Abstract
Expression of the Saccharomyces cerevisiae DPM1 gene (coding for dolichylphosphate mannose synthase) in Trichoderma reesei (Hypocrea jecorina) increases the intensity of protein glycosylation and secretion and causes ultrastructural changes in the fungal cell wall. In the present work, we undertook further biochemical and morphological characterization of the DPM1-expressing T. reesei strains. We established that the carbohydrate composition of the fungal cell wall was altered with an increased amount of N-acetylglucosamine, suggesting an increase in chitin content. Calcofluor white staining followed by fluorescence microscopy indicated changes in chitin distribution. Moreover, we also observed a decreased concentration of mannose and alkali-soluble β-(1,6) glucan. A comparison of protein secretion from protoplasts with that from mycelia showed that the cell wall created a barrier for secretion in the DPM1 transformants. We also discuss the relationships between the observed changes in the cell wall, increased protein glycosylation, and the greater secretory capacity of T. reesei strains expressing the yeast DPM1 gene.
The saprobic fungus Trichoderma reesei secretes a wide range of hydrolytic enzymes, such as cellulases and hemicellulases, which are widely used in the food, animal feed, and paper industries (10). Hence, stimulation of its secretory capacity is of considerable interest for biotechnology. Many, if not all, of these extracellular proteins are glycosylated. Our previous study showed a close correlation between protein secretion and the activity of dolichylphosphate mannose (DPM)-synthase (EC 2.4.1.83), a key enzyme in O glycosylation in T. reesei (15, 17). We have shown that in T. reesei, DPM, which is synthesized by DPM-synthase, donates the mannosyl residue that is transferred to the hydroxyl group of serine or threonine in protein O mannosylation (16). Moreover, T. reesei DPM-synthase, like its counterpart from rat liver (3), is activated in vitro by cyclic AMP-dependent protein kinase (18). An obligatory requirement for DPM-synthase in O mannosylation was demonstrated for Saccharomyces cerevisiae by the finding that a temperature-sensitive DPM-synthase mutant (dpm1) was completely blocked in O mannosylation of the model protein chitinase (24). Loss of DPM1 expression in yeast is lethal (24). DPM-synthase also participates in N glycosylation of protein, supplying the last four mannosyl residues during the assembly of the lipid-linked precursor oligosaccharide dolichol diphosphate-GlcNAc2Man9Glc3, and is required for the biosynthesis of glycosylphosphatidylinositol membrane anchors (11).
Our earlier data indicated that overexpression of the S. cerevisiae DPM1 gene encoding DPM-synthase in T. reesei elevated the enzyme activity twofold and resulted in an increased level of protein secretion. The secreted proteins were glycosylated to the same extent as in the control, although at a level up to seven times higher (15).
We have also isolated the dpm1 gene encoding DPM-synthase from T. reesei and tried, albeit unsuccessfully, to increase the DPM-synthase activity by overexpressing this gene (29). It must be stressed, however, that the DPM-synthase from T. reesei belongs to the human group of Dpm1 proteins and requires two other subunits (Dpm2p and Dpm3p) to be stably expressed in endoplasmic reticulum (ER) membranes (5, 19). This is in contrast to the S. cerevisiae DPM-synthase, which is a cytoplasmically oriented protein embedded in the ER membranes through its C-terminal hydrophobic domain.
Overexpression of the yeast DPM-synthase gene caused spectacular changes in the cell wall morphology of T. reesei through an unknown mechanism. Alterations in the cell wall structure reported in earlier publications were generated mostly by manipulation of genes coding for fungal enzymes engaged in the synthesis of major cell wall components. Disruption of the chitin synthase gene csmA or chsB in Aspergillus oryzae reduced chitin content and caused abnormalities in hyphal walls, tips, and septa, rendering the mutant strains sensitive to osmotic stress and chitin-binding dyes (21). The mutants formed fewer large inseparable clumps due to more-branched mycelia, which resulted in better aeration of the culture. A simple correlation was seen between chitin synthase and cell wall composition and morphology, in contrast to the more-complicated relationship between glycosylation and cell wall assembly. Nonetheless, experiments done on a Hansenula polymorpha mutant defective in GDP-mannose production revealed changes in cell wall morphology (1). The mutation reduced glycosylation and caused changes in cell wall morphology as well as in protein production and secretion.
Here, we present results of a biochemical characterization of T. reesei strains (JSK97/1, JSK97/2, JSK97/3, and JSK97/6) obtained by overexpression of the yeast DPM1 gene and discuss the role of cell wall morphology and increased glycosylation in protein secretion.
MATERIALS AND METHODS
The study was done on T. reesei strains with enhanced DPM-synthase activity by introduction of multiple copies of the S. cerevisiae DPM1 gene under the T. reesei pki1 (pyruvate kinase-encoding) gene promoter, as described previously (15).
T. reesei TU-6, a Δpyr4 mutant of T. reesei QM9414 (10) (control strain) and DPM1-transformed strains JSK97/1, JSK97/2, JSK97/3, and JSK97/6 were cultivated at 30°C on a rotary shaker (250 rpm) in 2-liter flasks containing 1 liter of minimal medium (MM) with 1 g MgSO4 · 7 H2O, 6 g (NH4)2SO4, 10 g KH2PO4, 3 g sodium citrate · 2 H2O, trace elements (25 mg FeSO4 · 7 H2O, 2.7 mg MnCl2 · 4 H2O, 6.2 mg ZnSO4 · 7 H2O, 14 mg CaCl2 · 2 H2O), and 1% lactose as a carbon source. For all experiments, T. reesei strains were cultivated for 200 h, when the protein secretion reached maximum, and all cultures were in a similar phase of growth.
Phase-contrast microscopy.
T. reesei transformants and the control strain were harvested by centrifugation, washed twice with sterile water, and observed under a Docuval phase-contrast microscope (Carl-Zeiss, Jena, Germany).
Fluorescence microscopy.
Mycelia were collected by centrifugation at 4,000 × g for 5 min and washed twice with sterile water. The cells were resuspended in an aqueous solution of calcofluor white M2R (1 mg ml−1) (26), incubated for 15 min at room temperature, and then centrifuged at 4 000 × g for 5 min. To remove the excess of calcofluor white, the mycelia were washed five times with water. The mycelia were examined under a Nikon Eclipse E 6800 fluorescent microscope using fluorescence filter UV-2A (excitation wavelength, 365/366 nm).
Colony growth rate.
Colony growth rates were measured as described by Oka et al. (23). Conidia from the strains were point inoculated into the center of agar MM plates with or without calcofluor white (300 μg ml−1) (23) and incubated at 30°C. Colony diameters were measured at 48, 72, 96, 120, and 144 h. Growth measurements for all strains were done six times. The data are presented as percentages of growth inhibition caused by calcofluor white compared to the growth on the control plates.
Staining of cellobiohydrolase I in the cell wall of T. reesei.
After being cultured, the T. reesei cells were washed twice with 0.1 M phosphate-buffered saline (PBS), pH 7.4, centrifuged for 2 min at 4,400 × g at 4°C, and subjected to immunostaining. Before the cells were incubated with the antibody, nonspecific binding was blocked by incubation for 2 h at 4°C with 5% (wt/vol) skimmed milk and 0.02% (wt/vol) sodium azide in PBS buffer. Following the washes with 0.1 M PBS, the mycelia were incubated overnight at 4°C in Tris-buffered saline (0.05 M Tris-0.15 M NaCl [pH 5.0]) containing a primary mouse monoclonal antibody against Cel7a (2). The liquid phase was removed by centrifugation, and the mycelia were washed five times in 0.1 M PBS buffer. Subsequently they were incubated for 2 h in the dark at 4°C with a secondary antibody (fluorescein isothiocyanate [FITC]-conjugated anti-mouse immunoglobulin G; Sigma) in PBS. The cells were then centrifuged, resuspended in a solution containing 90% (wt/vol) glycerol, 10% 0.1 M PBS buffer, and 0.06 M n-propylgallate, shaken, and centrifuged again. The FITC-stained hyphae were viewed in a fluorescence microscope at ×100 magnification, using a standard FITC filter (21).
Cell wall preparation.
Mycelia were harvested by centrifugation, homogenized in a Beadbeater with 0.5-mm glass beads in 50 mM Tris-HCl (pH 7.5) with 1 mM dithiothreitol and centrifuged at 1,500 × g for 10 min. The resulting pellet containing cell walls was washed with 1 M ice-cold NaCl until the supernatant was no longer absorbed at 260 to 280 nm in the UV light (22).
Determination of carbohydrate content.
Total hydrolysis of cell wall glycans was done with 2 M trifluoroacetic acid at 100°C with prior metanolysis in 1.5 M HCl in anhydrous methanol at 85°C for 16 h (30). Neutral sugars and hexosamines were determined in the hydrolysates by high-performance anion-exchange chromatography with pulsed amperometric detection, using a Dionex Series 4500i system. Neutral sugars and hexosamines were eluted with 18 mM NaOH at 1 ml min−1.
Determination of polysaccharides.
The amount of glucans in the cell was determined as described previously (8, 23), with a slight modification. For quantification of alkali-soluble β-(1,6) glucan, cell walls (200 mg) were suspended in 3% NaOH, heated at 75°C for 1 h, and centrifuged. The supernatant was dialyzed overnight at 4°C against distilled water and lyophilized, and the amount of alkali-soluble β-(1,6) glucan was estimated by the method described by Dubois et al. (9). The remaining pellet was washed twice with 0.1 M Tris-HCl [pH 7.4] and once with 10 mM Tris-HCl [pH 7.4] and digested overnight with zymolyase 20T (5 mg/ml in 10 mM Tris-HCl [pH 7.4]; ICN Biomedicals Inc.). Then the samples were centrifuged (13,000 rpm; 15 min), and the supernatant was used to estimate the amount of alkali-insoluble β-(1,3) glucan by the same method (9). The remaining pellets were incubated for 16 h with 70% sulfuric acid at 4°C and then diluted 10-fold with water and heated to 100°C for 8 h. After being neutralized with 2 M NaOH, the samples were used to estimate the amount of alkali-insoluble β-(1,6) glucan (9).
Protein secretion by protoplasts and mycelia.
T. reesei cells were cultivated for 200 h in 1-liter flasks containing 200 ml of MM with 0.5% glucose as a carbon source. The mycelia were then harvested, washed with water at 4°C, resuspended in 20 ml of 0.9 M KCl in 50 mM phosphate buffer [pH 6.0] supplemented with 100 mg of a lytic-enzyme mixture from T. harzianum (Sigma Chemical Co., St. Louis, MO) and 50 mg of yeast lytic enzyme (ICN Biomedicals Inc.) and incubated for 18 h at 28°C. The resultant protoplasts were filtered through a Schott 2 funnel, centrifuged for 10 min at 1,000 × g (4°C), and resuspended in 0.9 M KCl in 50 mM phosphate buffer [pH 6.0] at a concentration of 1 × 107 to 5 × 107 protoplasts per ml. Protein secretion was induced with 7 mM sophorose (Sigma Chemical Co., St. Louis, MO). To a total volume of 5 ml of protoplast suspension, 2 μCi [14C]leucine (74 kBq ml−1; Amersham) was added, and the mixture was incubated for 5 h at 28°C. Secreted proteins were precipitated with trichloroacetic acid, and leucine incorporation into the precipitate was measured in a scintillation cocktail (14). Protein secretion from the mycelia was measured as described for the protoplasts.
To exclude the possibility that the secreted proteins included cytosolic proteins, we examined the protoplasts under the microscope before and after the experiment, lysed the mycelia and protoplasts, and measured the remaining radioactivity. In addition, viability of the protoplasts was examined after the experiment by regeneration in top agar with sorbitol.
RESULTS
Microscopic analysis of mycelial branching.
T. reesei strains overexpressing the yeast DPM1 gene secrete up to seven times more protein than the control strain (15). Since secretion in filamentous fungi is believed to occur mostly at the advancing tip of hyphae (4, 20), it is possible that the mycelia of the transformants were more branched than those of the control. Thus, we examined the transformed cells by phase-contrast microscopy for possible occurrence of “extra branching.” One hundred pictures of every transformant and control were analyzed, but no statistically significant differences in the branching of the mycelia of the control and that of the transformed strains were found (about two branches per 2.5-μm hyphae).
Carbohydrate composition of the cell wall.
The morphological changes observed previously as a consequence of DPM1 overexpression could be generated by changes in the sugar composition of the cell wall. Therefore, we examined the carbohydrate content in the cell walls of the transformed strains and the control. The results revealed differences in the amounts of neutral and amino sugars. The cell walls of the transformed strains contained from 51 to 68% more N-acetylglucosamine (GlcNAc) than the control strain, and at the same time, 8 to 24% less glucose (Table 1). Moreover, mannose content decreased to just over 30% of that found in the control strain. These data suggest that overexpression of the yeast DPM1 gene in T. reesei influences the content of the cell wall carbohydrate polymers.
TABLE 1.
Sugar composition of the cell walls of T. reesei DPM1 transformants JSK97/1, JSK97/2, JSK97/3, and JSK97/6 and the parental strain TU-6a
| Sugar | Amt of sugar per indicated strain (μg sugar/mg cell wall)
|
||||
|---|---|---|---|---|---|
| TU-6 | JSK97/1 | JSK97/2 | JSK97/3 | JSK97/6 | |
| GlcNAc | 34.4 | 57.68 | 51.7 | 55.2 | 52.2 |
| GalNAc | 15.7 | 10.0 | 8.3 | ND | 8.4 |
| Gal | 165.9 | 101.8 | 91.8 | 96.5 | 98.2 |
| Gluc | 346.1 | 268.9 | 318.2 | 263.0 | 305.4 |
| Man | 126.9 | 47.1 | 41.1 | 48.2 | 43.4 |
Cell walls of each strain were isolated after 200 h of growth in minimal medium containing lactose as a carbon source. The values presented are the averages of the results of two independent experiments. ND, not detected.
Determination of levels of cell wall polymers.
Cell wall permeability is closely connected with the levels of mannoproteins and alkali-soluble β-(1,6) glucan (6, 7). We determined the amounts of cell wall glycans in the DPM1-overexpressing strains and compared them to that of the control (Table 2). The levels of alkali-soluble β-(1,6) glucan were decreased in all the transformants and varied from 77.4 to 81.4% of that in the control strain. No differences between strains were found in the amounts of β-(1,3) glucan and alkali-insoluble β-(1,6) glucan.
TABLE 2.
Glucans in the cell walls of T. reesei DPM1 transformants JSK97/1, JSK97/2, JSK97/3, and JSK97/6 and the parental strain TU-6a
| Strain | Αmt of indicated glucan per strain (μg/mg dry cell wall)
|
||
|---|---|---|---|
| β-(1,3) glucan | Alkali-soluble β-(1,6) glucan | Alkali-insoluble β-(1,6) glucan | |
| TU-6 | 248.78 ± 7.02 | 52.41 ± 0.49 | 29.40 ± 0.55 |
| JSK97/1 | 247.48 ± 1.53 | 40.73 ± 0.62 | 29.05 ± 0.01 |
| JSK97/2 | 248.13 ± 1.00 | 42.37 ± 0.47 | 29.06 ± 0.08 |
| JSK97/3 | 248.13 ± 0.03 | 40.57 ± 0.37 | 29.07 ± 0.06 |
| JSK97/6 | 248.38 ± 0.36 | 41.13 ± 0,06 | 29.15 ± 0.25 |
The data presented are the means ± the standard deviations of the results for three separate cultures. Cell walls were isolated from the strains after 200 h of growth in lactose minimal medium.
Calcofluor white staining of cell wall polymers.
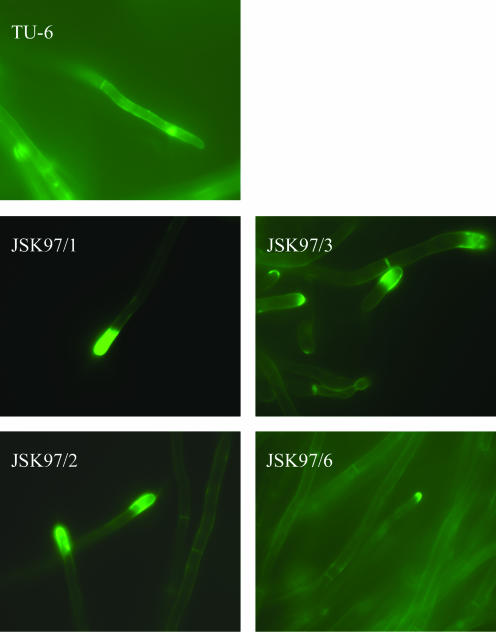
The elevated level of GlcNAc suggested that the chitin content was higher in the cell walls of the transformed cells. The increased amount of chitin should be expected to stiffen the cell wall, make it less permeable, and perturb secretion. Nonetheless, we observed up to seven-times-higher secretion levels in the strains bearing the yeast DPM1 gene (15). To elucidate this result, we stained the transformed and control cells with calcofluor white, a fluorochrome with high affinity for chitin, and examined the sugar polymers by fluorescence microscopy. We observed pronounced differences in chitin localization. In the DPM1-transformed cells, chitin was localized mainly in the cross walls, whereas in the control, it was distributed evenly along the cell wall (Fig. 1). This result explains why the cell wall of the transformants remains permeable despite the elevated overall content of chitin.
FIG. 1.
Calcofluor white staining of chitin in T. reesei DPM1 transformants JSK97/1, JSK97/2, JSK97/3, and JSK97/6 and the parental strain TU-6. Magnification, ×100. About 100 pictures were analyzed for each strain.
We also noticed different kinds of variations in hyphal diameter in the control and transformed T. reesei strains. Hyphae of the JSK97/3 strain were statistically about twice as wide as that of the control strain, but at the same time, JSK97/1 had mycelia only half the size of those of the control. On the other hand, the hyphae diameter of JSK97/2 and JKS97/6 stayed the same as in the control strain. Because these effects were not the same for every transformant, we concluded that they were not caused by DPM1 overexpression.
Hypersensitivity of DPM1-expressing strains to calcofluor white.
Calcofluor white is known to be absorbed into cell wall chitin and in consequence to exhibit antifungal properties. Hyphal growth of the DPM1 transformants was significantly inhibited by calcofluor white compared to hyphal growth of the control strain without calcofluor (Table 3). In contrast to that of the transformants, growth of the control strain was not altered.
TABLE 3.
Growth rate of DPM1-transformed T. reesei strains JSK97/1, JSK97/2, JSK97/3, and JSK97/6 and the parental strain TU-6a
| Strain | Growth rate in:
|
% Inhibition | |
|---|---|---|---|
| Minimal medium | Minimal medium + calcofluor white | ||
| TU-6 | 0.39 ± 0.024 | 0.41 ± 0.015 | 0 |
| JSK97/1 | 0.57 ± 0.021 | 0.32 ± 0.025 | 44 |
| JSK97/2 | 0.44 ± 0.017 | 0.27 ± 0.017 | 39 |
| JSK97/3 | 0.57 ± 0.019 | 0.38 ± 0.014 | 33 |
| JSK97/6 | 0.42 ± 0.029 | 0.27 ± 0.026 | 36 |
Growth rates are given in mm h−1. The data presented are the means ± the standard deviations of the results from six separate cultures.
Immunostaining of Cel7a in the cells.
Next, we considered the possibility that the transformants secrete proteins not only through the tip but through the whole mycelium. To show which part of the mycelium is engaged in protein secretion in the transformants, we stained the mycelia of Cel7a with FITC-conjugated antibodies and observed them by fluorescence microscopy (Fig. 2). A huge amount of Cel7a was detected as an intense fluorescence at the tips of the mycelia of all the transformants, while in the control strain the tips were only slightly fluorescent.
FIG. 2.
Fluorescence micrographs of FITC-stained Cel7a in the cell walls of T. reesei DPM1 transformants (JSK97/1, JSK97/2, JSK97/3, JSK97/6) and the control strain, TU-6. Magnification, ×100. About 100 pictures were analyzed for each strain.
Secretion from mycelia and protoplasts.
To examine the role of cell wall integrity in protein secretion, we compared the amounts of protein released from the protoplasts and mycelia of the control and the transformed strains. Protein secretion was induced by adding sophorose, and after 5 h, the incorporation of radioactive leucine into the secreted proteins was measured (see Materials and Methods). The results obtained for protoplasts were compared with those obtained for intact mycelia (Table 4). The rate of protein secretion by the control strain was only 7% higher for protoplasts than for mycelia, but in the case of the transformants it was up to 333% higher for protoplasts than for mycelia. These results clearly show that when protein production is up-regulated, the cell wall of the transformed strains, even though modified, still creates a strong barrier for secretion. When the amounts of secretion from the protoplasts of the control strain and the transformants were compared, the increase in the latter was up to 697% higher, which reveals the great potential of the transformed strains for protein production and secretion.
TABLE 4.
Protein secretion from mycelia and protoplasts of DPM1-transformed T. reesei strains JSK97/1, JSK97/2, JSK97/3, and JSK97/6 and the parental strain TU-6a
| Strain | Amount of protein secretion (cpm/g) from indicated source
|
|
|---|---|---|
| Mycelium | Protoplast | |
| TU-6 | 77,686 ± 2,140 | 83,071 ± 504 |
| JSK97/1 | 155,208 ± 11,639 | 621,137 ± 6,562 |
| JSK97/2 | 149,288 ± 3,569 | 603,817 ± 5,662 |
| JSK97/3 | 159,316 ± 8,114 | 603,878 ± 16,970 |
| JSK97/6 | 153,995 ± 4,168 | 662,467 ± 3,171 |
The data are presented as the means ± the standard deviations of the results from five separate cultures.
DISCUSSION
New methods of improving protein production and secretion are of immense interest for biotechnology. T. reesei strains obtained by overexpression of the S. cerevisiae DPM1 gene exhibited elevated production and secretion of glycosylated proteins. The mechanism of these changes remains difficult to explain. In this study, we established that in the transformed cells the sugar composition of the cell wall was changed in a manner similar to that in S. cerevisiae cells under stress (25, 28). In the transformed strains, an increased level of chitin was correlated with a decreased level of glucan, suggesting that the cells had turned on the cell wall compensatory mechanism as a reaction to stress conditions.
Hyperaccumulation of chitin, reaching 20% of the cell wall dry mass, has been shown for S. cerevisiae mutants defective in cell wall-related genes. The fks1 mutant, with a loss of a subunit of β-(1,3) glucan synthase, has a reduced level of β-(1,3) glucan and exhibits accumulation of chitin (12). This effect reveals that a decrease of glucan content is compensated for by an increase in other cell wall components. On the other hand, in yeast the increased amount of chitin is localized in the lateral wall not, as in T. reesei DPM1-transformed strains, in the septa. This difference, as well as the decrease of glucan content, has key importance for the increased secretion observed for these strains. It was shown that for S. cerevisiae, glucans determined the rigidity of the cell wall and that the external N-linked sugar chains of mannoproteins were responsible for the cell wall porosity (6, 7).
It was also shown that 84% of cell wall proteins (CWPs) were released by β-(1,6) glucanase and that only 14% more were released when β-(1,3) glucanase was used (13). This means that the majority of CWPs are linked to the cell wall via β-(1,6) glucan. Our results revealed a pronounced decrease of alkali-soluble β-(1,6) glucan content in the cell walls of the transformed strains, which should lead to decreased CWP cross-linking. Moreover, we observed a lower concentration of mannose in the cell walls of the transformants, suggesting that lower amounts of mannoproteins were present in the cell wall linked to the glucans and/or that the mannoproteins contained a decreased amount of mannose in the external N-linked sugar chains.
The reasons for these changes could be the increased secretion of fully glycosylated proteins in the DPM1-transformed strains and possible alterations in the cellular concentrations of nucleotide sugars needed for cell wall synthesis. In the DPM1-transformed cells of the JSK97/3 strain, we found a fourfold decrease in the intracellular concentration of GDP-mannose.
Similarly, the very low production of GDP-mannose in a Hansenula polymorpha strain with a mutation in the OPU24 gene, a homologue of the T. reesei mpg1 gene coding for GDP-mannose pyrophosphorylase, caused cell wall alterations and changes in the secretion of N- and O-glycosylated proteins (1). Foreign N-glycosylated proteins, expressed in the opu24 mutant, regardless of their source (yeast invertase or human urinary-type plasminogen activator), were secreted in large amounts but were unglycosylated.
Since the opu24 mutant exhibited cell wall alterations, the increased amount of secreted proteins could result from changes in cell wall permeability. We excluded, however, the key role of the cell wall structure in the efficiency of protein secretion in wild type T. reesei. Removal of the cell wall revealed that protoplasts obtained from the control strain secreted proteins in amounts similar to that of mycelia, indicating that the cell wall does not create a barrier for secretion in this strain. In contrast, protoplasts prepared from transformed strains exhibited a four-times-higher secretion level than mycelia. This result clearly showed that, in the strains with an elevated potential for protein production, secretion is strongly limited by the cell wall.
Accumulation of properly folded Cel7a in the cells, as revealed by Western blot analysis (15), could provoke cell responses manifested in cell wall alterations. Moreover, the stress response caused by protein accumulation in the secretory organelles could induce the secretory pathway (27).
On the other hand, massive accumulation of secreted proteins in the ER could disturb the quality control machinery, leading to a failure to degrade misfolded proteins and their transfer to the next stage of the secretory pathway (1). An unfavorable influence of limited O glycosylation on secretion was observed for chitinase produced by the opu24 mutant. The underglycosylated chitinase found in the cultivation medium was secreted in lower amounts or revealed lower affinity to chitin.
In summary, activation of glycosylation processes in T. reesei strains overexpressing the yeast DPM1 gene up-regulates processing of newly synthesized glycoproteins and increases protein production. Accumulation of properly folded secretory proteins in the secretory structures stimulates the secretory pathway. Moreover, the intensive glycosylation of the large amounts of secretory proteins generates a shortage of nucleotide sugars and limits the synthesis of cell wall carbohydrate polymers. The decreased content of alkali-soluble β-(1,6) glucan and mannose in the cell walls of the transformants results in higher porosity of the cell wall and facilitates secretion. The whole picture is complicated by a cell wall compensatory mechanism which stimulates chitin production and limits protein secretion. Nevertheless, we believe that our observations might be useful in the construction of hypersecretory strains.
Acknowledgments
We thank Marja Paloheimo of Roal Oy, Finland, for the antibody against Cel7a.
This work was supported by the State Committee for Scientific Research (KBN), Warsaw, Poland, project no. 6P04B00621 to J. S. Kruszewska.
Footnotes
Published ahead of print on 20 October 2006.
REFERENCES
- 1.Agaphonov, M. O., A. N. Packeiser, M. B. Chechenova, E. S. Choi, and M. D. Ter-Avanesyan. 2001. Mutation of the homologue of GDP-mannose pyrophosphorylase alters cell wall structure, protein glycosylation and secretion in Hansenula polymorpha. Yeast 18:391-402. [DOI] [PubMed] [Google Scholar]
- 2.Aho, S., V. Olkkonen, T. Jalava, M. Paloheimo, R. Buhler, M. Niku-Paavola, D. H. Bamford, and M. Korhola. 1991. Monoclonal antibodies against core and cellulose-binding domains of Trichoderma reesei cellobiohydrolase I and II and endoglucanase I. Eur. J. Biochem. 200:643-649. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee, D. K., E. E. Kousvelari, and B. J. Baum. 1987. cAMP mediated protein phosphorylation of microsomal membranes increases mannosylphosphodolichol synthase activity. Proc. Natl. Acad. Sci. USA 84:6389-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bocking, S. P., M. G. Wiebe, G. D. Robson, K. Hansen, L. H. Christiansen, and A. P. J. Trinci. 1999. Effect of branch frequency in Aspergillus oryzae on protein secretion and culture viscosity. Biotechnol. Bioeng. 65:638-648. [DOI] [PubMed] [Google Scholar]
- 5.Colussi, P. A., C. H. Taron, J. Mack, and P. Orlean. 1997. Human and Saccharomyces cerevisiae dolichyl phosphate mannose synthase represent two classes of the enzymes, but both function in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA 94:7873-7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Groot, P. W. J., A. F. Ram, and F. M. Klis. 2005. Features and function of covalently linked proteins in fungal cell wall. Fungal Genet. Biol. 42:657-675. [DOI] [PubMed] [Google Scholar]
- 7.De Nobel, J. G., F. M. Klis, J. Priem, T. Munnik, and H. Van der Ende. 1990. The glucanase-soluble mannoproteins limit cell wall porosity in Saccharomyces cerevisiae. Yeast 6:491-499. [DOI] [PubMed] [Google Scholar]
- 8.Dijkgraaf, G. J., J. L. Brown, and H. Bussey. 1996. The KNH1 gene of Saccharomyces cerevisiae is a functional homolog of KRE9. Yeast 12:683-692. [DOI] [PubMed] [Google Scholar]
- 9.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Robers, and F. Smith. 1956. Colorimetric method for determination of sugar and related substrates. Anal. Biochem. 28:350-356. [Google Scholar]
- 10.Harman, G. E., and C. P. Kubicek. 1998. Enzymes, biocontrol and commercial applications, p. 129-163. In E.G. Harman and C.P. Kubicek (ed.), Trichoderma and Glocladium, vol. 2. Taylor and Francis Ltd., London, United Kingdom. [Google Scholar]
- 11.Herscovics, A., and P. Orlean. 1993. Glycoprotein biosynthesis in yeast. FASEB J. 7:540-550. [DOI] [PubMed] [Google Scholar]
- 12.Kapteyn, J. C., A. F. J. Ram, E. M. Groos, R. Kollar, R. C. Montijn, H. Van Der Ende, A. Llobell, E. Cabib, and F. M. Klis. 1997. Altered extent of cross-linking of β1,6-glucosylated mannoproteins to chitin in Saccharomyces cerevisiae mutants with reduced cell wall β1,3-glucan content. J. Bacteriol. 179:6279-6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapteyn, J. C., H. Van Den Ende, and F. M. Klis. 1999. The contribution of cell wall proteins to the organization of the yeast cell wall. Biochim. Biophys. Acta 1426:373-383. [DOI] [PubMed] [Google Scholar]
- 14.Kolar, H., H. Mischak, W. P. Kammel, and C. P. Kubicek. 1985. Carboxymethylcellulase and β-glucosidase secretion by protoplasts of Trichoderma reesei. J. Gen. Microbiol. 131:1339-1347. [Google Scholar]
- 15.Kruszewska, J. S., A. H. Butterweck, W. Kurza̧tkowski, A. Migdalski, C. P. Kubicek, and G. Palamarczyk. 1999. Overexpression of the Saccharomyces cerevisiae mannosylphosphodolichol synthase-encoding gene in Trichoderma reesei results in an increased level of protein secretion and abnormal cell ultrastructure. Appl. Environ. Microbiol. 65:2382-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruszewska, J., R. Messner, C. P. Kubicek, and G. Palamarczyk. 1989. O-glycosylation of proteins by membrane fraction of Trichoderma reesei QM9414. J. Gen. Microbiol. 135:301-307. [Google Scholar]
- 17.Kruszewska, J., G. Palamarczyk, and C. P. Kubicek. 1990. Stimulation of exoprotein secretion by choline and Tween 80 in Trichoderma reesei QM 9414 correlates with increased activity of dolichol phosphate mannose synthase. J. Gen. Microbiol. 136:1293-1298. [Google Scholar]
- 18.Kruszewska, J., G. Palamarczyk, and C. P. Kubicek. 1991. Mannosyl-phospho-dolichol synthase from Trichoderma reesei is activated by protein kinase dependent phosphorylation in vitro. FEMS Lett. 80:81-86. [Google Scholar]
- 19.Kruszewska, J. S., M. Saloheimo, A. Migdalski, P. Orlean, M. Penttilä, and G. Palamarczyk. 2000. Dolichol phosphate mannose synthase from the filamentous fungus Trichoderma reesei belongs to the human and Schizosaccharomyces pombe class of the enzyme. Glycobiology 10:983-991. [DOI] [PubMed] [Google Scholar]
- 20.McIntyre, M., C. Muller, J. Dynesen, and J. Nielsen. 2001. Metabolic engineering of the morphology of Aspergillus. Adv. Biochem. Eng. Technol. 73:103-128. [DOI] [PubMed] [Google Scholar]
- 21.Müller, C., M. McIntyre, K. Hansen, and J. Nielsen. 2002. Metabolic engineering of the morphology of Aspergillus oryzea by altering chitin synthesis. Appl. Environ. Microbiol. 68:1827-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemcovic, M., and V. Farkas. 2001. Cell wall composition and polysaccharide synthase activity changes following photoinduction in Trichoderma viride. Acta Biologica Hungarica 52:281-288. [DOI] [PubMed] [Google Scholar]
- 23.Oka, T., T. Hamaguchi, Y. Sameshima, M. Goto, and K. Furukawa. 2004. Molecular characterization of protein O-mannosylation and its involvement in cell-wall synthesis in Aspergillus nidulans. Microbiology 150:1973-1982. [DOI] [PubMed] [Google Scholar]
- 24.Orlean, P. 1990. Dolichol phosphate mannose synthase is required in vivo for glycosyl phosphatidylinositol membrane anchoring, O mannosylation, and N glycosylation of protein in Saccharomyces cerevisiae. Mol. Cell. Biol. 10:5796-5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popolo, L., D. Gilardelli, P. Bonfante, and M. Vai. 1997. Increase in chitin as an essential response to defects in assembly of cell wall polymers in the ggp1Δ mutant of Saccharomyces cerevisiae. J. Bacteriol. 179:463-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pringle, J. R. 1991. Staining of bud scars and cell wall chitin with Calcofluor. Methods Enzymol. 194:732-735. [DOI] [PubMed] [Google Scholar]
- 27.Saloheimo, M., H. Wang, M. Valkonen, T. Vasara, A. Huuskonen, M. Riikonen, T. Pakula, M. Ward, and M. Penttilä. 2004. Characterization of secretory genes ypt1/yptA and nsf1/nsfA from two filamentous fungi: induction of secretory pathway genes of Trichoderma reesei under secretion stress conditions. Appl. Environ. Microbiol. 70:459-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valdivieso, M. H., L. Ferrario, M. Vai, A. Duran, and L. Popolo. 2000. Chitin synthesis in a gas1 mutant of Saccharomyces cerevisiae. J. Bacteriol. 182:4752-4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zakrzewska, A., G. Palamarczyk, H. Krotkiewski, E. Zdebska, M. Saloheimo, M. Penttilä, and J. S. Kruszewska. 2003. Overexpression of the gene encoding GTP:mannose-1-phosphate guanyltransferase, mpg1, increases cellular GDP-mannose levels and protein mannosylation in Trichoderma reesei. Appl. Environ. Microbiol. 69:4383-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zdebska, E., and J. Kościelak. 1999. A single-sample method for determination of carbohydrate and protein contents in glycoprotein bands separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal. Biochem. 275:171-179. [DOI] [PubMed] [Google Scholar]