Abstract
A wastewater tertiary treatment system based on membrane ultrafiltration and fed with secondary-treated municipal wastewater was evaluated for its Giardia cyst and Cryptosporidium oocyst removal efficiency. Giardia duodenalis (assemblages A and B) and Cryptosporidium parvum were identified in feed water but were found in filtered water only during occasional failure of the filtration system.
Treated wastewater can be utilized as an alternative water source for agricultural irrigation, but advanced treatment is required to minimize its potentially negative impact on public health. In particular, chlorine-based disinfection tools cannot destroy the resistant stages of protozoan parasites Giardia and Cryptosporidium (7, 11), and there is an urgent need for specific treatments to remove these protozoa from wastewater. Membrane technologies have a strong potential in this respect, and ultrafiltration (pore size of 0.002 to 0.1 μm) can achieve complete removal of protozoan cysts (4 to 15 μm) by physical sieving (2, 16). This is especially interesting for wastewater reclamation and makes it possible to avoid chemical disinfection and consequent possible formation of toxic by-products (14).
In a previous investigation, a pilot-scale membrane system was evaluated for reuse of treated wastewater in irrigation and gave very good performances in terms of suspended solids and bacterial removal (17). This work refers to the same experimental plant and reports results on (i) the presence of Giardia cysts and Cryptosporidium oocysts in well water used for irrigation in southern Italy and in secondary-treated wastewater from urban areas, (ii) the (oo)cyst removal efficiency of the tertiary treatment system based on membrane filtration, and (iii) the presence of (oo)cysts in soil and vegetables irrigated with well water and water filtered by the above-mentioned method.
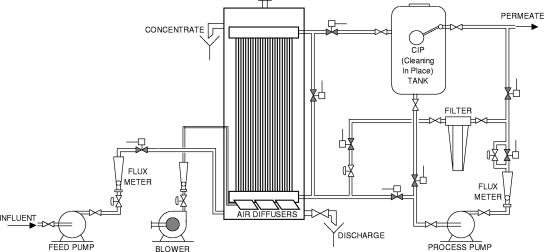
Wastewater tertiary treatment was carried out at the pilot scale by membrane ultrafiltration using a submerged hollow-fiber system. All details of the pilot plant, equipped with a Zenon ZeeWeed membrane module (Zenon Environmental Inc., Canada), were provided in a previous article (17). A simplified scheme of the pilot plant is shown in Fig. 1. The permeate after filtration was collected and stored in six tanks (5 m3 each). Although each irrigation required about 15 m3 of water, a total stored volume of 30 m3 was always available in order to match the continuous plant operation with the discontinuous demand for treated water for irrigation and to have a buffer of treated water in case of possible equipment failures. The test field was located about 100 m from the pilot plant and was connected to the storage tanks by a pipeline. In this experimental field, 2-year studies were carried out to compare, on four crops in succession (fennel, lettuce, chicory, and processing tomato), two types of water: tertiary filtered municipal wastewater and well water commonly used for irrigation.
FIG. 1.
Scheme of the membrane filtration pilot plant.
Giardia cyst and Cryptosporidium oocyst detection in water and wastewater was performed according to the ICR protozoan method for Giardia cysts and Cryptosporidium oocysts (20), except that all centrifugation steps were performed at 1,800 × g for 15 min. Briefly, 50-liter samples were filtered through a yarn-wound cartridge filter of 1-μm nominal porosity. The fluid obtained from filter washing was concentrated by centrifugation, and the sediment was subjected to Percoll-sucrose (specific gravity, 1.10) flotation. (Oo)cysts were identified by immunofluorescence (IF), using a commercially available kit (Meridian Diagnostics, Cincinnati, Ohio) and a fluorescence microscope (Nikon) at ×400 magnification. Most samples were also subjected to molecular analysis after genomic DNA extraction using a QIAamp DNA stool mini kit (QIAGEN). For Giardia spp., a nested-PCR procedure was carried out to amplify a 130-bp region from the small-subunit ribosomal DNA by use of the primers RH11 and RH4 (10) and the primers GiarR and GiarF (18). Regarding Cryptosporidium spp., an ∼400-bp region of the N-terminal domain of the COWP gene was amplified by a seminested PCR using the primers CRY15D and CRY9D (19) and the primer CRYINT2D (15). For both Giardia and Cryptosporidium spp., positive and negative controls were included in each batch of PCR. The final products were visualized on 2% agarose gel with ethidium bromide. PCR products were then purified using a NucleoSpin extract (Macherey-Nagel) purification kit and sequenced by the MWG DNA sequencing service (MWG Biotech AG, Germany). The obtained sequences were aligned using Clustal W and compared with those available in GenBank for Giardia and Cryptosporidium species and genotype identification.
In a first set of experiments, the presence of Giardia cysts and Cryptosporidium oocysts was investigated in secondary-treated municipal wastewater after activated sludge and sedimentation. Giardia cysts were found in four of four wastewater samples at a concentration of 1.83 × 103 ± 1.81 × 103/liter (mean ± standard deviation). These samples were also PCR positive and after sequencing were determined to belong to the Giardia duodenalis assemblages A (three isolates) and B (one isolate). Cryptosporidium oocysts were detected in two of four of the same samples at a concentration of 5.62 × 101 ± 8.06 × 101/liter (mean ± standard deviation). These microscopically positive samples were successfully amplified by PCR and sequenced, aligning with the Cryptosporidium parvum COWP sequence available in GenBank.
The secondary-treated wastewater was used as feed water for the tertiary treatment based on membrane filtration. Table 1 shows the results in terms of Giardia cysts and Cryptosporidium oocysts detected by IF and PCR in filtered water. In the first period of this investigation, from February 2004 to February 2005, preliminary results indicated a good (oo)cyst removal efficiency of Giardia cysts by the ultrafiltration system (99.98%, 3.6 log), while the Cryptosporidium oocyst removal results were limited by the low amount in the feed and in the permeate. However, the presence of (oo)cysts in the permeate suggested the need for complete examination of the filtration plant and the membrane module for possible failures, since the membrane nominal porosity (0.03 μm) should guarantee complete (oo)cyst removal. A thorough test of the system revealed the imperfect sealing of a connection on the permeate extraction pipeline, which had probably caused partial leakages of the wastewater contained in the filtration tank, with consequent contamination of the permeate.
TABLE 1.
Giardia cysts and Cryptosporidium oocysts detected by direct IF microscopy and PCR in filtered water (FW) before and after plant overhaul and in well water
| Type of water |
Giardia
|
Cryptosporidium
|
||||
|---|---|---|---|---|---|---|
| No. of samples positive/no. of samples examined by IF | Mean no. of cysts/liter (±SD) | No. of samples positive/no. of samples examined by PCR | No. of samples positive/no. of samples examined by IF | Mean no. of oocysts/liter | No. of samples positive/no. of samples examined by PCR | |
| FW before plant overhaul | 9/13 | 0.43 ± 0.1 | 2/3 | 1/13 | 0.2 | 0/1 |
| FW after plant overhaul | 0/10 | 0 | 0/10 | 0/10 | 0 | 0/10 |
| Well water | 2/19 | 0.55 ± 0.07 | 2/8 | 0/19 | 0 | 0/8 |
After a complete overhaul of the pilot plant, 10 permeate samples were collected between April 2005 and July 2006 and tested negative for the presence of Giardia cysts and Cryptosporidium oocysts, thus indicating the effectiveness of the measures adopted. All of these samples were also subjected to nested PCR for Giardia and Cryptosporidium and tested negative (Table 1).
Moreover, Giardia cysts belonging to G. duodenalis assemblage A were present in 2 of 19 water samples collected between June 2004 and July 2006 from a phreatic well used for irrigation (Table 1).
In addition, soil samples, collected as cylinders at a depth of 0 to 10 cm, were taken from the areas watered with well water (16 samples) or filtered water (16 samples). To search for (oo)cysts in soil, 30 g of the soil samples was diluted in 50 ml of buffered detergent solution and vortexed by magnetic stirring for 15 min. The suspension was then filtered through a double gauze. After further washing of the gauze with buffered detergent solution, the filtrate was centrifuged at 1,800 × g for 10 min. Then, the pellet was clarified by Percoll-sucrose and parasites were identified by IF as described above. Giardia cysts (53.5/10 g) and Cryptosporidium oocysts (82/10 g) were found in one sample watered with well water, while (oo)cysts were never found in soil samples watered with filtered wastewater.
Finally, 22 different vegetable samples, 11 irrigated with filtered water and 11 with well water, were collected at harvesting time. After extensive washing of 100 g from all items with buffered detergent solution, the washing water was centrifuged at 1,800 × g for 15 min, the pellet was subjected to Percoll-sucrose flotation, and then (oo)cysts were identified by IF. All of these samples were negative for the presence of Giardia cysts, while Cryptosporidium oocysts were found in two samples (lettuce and fennel) watered with filtered water before the definitive overhaul of the filtration plant.
Regarding the agreement between immunofluorescence microscopy and PCR for (oo)cyst detection, the results obtained with Giardia were in accordance in all but two well water samples, one IF positive (with low cyst concentration) and PCR negative and the other IF negative but PCR positive. For Cryptosporidium, only one sample of filtered water that tested IF positive with very low oocyst numbers was found to be PCR negative.
The results of the present study suggest that the membrane filtration system under investigation is useful for removal of potentially pathogenic protozoan cysts from wastewater to be used in agriculture. This confirms previous investigations showing that ultrafiltration membranes provide high log removal of Cryptosporidium oocysts and Giardia cysts (12, 13), also in the context of the U.S. EPA Environmental Technology Verification (ETV) program (http://www.epa.gov/etv/pdfs/vrvs/02_vr_zenon_or.pdf; http://www.epa.gov/etv/pdfs/vrvs/02_vs_zenon_pa.pdf).
In the present work, higher concentrations of Giardia cysts than Cryptosporidium oocysts were found in municipal wastewater. This confirms previous studies on wastewater carried out in Italy (5) and suggests a high circulation of Giardia infection in humans. In this respect, giardiasis and cryptosporidiosis are not notifiable diseases in Italy and epidemiological data are based on specialized studies which suggest an overall prevalence of Giardia infection of 2.7% (8). Regarding cryptosporidiosis, 1.7% of 359 immunocompetent hospitalized children with enteritis were infected in southern Italy (3), but a higher prevalence (11%) was found in human immunodeficiency virus-infected adult patients before the introduction of highly active antiretroviral therapy (4). In addition, our results have demonstrated that Giardia cysts present in well water commonly used for irrigation belonged to zoonotic assemblages A and B, thus suggesting a possible risk for human health from this water source for irrigation (1, 6).
Finally, the results of this investigation underline the importance of continuous monitoring of the ultrafiltration system integrity by searching for (oo)cysts in the permeate in order to allow rapid detection of possible failures in the filtration plant. It should also be noted that the search for (oo)cysts in reclaimed water cannot be surrogated efficiently by the search for other indicator organisms, such as Clostridium perfringens, as was previously reported (9).
In conclusion, the tested ultrafiltration system gave good results in terms of Giardia and Cryptosporidium removal from wastewater for irrigation, suggesting that membrane-based technologies are suitable for water reclamation in semiarid areas, which often lack natural water resources.
Acknowledgments
Monitoring of Giardia cysts and Cryptosporidium oocysts was supported in part by a grant from the Ministero dell'Istruzione, dell'Università e della Ricerca, Rome, Italy, COFIN-PRIN 2004, project Giardia and Cryptosporidium: analysis of zoonotic potential of environmental isolates and evaluation of removal efficiency of new filtration method for wastewater.
Pilot plant and reuse experiments were performed within the project AQUATEC, funded by the Italian Ministry of Research (PON Ricerca 2000-2006).
Footnotes
Published ahead of print on 20 October 2006.
REFERENCES
- 1.Berrilli, F., D. Di Cave, C. De Liberato, A. Franco, P. Scaramozzino, and P. Orecchia. 2004. Genotype characterisation of Giardia duodenalis isolates from domestic and farm animals by SSU-rRNA gene sequencing. Vet. Parasitol. 122:193-199. [DOI] [PubMed] [Google Scholar]
- 2.Betancourt, W. Q., and J. B. Rose. 2004. Drinking water treatment processes for removal of Cryptosporidium and Giardia. Vet. Parasitol. 126:219-234. [DOI] [PubMed] [Google Scholar]
- 3.Brandonisio, O., A. Marangi, M. A. Panaro, R. Marzio, M. I. Natalicchio, P. Zizzadoro, and U. De Santis. 1996. Prevalence of Cryptosporidium in children with enteritis in southern Italy. Eur. J. Epidemiol. 12:187-190. [DOI] [PubMed] [Google Scholar]
- 4.Brandonisio, O., P. Maggi, M. A. Panaro, S. Lisi, A. Andriola, A. Acquafredda, and G. Angarano. 1999. Intestinal protozoa in HIV-infected patients in Apulia, south Italy. Epidemiol. Infect. 123:457-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caccio, S. M., M. De Giacomo, F. A. Aulicino, and E. Pozio. 2003. Giardia cysts in wastewater treatment plants in Italy. Appl. Environ. Microbiol. 69:3393-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caccio, S. M., R. C. Thompson, J. McLauchlin, and H. V. Smith. 2005. Unravelling Cryptosporidium and Giardia epidemiology. Trends Parasitol. 21:430-437. [DOI] [PubMed] [Google Scholar]
- 7.Carey, C. M., H. Le, and J. T. Trevors. 2004. Biology, persistence and detection of Cryptosporidium parvum and Cryptosporidium hominis oocysts. Water Res. 38:818-862. [DOI] [PubMed] [Google Scholar]
- 8.Crotti, D., M. L. D'Annibale, G. Fonzo, M. Lalle, S. M. Cacciò, and E. Pozio. 2005. Dientamoeba fragilis is more prevalent than Giardia duodenalis in children and adults attending a day care centre in central Italy. Parasite 12:165-170. [DOI] [PubMed] [Google Scholar]
- 9.Harwood, V. J., A. D. Levine, T. M. Scott, V. Chivukula, J. Lukasik, S. R. Farrah, and J. B. Rose. 2005. Validity of the indicator organism paradigm for pathogen reduction in reclaimed water and public health protection. Appl. Environ. Microbiol. 71:3163-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopkins, R. M., B. P. Meloni, D. M. Groth, J. D. Wetherall, J. A. Reynoldson, and R. C. Thompson. 1997. Ribosomal RNA sequencing reveals differences between the genotypes of Giardia isolates recovered from humans and dogs living in the same locality. J. Parasitol. 83:44-51. [PubMed] [Google Scholar]
- 11.Hunter, P. R., and R. C. Thompson. 2005. The zoonotic transmission of Giardia and Cryptosporidium. Int. J. Parasitol. 35:1181-1190. [DOI] [PubMed] [Google Scholar]
- 12.Jacangelo, J. G., R. Rhodes Trussell, and M. Watson. 1997. Role of membrane technology in drinking water treatment in the United States. Desalination 113:119-127. [Google Scholar]
- 13.Jacangelo, J. G., S. S. Adham, and J. M. Laine. 1995. Mechanism of Cryptosporidium, Giardia, and MS2 virus removal by MF and UF. J. Am. Water Works Assoc. 87:107-121. [Google Scholar]
- 14.Lawrence, P., S. Adham, and L. Barrot. 2002. Ensuring water re-use projects succeed institutional and technical issues for treated wastewater re-use. Desalination 152:291-298. [Google Scholar]
- 15.Molini, U., D. Traversa, G. Cerchia, R. Iorio, L. Boffo, A. Zentilin, G. Capelli, and A. Giangaspero. Temporal occurrence of Cryptosporidium in the Asian clam Ruditapes philippinarum in the northern Adriatic Italian lagoons. J. Food Prot., in press. [DOI] [PubMed]
- 16.Ottoson, J., A. Hansen, B. Bjorlenius, H. Norder, and T. A. Stenstrom. 2006. Removal of viruses, parasitic protozoa and microbial indicators in conventional and membrane processes in a wastewater pilot plant. Water Res. 40:1449-1457. [DOI] [PubMed] [Google Scholar]
- 17.Pollice, A., A. Lopez, G. Laera, P. Rubino, and A. Lonigro. 2004. Tertiary filtered municipal wastewater as alternative water source in agriculture: a field investigation in southern Italy. Sci. Total Environ. 324:201-210. [DOI] [PubMed] [Google Scholar]
- 18.Read, C., J. Walters, I. D. Robertson, and R. C. A. Thompson. 2002. Correlation between genotype of Giardia duodenalis and diarrhoea. Int. J. Parasitol. 32:229-231. [DOI] [PubMed] [Google Scholar]
- 19.Traversa, D., A. Giangaspero, U. Molini, R. Iorio, B. Paoletti, D. Otranto, and C. Giansante. 2004. Genotyping of Cryptosporidium isolates from Chamelea gallina clams in Italy. Appl. Environ. Microbiol. 70:4367-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Environmental Protection Agency. 1995. ICR protozoan method for Giardia cysts and Cryptosporidium oocysts. EPA/814-B-95-003, June 1995. U.S. Environmental Protection Agency, Washington, D.C.