATTCATAATACAAACAGACCAGATTGTCTGTTTGTTGCCCTTAGGATCCGCGGTATC-3′) (the EcoRI site is underlined). The 403-bp PCR fragment containing PmxaF+operator was cloned into pCR2.1 to create pCR-MDHOP. Next, the kanamycin resistance gene of a 1,218-bp PstI fragment from pBK-miniTn7-ΩSm2 was cloned into the PstI site of pCM110, and then tetA and tetR were removed by restriction with BclI and self-ligation yielded pCHOI3. A 403-bp PstI-EcoRI fragment from pCR-MDHOP was then ligated between the PstI and EcoRI sites of pCHOI3; this replaced PmxaF with PmxaF+operator to form the cumate-inducible expression vector pCUM50.
To test heterologous protein production using this cumate switch system, we obtained an XbaI-ClaI fragment containing the gfp gene from pQBI63 and cloned it into SpeI-ClaI sites of pCUM50 to generate pCUM-gfp. The 2,100-bp fragment carrying the lactase gene (bgl) from Bifidobacterium infantis was amplified from pEBIG4 (15) using primers BGL-F-Nhe (5′-CGCTAGCGAACATAGAGCGTTCAAGTGGC-3′) (the NheI site is underlined) and BGL-R-Cla (5′-CATCGATTTACAGCTTGACGACGAGTACGCCG-3′) (the ClaI site is underlined). For the amplification of the esterase gene (1,800 bp; estI) of Lactobacillus casei, pCESTa (9) was used as a template with primers EST-F-Nhe (5′-GGCTAGCGATCAATCTAAAACAAATC-3′) (the NheI site is underlined) and EST-R-Cla (5′-CATCGATTTATTTATTTGTAATACCGTCTGC-3′) (the ClaI site is underlined). These NheI-ClaI fragments of bgl and est were then replaced with a gfp gene in pCUM-gfp to form pCUM-bgl and pCUM-est, respectively. The different proteins tested in the pCUM system were cloned via SpeI and ClaI (pCUM-gfp) or via NheI and ClaI (pCUM-bgl and pCUM-est).
Detection of gene expression.
Detection of GFP was carried out by fluorescence microscopy, and quantification was done using a microplate spectrofluorometer (SPECTRAFluor Plus; TECAN) under excitation and emission wavelengths of 485 and 508 nm, respectively. The concentration of GFP was calculated based on a linear relationship between concentration and fluorescence units determined using solutions of purified GFP (Qbiogene). The biomass was determined by cell dry weight measurement of the samples (Moisture Analyzer MA 30; Sartorius).
Esterase activity was determined by a spectrophotometric method using para-nitrophenyl caprylate as a substrate. The rate of hydrolysis of para-nitrophenyl caprylate at 37°C was measured in 50 mM sodium phosphate buffer (pH 7.0) according to the method described previously (9, 16). One unit of activity was defined as the amount of enzyme that liberated 1 μmol of p-nitrophenol per min under the given assay conditions. The β-galactosidase activity was measured with o-nitrophenol-β-d-galactopyranoside as a substrate, and one unit of activity was defined as the amount of enzyme that liberated 1 μmol of o-nitrophenol per min (25). The protein concentration was estimated by the method of Bradford (5) using the Bio-Rad protein assay kit with bovine serum albumin as a standard.
Western blotting.
Integrative expression of the repressor protein (CymR) was determined by Western blotting using a standard protocol. CymR was detected with rabbit anti-bCymR no. 422 antibody (0.1 g ml−1; in-house antibody generated by our group) and a goat anti-rabbit immunoglobulin G (heavy plus light chains) horseradish peroxidase conjugate (0.1 μg ml−1; catalog no. 31460; Pierce, West Grove, PA). Cells were lysed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer.
RESULTS AND DISCUSSION
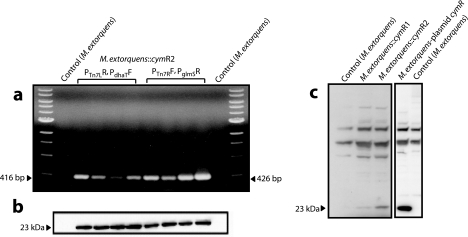
The construction of the cumate-regulated/inducible system in M. extorquens required the engineering both of the chromosome and of the cloned PmxaF promoter, described hereafter. The structural components required for an operational cumate-regulated expression system are the cymR gene, which encodes the CymR repressor protein, and the CymR binding DNA region fused to the 3′ end of a selected promoter. For the cumate-regulated system to work effectively in M. extorquens, the culture must be able to express and produce the functional repressor protein (CymR) constitutively. First, the 612-bp cymR gene from P. putida F1 was cloned into pCM110 under the control of the PmxaF promoter. CymR expression was confirmed by Western blot analysis (Fig. 1c). However, the culture grew very poorly (data not shown), indicating a potential toxicity of CymR to the host. In order to reduce the level of CymR production and, by inference, its toxicity, an alternative to the plasmid-based cymR expression was adopted. In the absence of information on the minimal amount of CymR required for optimal repression of transcription, DNA fragments containing either one or two copies of the cymR gene were integrated into the chromosome of M. extorquens, generating the clones M. extorquens (CymR1) and M. extorquens (CymR2), respectively. Integration was achieved using the mini-Tn7 transposon system described elsewhere (7, 8). Insertion of mini-Tn7-PmxaF-cymR into the unique Tn7 attachment site (attTn7) of M. extorquens was verified by colony PCR (Fig. 1a). Expression was once again confirmed by Western blot analysis (Fig. 1b). Importantly, growth of the cymR-expressing clones was indistinguishable from that of wild-type M. extorquens (data not shown). To verify if the levels of CymR produced by the transformants were sufficient to repress transcription and thereby regulate expression, an expression vector possessing the engineered PmxaF promoter had to be constructed. The expression vector (pCUM50) was constructed containing the operator sequence of the cmt operon from P. putida F1 downstream of the cloned homogeneous promoter, PmxaF, in pCHOI3, a derivative of pCM110 where the tetracycline marker was exchanged for kanamycin (Table 1). Three different constructs containing a gene encoding GFP, esterase, or β-galactosidase were inserted into the multiple cloning site downstream of the operator, generating pCUM-gfp, pCUM-est, or pCUM-bgl, respectively (Fig. 2). Expression of GFP in the recombinant host M. extorquens (CymR2) transformed with the expression vector pCUM-gfp was not detected in the absence of the inducer, cumate. However, the recombinant host M. extorquens (CymR1) transformed with the expression vector pCUM-gfp grown in the absence of the inducer showed leaky expression, amounting to 15 mg GFP/g dry biomass. This leaky expression represents about 20% of the GFP yield of a fully cumate-induced culture (80 mg GFP/g dry biomass). Incomplete repression in M. extorquens (CymR1) hosts was assumed to be due to the insufficient CymR production (Fig. 1c). Hence, competent cells containing two cymR copies [M. extorquens (CymR2)] were used for further studies.
FIG. 1.
Analysis of intracellular polypeptides from recombinant strains of M. extorquens::cymR expressing repressor protein. (a) Colony PCR profiles. The physical presence of the integrated cymR gene in the chromosome of M. extorquens was tested in eight randomly selected M. extorquens (CymR2) clones by colony PCR utilizing the primers described in Materials and Methods. The position and size of the expected PCR products are marked. PTn7LR, PdhaTF, PTn7RF, and PglmSR represent the primers used to generate PCR products. (b) The presence of the repressor protein in eight randomly selected clones (see panel a) was confirmed by Western blot analysis. Arrows indicate the position and the molecular size (23 kDa) of the repressor protein. (c) Western blot analysis of repressor protein expressed by native M. extorquens, a single-copy cymR integrant, a double-copy cymR integrant, or plasmid (pCM110)-expressed cymR, respectively.
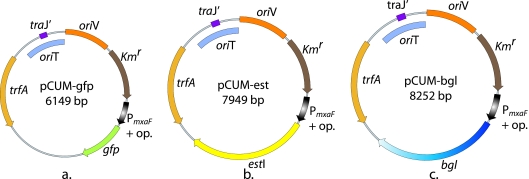
FIG. 2.
Maps of recombinant plasmids containing the required regulatory elements. Abbreviations: traJ′, mutated conjugal transfer gene that is missing the final 85 of 123 amino acids; oriT, origin of transfer; oriV, vegetative origin; trfA, replication initiator gene; Kmr, kanamycin resistance gene; PmxaF + op., methanol dehydrogenase combined with operator sequence from cmt operon of P. putida F1; gfp, green fluorescent protein-encoding gene; estI, esterase-encoding gene; and bgl, β-galactosidase-encoding gene.
M. extorquens (CymR2) transformed with the plasmids pCUM-gfp, pCUM-est, and pCUM-bgl grew well, and expression could not be detected in the absence of cumate. The addition of cumate to the culture reduces the binding capacity of the repressor protein (CymR) for the operator region, and transcriptional repression is thereby alleviated. Tight inducible expression of the reporter gene is thereby attained. The basic mechanism of this system is summarized in Fig. 3. The optimal inducer concentration for recombinant M. extorquens (CymR2) transformed with pCUM-gfp was determined in shake flask cultures grown to the mid-log phase (0.8 to 1.0 units at an optical density at 600 nm [OD600]) at 30°C, followed by induction with cumate at different concentrations, ranging from 1 to 30 μg cumate/ml. GFP expression was detectable at 4 h postinduction at all concentrations tested. Cumate induction was shown to be very sensitive, since the addition of 1 μg cumate/ml resulted in GFP production representing approximately 47% of the GFP yield of a culture fully induced with 15 μg cumate/ml (data not shown). Growth inhibition was not observed with the cumate concentrations tested (1 to 30 μg/ml), indicating that cumate does not negatively affect cell metabolism at these concentrations in shake flask experiments, where typical biomass yields are expected to reach 1.5 to 1.7 g of cells (dry weight)/liter. The optimal cumate concentration for effective induction of GFP expression in shake flask growth experiments was 15 μg/ml (approximately 0.1 mM). Understandably, in high-cell-density fermentations, where biomass yields can reach over 50 g of cells (dry weight) per liter, higher concentrations of cumate may be required.
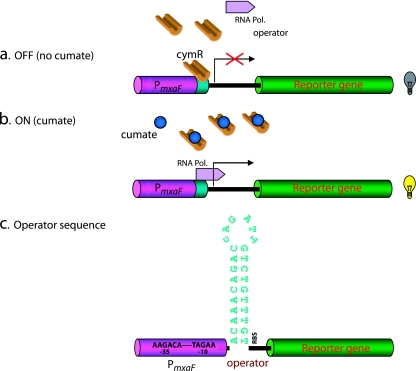
FIG. 3.
A schematic diagram of the mechanism of action of the cumate-switchable expression system. (a) In the absence of the inducer, cumate, the repressor protein (CymR) is bound to the operator site upstream of the reporter gene or gene of interest and inhibits transcription. (b) The presence of cumate is necessary for transcription of the gene of interest. The addition of cumate rapidly alters the binding of the operator to the repressor CymR, thereby facilitating the formation of the CymR-cumate complex. The detachment of CymR from the operator site activates transcription of the downstream reporter gene. The CymR-cumate complex is unable to bind to the operator site. (c) Nucleotide sequence of the operator, which represents the putative binding site for the helix-turn-helix transcription factor (see Materials and Methods for details of the construction of the PmxaF+operator fragment).
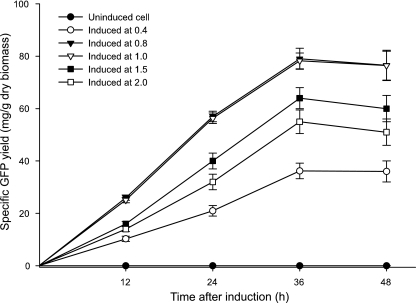
To determine the optimal time of induction for maximal protein production, M. extorquens (CymR2) cells stably transformed with pCUM-gfp were induced at different stages of growth (OD600 of 0.4, 0.6, 0.8, 1.0, and 1.2) in shake flask experiments with 15 μg cumate/ml. Cells induced at an OD600 of 0.8 to 1.0 generated maximal specific yields of GFP. At these induction points, the specific yield of the recombinant protein was consistently 20 to 50% greater than at other induction points (Fig. 4). However, it should be noted that stationary-phase cultures could still be induced, albeit resulting in reduced levels of expression (data not shown).
FIG. 4.
Identification of the optimal time of induction. Maximum gene expression was obtained when the culture was induced with 15 μg/ml cumate at an OD600 of 0.8 to 1.0. Induction at an OD600 of 0.4, 1.5, and 2.0 resulted in reduced expression of the target gene.
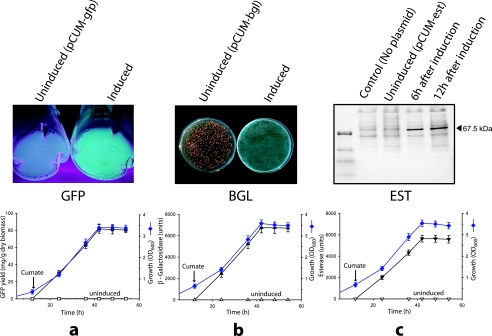
To further validate cumate inducibility, we expressed two other proteins (esterase and β-galactosidase) in the recombinant host strain M. extorquens (CymR2). These transformants were analyzed for induction profiles and expression levels and were compared to GFP-expressing cultures. All clones, cultured up to an OD600 of 0.8 to 1.0, did not produce detectable levels of recombinant proteins in the absence of cumate (Fig. 5). In all cases, at 4 h postinduction with 15 μg cumate/ml, expression of the recombinant proteins was detected. Production of GFP, esterase, and β-galactosidase remained active even after 24 h postinduction, with maximum expression at 30 h postinduction (Fig. 5a, b, and c). The recombinant protein expression was not detected in noninduced cells, and the expression occurred as long as there was cell growth. Using this system, 6.1 g GFP/liter and 5,637 ± 320 and 6,742 ± 480 U of recombinant esterase and β-galactosidase were obtained, respectively (Fig. 5a, b, and c).
FIG. 5.
Induction and production of recombinant proteins by the cumate-regulated expression system. The inducible expression system was validated with three different heterologous genes, encoding green fluorescent protein (a), β-galactosidase (b), and esterase (c). Without cumate induction, target proteins were not detected, as analyzed by UV for GFP, the chromogenic substrate (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) for β-galactosidase, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis for esterase. The lower lane shows the recombinant protein production and bacterial growth profiles from each construct. The arrow indicates the point of induction with cumate (15 μg/ml). Protein yield or enzyme activities for induced cultures, closed symbols (▪, ▴, and ▾); for uninduced cultures, open symbols (□, ▵, and ▿).
The well-characterized promoters (T5, T7, and lac) utilized in E. coli expression vectors require isopropyl-β-d-thiogalactopyranoside for induction. Although isopropyl-β-d-thiogalactopyranoside is an effective inducer for E. coli, it is expensive and can elicit cellular toxicity at concentrations which are deemed optimal for expression of recombinant proteins (14, 23). The innovative aspect of our inducible system is the fact that we have kept a powerful native promoter for heterologous gene expression, added an operator region downstream of the promoter, and integrated a repressor-encoding sequence in the chromosome which expresses sufficient repressor protein to repress expression of the target gene completely. Furthermore, cumate, a nontoxic organic and nonmetabolizable compound for M. extorquens, at low concentrations is capable of traversing the cell's outer membranes and alleviates repression effectively. Cumate did not cause any growth inhibition even at concentrations twofold higher than that required for optimal induction. This is the first documented description of a tightly regulated recombinant expression system in M. extorquens. Temporal expression of selected gene products and applications in metabolic flux and pathway engineering may now be possible with M. extorquens.
Acknowledgments
We gratefully acknowledge A. R. Theisen and M. Pacheco-Oliver for helpful comments and critical review of the manuscript.
Footnotes
Published ahead of print on 13 October 2006.
REFERENCES
- 1.Anthony, C. 1993. Methanol dehydrogenase in Gram-negative bacteria, p. 17-45. In V. Davidson (ed.), Principles and applications of quinoproteins. Dekker, New York, N.Y.
- 2.Bélanger, L., M. M. Figueira, D. Bourque, L. Morel, M. Béland, L. Laramée, D. Groleau, and C. B. Míguez. 2004. Production of heterologous proteins by Methylobacterium extorquens in high cell density fermentation. FEMS Microbiol. Lett. 231:197-204. [DOI] [PubMed] [Google Scholar]
- 3.Bourque, D., B. Ouellette, G. Andre, and D. Groleau. 1992. Production of poly-β-hydroxybutyrate from methanol: characterization of a new isolate of Methylobacterium extorquens. Appl. Microbiol. Biotechnol. 37:7-12. [Google Scholar]
- 4.Bourque, D., Y. Pomerleau, and D. Groleau. 1995. High cell density production of poly-beta-hydroxybutyrate (PHB) from methanol by Methylobacterium extorquens: production of high-molecular-mass PHB. Appl. Microbiol. Biotechnol. 44:367-376. [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Chistoserdova, L., S. W. Chen, A. Lapidus, and M. E. Lidstrom. 2003. Methylotrophy in Methylobacterium extorquens AM1 from a genomic point of view. J. Bacteriol. 185:2980-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi, K. H., J. B. Gaynor, K. G. White, C. Lopez, C. M. Bosio, R. R. Karkhoff-Schweizer, and H. P. Schweizer. 2005. A Tn7-based broad-range bacterial cloning and expression system. Nat. Methods 2:443-448. [DOI] [PubMed] [Google Scholar]
- 8.Choi, Y. J., D. Bourque, L. Morel, D. Groleau, and C. B. Míguez. 2006. Multicopy integration of heterologous genes and expression in the methylotroph Methylobacterium extorquens ATCC 55366. Appl. Environ. Microbiol. 72:753-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi, Y. J., C. B. Míguez, and B. H. Lee. 2004. Characterization and heterologous gene expression of a novel esterase from Lactobacillus casei CL96. Appl. Environ. Microbiol. 70:3213-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson, V. L. 1993. Methylamine dehydrogenase, p. 73-85. In V. Davidson (ed.), Principles and applications of quinoproteins.. Dekker, New York, N.Y.
- 11.Eaton, R. W. 1996. p-Cumate catabolic pathway in Pseudomonas putida F1: cloning and characterization of DNA carrying the cmt operon. J. Bacteriol. 178:1351-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaton, R. W. 1997. p-Cymene catabolic pathway in Pseudomonas putida F1: cloning and characterization of DNA encoding conversion of p-cymene to p-cumate. J. Bacteriol. 179:3171-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figueira, M. M., L. Laramée, J. C. Murrell, D. Groleau, and C. B. Míguez. 2000. Production of green fluorescent protein by the methylotrophic bacterium Methylobacterium extorquens. FEMS Microbiol. Lett. 193:195-200. [DOI] [PubMed] [Google Scholar]
- 14.Gombert, A. K., and B. V. Kilikian. 1998. Recombinant gene expression in Escherichia coli cultivation using lactose as inducer. J. Biotechnol. 60:47-54. [DOI] [PubMed] [Google Scholar]
- 15.Hung, M. N., Z. Xia, N. T. Hu, and B. H. Lee. 2001. Molecular and biochemical analysis of two β-galactosidases from Bifidobacterium infantis HL96. Appl. Environ. Microbiol. 67:4256-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kademi, A., L. Fakhreddine, N. Abdelkader, and J. C. Baratti. 1999. Effect of culture condition on growth and esterase production by the moderate thermophile Bacillus circulans MAS2. J. Ind. Microbiol. Biotechnol. 23:188-193. [Google Scholar]
- 17.Koch, B., L. E. Jensen, and O. Nybroe. 2001. A panel of Tn7-based vectors for insertion of the gfp marker gene or for delivery of cloned DNA into Gram-negative bacteria. J. Microbiol. Methods 45:187-195. [DOI] [PubMed] [Google Scholar]
- 18.Laukel, M., M. Rossignol, G. Borderies, U. Völker, and J. A. Vorholt. 2004. Comparison of the proteome of Methylobacterium extorquens AM1 grown under methylotrophic and nonmethylotrophic conditions. Proteomics 4:1247-1264. [DOI] [PubMed] [Google Scholar]
- 19.Marx, C. J., and M. E. Lidstrom. 2001. Development of improved versatile broad host-range vectors for use in methylotrophs and other Gram-negative bacteria. Microbiology 147:2065-2075. [DOI] [PubMed] [Google Scholar]
- 20.Marx, C. J., and M. E. Lidstrom. 2002. Broad-host-range cre-lox system for antibiotic marker recycling in Gram-negative bacteria. BioTechniques 33:1062-1067. [DOI] [PubMed] [Google Scholar]
- 21.Marx, C. J., and M. E. Lidstrom. 2004. Development of an insertional expression vector system for Methylobacterium extorquens AM1 and generation of null mutants lacking mtdA and/or fch. Microbiology 150:9-19. [DOI] [PubMed] [Google Scholar]
- 22.Marx, C. J., B. N. O'Brien, J. Breezee, and M. E. Lidstrom. 2003. Novel methylotrophy genes of Methylobacterium extorquens AM1 identified by using transposon mutagenesis including a putative dihydromethanopterin reductase. J. Bacteriol. 185:669-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menzella, H. G., E. A. Ceccarelli, and H. C. Gramajo. 2003. Novel Escherichia coli strain allows efficient recombinant protein production using lactose as inducer. Biotechnol. Bioeng. 82:809-817. [DOI] [PubMed] [Google Scholar]
- 24.Omer, Z. S., R. Tombolini, and B. Gerhardson. 2004. Plant colonization by pink-pigmented facultative methylotrophic bacteria (PPFMs). FEMS Microbiol. Ecol. 47:319-326. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., and D. W. Russell. 2000. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 26.Sy, A., A. C. Timmers, C. Knief, and J. A. Vorholt. 2005. Methylotrophic metabolism is advantageous for Methylobacterium extorquens during colonization of Medicago truncatula under competitive conditions. Appl. Environ. Microbiol. 71:7245-7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sy, A., E. Giraud, P. Jourand, N. Garcia, A. Willems, P. de Lajudie, Y. Prin, M. Neyra, M. Gillis, C. Boivin-Masson, and B. Dreyfus. 2001. Methylotrophic Methylobacterium bacteria nodulate and fix nitrogen in symbiosis with legumes. J. Bacteriol. 183:214-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trotsenko, Y. A., E. G. Ivanova, and N. V. Doronina. 2001. Aerobic methylotrophic bacteria as phytosymbionts. Microbiology 70:623-632. [Google Scholar]
- 29.Van Dien, S. J., C. J. Marx, B. N. O'Brien, and M. E. Lidstrom. 2003. Genetic characterization of the carotenoid biosynthetic pathway in Methylobacterium extorquens AM1 and isolation of a colorless mutant. Appl. Environ. Microbiol. 69:7563-7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang, M., and M. E. Lidstrom. 2003. Promoters and transcripts for genes involved in methanol oxidation in Methylobacterium extorquens AM1. Microbiology 149:1033-1040. [DOI] [PubMed] [Google Scholar]