Abstract
The Adenoviridae and Polyomaviridae families comprise a wide diversity of viruses which may be excreted for long periods in feces or urine. In this study, a preliminary analysis of the prevalence in the environment and the potential usefulness as source-tracking tools of human and animal adenoviruses and polyomaviruses has been developed. Molecular assays based on PCR specifically targeting human adenoviruses (HAdV), porcine adenoviruses (PAdV), bovine adenoviruses (BAdV), and bovine polyomaviruses (BPyV) were applied to environmental samples including urban sewage, slaughterhouse, and river water samples. PAdV and BPyV were detected in a very high percentage of samples potentially affected by either porcine or bovine fecal contamination, respectively. However, BAdV were detected in only one sample, showing a lower prevalence than BPyV in the wastewater samples analyzed. The 22 slaughterhouse samples with fecal contamination of animal origin showed negative results for the presence of HAdV. The river water samples analyzed were positive for the presence of both human and animal adenoviruses and polyomaviruses, indicating the existence of diverse sources of contamination. The identities of the viruses detected were confirmed by analyses of the amplified sequences. All BPyV isolates showed a 97% similarity in nucleotide sequences. This is the first time that PAdV5, BAdV6, and BPyV have been reported to occur in environmental samples. Human and porcine adenoviruses and human and bovine polyomaviruses are proposed as tools for evaluating the presence of viral contamination and for tracking the origin of fecal/urine contamination in environmental samples.
Environmental contamination of fecal origin and the potential transmission of diseases through water and food have been concerning the scientific community for more than a century. Fecal coliform bacteria and other fecal bacterial indicators were established as safety standards to assess water quality. However, they often fail to predict the risk for waterborne pathogens, especially protozoan parasites and enteric viruses (15, 26). Outbreaks of viral diseases have occurred as a result of the consumption of water with accepted values of coliform standards (8, 18), and it has been proven that bacterial standards do not correlate with the presence or absence of viral pathogens.
Enteric viruses are excreted in high concentrations in human and animal feces of individuals showing clinical syndromes but also in lower concentrations in the feces and in some cases urine in a high proportion of the healthy population (4, 34). The Adenoviridae family includes a group of icosahedral nonenveloped viruses containing a double-stranded DNA genome. The widely diverse population of adenoviruses infects a broad spectrum of species, and 51 human, 6 porcine, and 10 bovine serotypes have been described along with adenoviruses infecting other hosts, such as birds or amphibians. The Adenoviridae were classically divided into the Mastadenovirus and Aviadenovirus genera, comprising mammalian and avian isolates, respectively. Recently, according to the available data from DNA sequencing, phylogenetic analyses, and the genome arrangements observed, two new genera, Atadenovirus and Siadenovirus, have been accepted as including some bovine serotypes and the increased number of serotypes identified from amphibians and reptiles in particular (2, 3, 9).
Human polyomaviruses JCPyV and BKPyV have been previously reported as highly prevalent in sewage samples from widely divergent urban areas, which also suggests the usefulness of these viruses as potential indicators of human fecal pollution (5).
The Polyomaviridae constitute a family of DNA viruses that infect different vertebrate species, including rodents, birds, rabbits, cattle, humans, and nonhuman primates. The members of this family present an approximately 5,000-bp circular doubled-stranded super-coiled and covalently closed DNA genome. The polyomaviruses are highly specific for their hosts, and all the members share a similar organization. The murine polyomavirus (MPyV) and a simian polyomavirus (simian virus 40) have been extensively used as models for understanding replication, oncogenesis, and other cellular mechanisms due to their possession of a genome which assembles a minichromosome and due to their oncogenic properties when assayed in cell culture and experimental animals.
Bovine polyomavirus (BPyV) was originally isolated as a contaminant of cell culture (44), and its nucleotide sequence was reported in 1990 (37) under EMBL/GenBank/DDJB accession number D00755. BPyV sequences have been reported by diverse authors to be found in calf serum batches (24, 32, 38, 39). Although antibodies have been detected in cattle (32, 44) and in humans in close contact with cattle (31), no specific illness has been attributed to BPyV.
JCPyV and BKPyV are the two members of the Polyomaviridae family which produce persistent infections in humans, and they are frequently excreted in urine, especially in the case of JCPyV, which is excreted by about 40 to 80% of the population (6). The human polyomaviruses are associated with important diseases in immunocompromised people and have also been related to some types of human cancer, e.g., colorectal cancer.
Human adenoviruses (HAdV) are easily detected in sewage and river water with fecal contamination and are reported to be more stable than enteroviruses when subjected to UV irradiation and chlorination (14, 29, 34, 41, 42). This agrees with their marked prevalence in aquatic environments of and shellfish from widely divergent geographical areas (11, 13, 20, 34).
Several viral parameters, such as F+ phages (17), bovine enteroviruses (22, 25), and phages infecting Bacteroides fragilis (33, 40), have been also proposed for tracking either human or animal contamination. Detection of HAdV by a specific nested-PCR test has also been proposed as a molecular index of human viral contamination in the environment (34, 35). More recently, specific nested primer sets have been designed for the detection of porcine adenoviruses (PAdV) and bovine adenoviruses (BAdV), applied to the analysis of urban sewage and animal fecal samples, and suggested as environmental indicators for tracking porcine and bovine sources of viral contamination (27).
In this study, the distribution of HAdV, PAdV, BAdV, and BPyV has been evaluated for slaughterhouse sludge and wastewater, for urban sewage, and for samples from a river potentially affected by animal fecal pollution. The results obtained suggest that the assessment of HAdV, PAdV, and BPyV distribution may be a very useful tool for tracking the source of fecal contamination in the environment and in water.
MATERIALS AND METHODS
Slaughterhouse samples.
Twenty-two samples from two slaughterhouses located in different areas of Catalonia (Spain) were collected from May to July 2004. The first slaughterhouse (A) processed bovine and ovine adult animals. The second slaughterhouse (B) processed bovine and, in higher numbers, porcine adult animals. Samples were collected in sterile 500-ml polyethylene containers and kept at 4°C for less than 8 h prior to analysis. The types of samples collected were related to the treatments used in the two slaughterhouse studied and included raw wastewater, flocculated sediment after a chemical flocculation treatment, and sludge collected from the tank of the aerobic activated sludge treatment of the supernatant produced after flocculation.
Urban sewage samples.
Nine urban sewage samples were collected at the entry of a wastewater treatment plant receiving 670,000 m3/day from the urban area of Barcelona, with a population of approximately 1,800,000 inhabitants, without significant animal wastewater content. Samples were collected monthly from May 2003 to February 2004. All samples were collected in sterile 500-ml polyethylene containers and kept at 4°C for less than 8 h prior to analysis.
River water samples.
Nine river water samples were collected from two different rivers, four samples from the Ter River at a point close to an important farming area in Catalonia and five from the Llobregat River in an area close to the estuary.
Virus concentration from river water.
One hundred liters of river water was filtered through a Zeta Plus MK electropositive filter at 1 liter/minute by use of a Millipore peristaltic pump. The protocol used for processing river water samples is a combination of the EPA method (EPA 600/4-84/013 [N14]) with minor modifications and the method based on ultracentrifugation and elution in glycine buffer (0.25 N, pH 9.5) developed in previous studies (35). Viruses retained in the filter were eluted in 900 ml of glycine buffer (0.25 N, pH 9.5) 1% beef extract (Becton, Dickinson & Co., Sparks, MD) by reverse flow with a Millipore peristaltic pump at 0.4 liters/minute for 45 min. For the flocculation, 3% beef extract was added, the pH was adjusted to 3.5 using 5 M HCl, and the resultant suspension was magnetically stirred for 30 min and centrifuged at 12,800 × g for 25 min at 4°C. The sedimented flock was then eluted in 42 ml of phosphate-buffered saline. From this step, the sample was treated using the protocol previously applied for the recovery of viruses from urban sewage as described in the following section. The efficiencies of the methods applied for determining the concentrations of adenoviruses and polyomaviruses from river water samples have been assayed in previous studies using HAdV and the human polyomavirus JCPyV and were estimated to be between 2 and 25% for HAdV and 0.15% for JCPyV (1).
Virus concentration from slaughterhouse and urban sewage samples.
Recovery of viral particles was performed as described in previous studies (34, 35). Briefly, 42 ml of sewage was ultracentrifuged at 110,000 × g for 1 h at 4°C to pellet all the viral particles together with any suspended material. The pellet was eluted with 4 ml of 0.25 N glycine buffer, pH 9.5, and suspended solids were separated by centrifugation at 12,000 × g for 20 min. Finally, viruses were concentrated by ultracentrifugation at 110,000 × g for 1 h at 4°C, resuspended in 100 μl phosphate-buffered saline, and stored at −80°C.
Experiments to determine the recovery of polyomavirus JCPyV from sewage samples have been previously carried out by a real-time quantitative PCR assay, and 37% recovery was shown (1).
Nucleic acid extraction.
The nucleic acids from viral concentrates were extracted by using a procedure selected in previous studies for its high sensitivity and efficiency (7, 35). This procedure is based on the use of guanidinium isothiocyanate to denature viral capsids and silica particles to bind viral nucleic acids until their final elution in TE buffer (10 mM Tris, 0.1 mM EDTA, pH 7.4) from this support and storage at −20°C.
Enzymatic amplification.
Ten-microliter aliquots of the extracted nucleic acids, corresponding to 4.2 ml of slaughterhouse and sewage samples and to a 5-liter portion of river water, were analyzed in each nested-PCR test.
Primers designed by Maluquer de Motes et al. (27) were used to amplify PAdV and BAdV. For HAdV detection, two different sets of primers previously described by Allard et al. (1a), Pina et al. (34), and Puig et al. (35) were used. Both sets are known from previous studies to detect all human adenoviral species and were tested in order to produce more information on the specificity of these assays for the detection of the contamination of human origin. For the detection of BPyV, two different sets of primers which anneal to different genome regions were used, VP1-based primers designed by Wang et al. (43) and primers designed for amplification of the agnoprotein region (Table 1). For HAdV, PAdV, and BAdV, 10 μl of the extracted nucleic acids and a 10-fold dilution were analyzed in a 40-μl reaction mixture containing 1× PCR buffer [160 mM (NH4)2SO4, 670 mM Tris-HCl, pH 8.8] at 25°C, MgCl2 (1.5 mM), 0.025 mM of each deoxynucleoside triphosphate, 25 pmol of each primer, and 2 units of Taq DNA polymerase (Bioron GmbH, Germany). In all PCR assays for the detection of HAdV, PAdV, BAdV, and BPyV, annealing temperatures were determined based on the conditions described by the authors or by calculating 4°C below the melting temperatures estimated for the primers. The reaction conditions were as follows: 94°C for 4 min and then 30 cycles of 92°C for 60 s, 60 s at the corresponding annealing temperature (Table 1), and extension at 72°C for 75 s. Amplifications were completed with a 7-min extension step at 72°C. The reaction conditions for BPyV were as follows: 94°C for 2 min and then 35 cycles of 94°C for 1 min, 58°C for 75 s, and 72°C for 75 s. Amplifications were completed with a 5-min extension step at 72°C. The nested PCR was performed using the same conditions as in the first-round PCR, except that 1 μl of the PCR products was added to a new 49-μl PCR mixture, and 30 amplification cycles were performed. Thermal cycling of the amplification mixture was performed by use of a programmable heat block (Gene Amp PCR System 2400; Applied Biosystems). The results were analyzed by electrophoresis on a 2% agarose gel stained with ethidium bromide.
TABLE 1.
Oligonucleotide primers used for PCR amplification and sequencing of HAdV, PAdV, BAdV, and BPyV
| Virus (region) | Position | Primer | Product size (bp) | Annealing temp (°C) | Sequence |
|---|---|---|---|---|---|
| HAdV set 1a | |||||
| HAdV40 (hexon) | 18858-18883 | HL | 301 | 55 | 5′-GCCGCAGTGGTCTTACATGCACATC-3′ |
| HAdV41 (hexon) | 19136-19158 | HR | 5′-CAGCACGCCGCGGATGTCCAAAGT-3′ | ||
| HAdV2 (hexon) | 18937-18960 | NHL | 143 | 55 | 5′-GCCACCGAGACGTACTTCAGCCTG-3′ |
| 19051-19079 | NHR | 5′-GTACGAGTACGCGGTATCCTCGCGGTC-3′ | |||
| HAdV set 2b | |||||
| HAdV2 (hexon) | 18858-18882 | Hex1deg | 301 | 55 | 5′-GCCSCARTGGKCWTACATGCACAT-3′f |
| 19138-19158 | Hex2deg | 5′-CAGCACSCCNCGRATGTCAAA-3′f | |||
| 18931-18954 | Hex3deg | 171 | 55 | 5′-GCCCGYGCMACNGANACSTACTTC-3′f | |
| 19077-19102 | Hex4deg | 5′-CYACRGCCAGNGTRWANCGMRCYTTGTA-3′f | |||
| PAdVc | |||||
| PAdV (hexon) | 20627-20647 | PALF | 612 | 54 | 5′-GATGTCATGGAYAACGTCAAC-3′f |
| 21217-21238 | PARF | 5′-CACGGAGGAGTCRAACTGGATG-3′f | |||
| 20711-20733 | PALN | 344 | 57 | 5′-TACTGCMAGTTYCACATCCAGGT-3′f | |
| 21035-21054 | PARN | 5′-GGAATGGAGATGGGCAGGTT-3′ | |||
| BAdVd | |||||
| BAdV (hexon) | 19235-19256 | BALF | 641 | 52 | 5′-GRTGGTCIYTRGATRTRATGGA-3′f |
| 19852-19872 | BARF | 5′-AAGYCTRTCATCYCCDGGCCA-3′f | |||
| 19347-19370 | BALN | 430 | 51 | 5′-ATTCARGTWCCWCARAARTTTTTTGC-3′f | |
| 19747-19769 | BARN | 5′-CCWGAATAHRIAAARTTKGGATC-3′f | |||
| BPyVe | |||||
| BPyV (VP) | 11940-1963 | VP1F | 527 | 58 | 5′-GGTATTCGCCCTCTGCTGGTCAAG-3′ |
| 12466-2444 | VP1R | 5′-GCTGGCAATGGGGTATGGGTTCT-3′ | |||
| 12048-2068 | VP2F | 263 | 58 | 5′-ATTTCAAAGCCCCCTATCATC-3′ | |
| 12310-2290 | VP2R | 5′-GCCTACGCCATTCTCATCAAG-3′ | |||
| BPyV | |||||
| BPyV (agnoprotein) | 281-304 | BP1F-L | 241 | 47 | 5′-GTGTAGAATAATGATTGAACTAT-3′ |
| 563-586 | BP2F-R | 5′-TGGCCTACCTTTAGTTAAAATCT-3′ | |||
| 322-342 | BP3N-L | 201 | 54 | 5′-TTCTGGACAGTGGGGACTAT-3′ | |
| 503-523 | BP4N-R | 5′-ATTTCAAAGCCCCCTATCATC-3′ |
In all PCR assays, standard precautions were applied. Negative controls were added after every two samples, and positive results were confirmed by sequencing analysis of the amplified DNA. No negative controls were recorded to be contaminated in these specific assays during this study, probably due to the low concentrations of the positive controls used and all the strict manipulation rules applied. However, sporadic contamination may occur in any laboratory when nested PCR is used, and the PCRs must then be repeated using new solutions and materials and carefully analyzing negative controls until it becomes clear that the contaminated material has been removed from the laboratory.
Sequencing and analysis of viral genomes.
Amplicons obtained after nested PCR were purified using a QIAquick purification kit (QIAGEN, Inc.) following the manufacturer's instructions. Both strands of the purified DNA amplicons were sequenced with the ABI PRISM BigDye Terminator cycle sequencing ready reaction kit with AmpliTaq DNA polymerase FS (Applied Biosystems) according to the manufacturer's instructions. Products were checked using an ABI PRISM 377 analyzer (Applied Biosystems) by Scientific and Technical Services of the University of Barcelona, and the sequences were compared with the nucleotide sequences present in the GenBank using the BLAST program of the NCBI (National Center for Biotechnology Information; www.ncbi.nlm.nih.gov/BLAST) and aligned using the ClustalX 1.8 program. Phylogenetic tree reconstructions were performed with Mega 3.1 (www.megasoftware.net). Evolutionary distances were determined by use of the Kimura two-parameter equation, and the tree was constructed by using the neighbor-joining (NJ) algorithm.
Quantification of Escherichia coli in the analyzed samples.
The procedure for the quantification of E. coli has been described elsewhere (16). Slaughterhouse wastewater samples were diluted and inoculated in triplicate into 10 ml mineral modified medium base medium. Tryptone bile X-glucoronide agar medium was used to confirm potential positives. The most probable number (MPN) technique was used to estimate the number of E. coli cells/100 ml of sample according to standard methods.
Nucleotide sequence accession numbers.
The sequences reported in this paper have been deposited in the GenBank database under accession no. DQ531655 to DQ531660 for BPyV-Sh1 to BPyV-Sh7 and DQ531661 to DQ531667 for PAdV-Sh1 to PAdV-Sh-4 and PAdV-TR1, PAdV-TR2, and PAdV-LlR1, respectively.
RESULTS
Sensitivity and specificity of the PCR assays.
A preliminary study has shown that the assays developed for the specific detection of HAdV, PAdV, and BAdV produced positive results in fecal samples only when the corresponding strain was present in the sample (27). In that study, fecal samples containing PAdV (serotype 3) and BAdV (serotypes 2, 4, and 7) as well as human adenovirus working stocks (serotypes 2, 5, 12, and 41) were tested using all primer sets designed for the specific detection of porcine, bovine, and human adenoviruses. The assay for the identification of BPyV has also proven to be specific according to the analysis by sequence comparison in the nucleotide sequence database (NCBI BLAST) and by experimental assays using nested PCR in which no amplification was observed when human polyomaviruses JCPyV and BKPyV obtained from cell culture were analyzed. In the same way, the nested PCR designed for the detection of JCPyV produced positive results only when this virus was present in the sample. The specificity of the results was confirmed by sequencing the amplified genome regions when field samples were analyzed. The sensitivities of the procedures applied in wastewater have been estimated to be of 1 to 10 genome copies for HAdV and JCPyV in the 10 μl of extracted nucleic acids assayed (6).
Viruses in slaughterhouse sewage samples.
All samples were analyzed for the presence of HAdV, PAdV, BAdV, and BPyV. Although diverse type of wastewater samples and sludge were collected, all were tested using the same procedures, and no significant differences were observed in the results, which are summarized in Table 2. None of the 22 samples were positive for HAdV when the two sets of primers described in Table 1 were used. The 10 samples analyzed from slaughterhouse B were found to be positive for PAdV. The number of detected PAdV viral genomes was approximately 101 to 103 genome equivalents/ml of sample, as estimated by end-point decimal dilution analysis. Only 1 out of 22 samples analyzed was found to be positive for BAdV. BPyV were detected in 21 out of the 22 samples analyzed at a concentration of 101 to 102 genome equivalents/ml of sample, thus proving to be more abundant in the wastewater analyzed. These results suggest that BPyV could be more frequently excreted in bovine cattle and a better parameter than BAdV for the identification of this source of contamination in the environment. Observed mean values for E. coli were 4.4 × 105 MPN and 9.3 × 105 MPN/100 ml in the slaughterhouse sewage samples from locations A and B, respectively.
TABLE 2.
Viruses detected in river water and in slaughterhouse wastewater samples expressed as positive samples out of the total samples analyzed
| Type of sample | Virusa
|
|||
|---|---|---|---|---|
| HAdV | PAdV | BAdV | BPyV | |
| Slaughterhouse A (bovine, ovine) | 0/12 | NT | 1/12 | 10/10 |
| Slaughterhouse B (porcine, bovine) | 0/10 | 10/10 | 0/10 | 7/8 |
| Ter River | NT | 3/4 | NT | 4/4 |
| Llobregat River | 5/5 | 4/5 | NT | 1/5 |
NT, not tested.
Viruses in river water samples.
Seven samples out of the nine tested proved to be positive for PAdV at concentrations ranging from 101 to 102 genome equivalents/5 liters in both rivers. The sampling point at the Ter River was selected because his proximity to an important farming area in Catalonia having intensive bovine and porcine farms. The four analyzed Ter River samples were positive for BPyV at a concentration ranging from 101 to 102 genome equivalents per 5 liters. Only 1/5 of samples collected from the Llobregat River was positive for BPyV. HAdV and JCPyV were tested for in the Llobregat River samples by using real-time PCR as described by Albinana-Gimenez et al. (1), and all samples were found to be positive with average concentrations of 102 and 101 genome copies/liter, respectively. These results are shown in Table 2. The mean values for E. coli in the Llobregat River and the Ter River were 4.8 × 104 MPN and 5.1 × 103 MPN, respectively, per 100 ml.
Viruses in urban sewage samples.
Nine raw urban sewage samples were analyzed and, as expected, all were shown to be positive for HAdV and JCPyV. BPyV were not detected in any of the samples analyzed. Additionally, previous studies had failed to detect animal adenoviruses in urban sewage samples (27), confirming the low animal fecal content present in these samples, and the specificity of the primers used as urban sewage samples have been described as containing high concentrations of human adenoviruses and polyomaviruses BKPyV and JCPyV (6).
Characterization of the porcine adenoviruses detected.
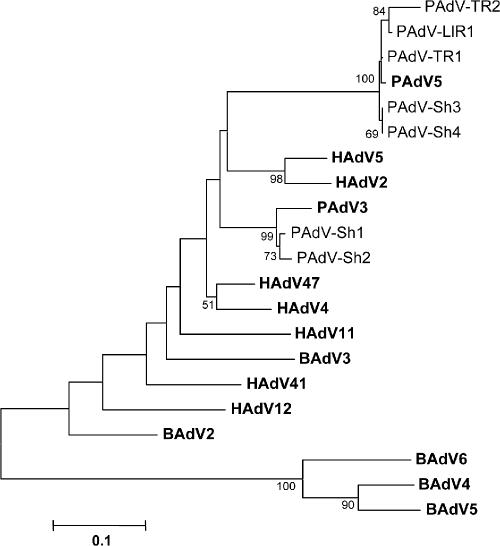
The amplified region within the hexon gene was 344 bp long. The seven sequences obtained from 10 positive samples were named PAdV-Sh1 to PAdV-Sh4, PAdV-TR1, PAdV-TR2, and PAdV-LlR1 (four strains from slaughterhouse sewage samples, two strains from the Ter River, and one strain from the Llobregat River) and were compared to those available at GenBank. The nucleotide sequence alignment showed that two, PAdV-Sh1 and PAdV-Sh2, were closely related to porcine adenovirus type 3 (PAdV3) (accession no. AY288807.1) and that five sequences, i.e., those of PAdV-Sh3, PAdV-Sh4, PAdV-TR1, PAdV-TR2, and PAdV-LlR1, were closely related to porcine adenovirus type 5 (accession no. AF289262), all of them sharing identities ranging 94 to 98%. PAdV3 has commonly been detected in swine fecal samples (27). This is the first time that PAdV5 has been found in environmental samples. The 356-bp amplicon of the protease gene obtained from one of the isolates showed a 98% similarity to the PAdV5 protease sequence present at GenBank, confirming the identification obtained by the analysis of the amplified hexon region. Figure 1 shows the phylogenetic tree of the strains identified that was constructed by using Mega 3.1 (www.megasoftware.net).
FIG. 1.
NJ tree constructed to represent phylogenetic relationships among PAdV isolated from slaughterhouse wastewater and river samples (PAdV-Sh1 to PAdV-Sh4 and PAdV TR1, PAdV-TR2, and PAdV-LIR1) and other reference human (HAdV2, -4, -5, -11, -12, -41, and -47), porcine (PAdV3 and -5), and bovine (BAdV2, -3, -4, -5, and -6) adenoviruses, which are represented in bold. A fragment of 171 nucleotides amplified from the hexon gene was used in this analysis. Evolutionary distances were determined by use of the Kimura two-parameter equation. Bootstrap values indicating nodes supported by more than 50% replicate trees are represented.
Characterization of the bovine adenoviruses detected.
The amplified hexon fragment was 430 bp long and was sequenced and identified as BAdV type 6 (BAdV6; accession no. AF207659) with 97% similarity. In order to confirm the identity of the isolated strain as an atadenovirus, the protease region was further amplified and characterized. Since the BAdV6 protease region is not available in the GenBank, the 525-bp-long amplicon obtained was shown to be 84% similar to BAdV type 4 (accession no. AY288820), the only protease sequence of Atadenovirus members available, confirming the isolate as a member of this genus. Since all the main described features of the protease gene of adenoviruses belonging to the Atadenovirus genus are in accordance, it might be considered as the first reported sequence of a region in the protease gene of this viral serotype.
Characterization of the bovine polyomaviruses detected.
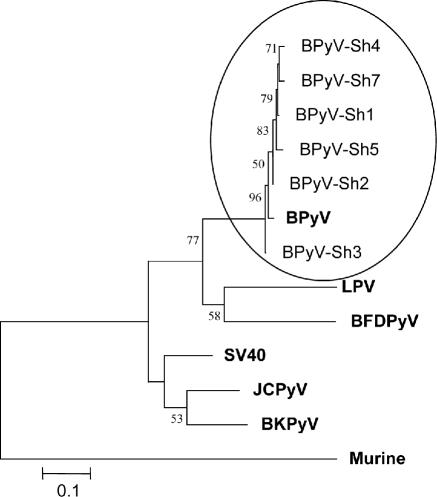
The amplicon obtained within the VP-1 protein was 263 bp long. Six samples were sequenced for VP1 and named BPyV-Sh1 to BPyV-Sh5 and BPyV-Sh7. The sequences obtained were aligned to the sequence of the BPyV genome available in GenBank (accession no. D13942). Nucleotide identity between these amplicons ranged from 95 to 99%. The phylogenetic tree constructed using Mega 3.1 (www.megasoftware.net) shows the close relation between all the bovine strains identified in this study and the strain deposited in GenBank under accession no. D13942 (Fig. 2). Agnoprotein-based amplicon alignment provided similar nucleotide identity values (data not shown).
FIG. 2.
NJ tree constructed to represent phylogenetic relationships among BPyV isolated from slaughterhouse wastewater samples (BPyV-SH1 to BPyV-Sh7; large oval) and other reference human (JCPyV and BKPyV) and monkey (simian virus 40 and lymphotropic polyomavirus [LPV]) as well as murine and avian (Budgerigar fledging polyomavirus [BFDPyV]) polyomaviruses, which are represented in bold. A fragment of 193 nucleotides amplified from the VP1 gene was used in this analysis. Evolutionary distances were determined by use of the Kimura two-parameter equation. Bootstrap values indicating nodes supported by more than 50% replicate trees are represented.
DISCUSSION
Animal enteric viruses may contaminate the environment and water via runoff, drainage, or direct depositions or by contamination with slaughterhouse wastewater or sludge produced in the wastewater treatment plants that could be used as a fertilizer in agricultural practices. Tracking the source of the fecal contamination is an essential issue in the assessment of water quality standards and the corresponding sanitary risks for human and animal health. Available microbial markers include diverse enteric bacteria and viruses and also include bacteriophages (11, 23, 30). The use of highly prevalent DNA viruses commonly excreted in feces or urine, stable in the environment and specific for human or animal hosts, represents a very interesting concept as a tool for source tracking in studies of environmental contamination, especially in relation to viral pathogens. In previous studies, the detection of human, porcine, and bovine adenoviruses by nested PCR was proposed as a source-tracking tool for environmental samples (27, 34). Nested PCR is a highly specific and sensitive technique. If it is applied with the appropriate quality controls, it allows for evaluation of the prevalence and typing after sequencing analysis of viruses that are not efficiently cultured in cell lines.
HAdV were reported to be more prevalent than enteroviruses and hepatitis A virus in different aquatic environments and more prevalent than noroviruses in shellfish from different countries in Europe (12, 34). The former are also more stable when subjected to UV irradiation and natural inactivating processes (20, 29) than the latter. Viral particles of human adenoviruses and polyomavirus JCPyV have been shown to be remarkably stable in sewage samples, showing t90 (time required to observe a reduction of 90% in the initial viral concentration) values of 60.9 and 63.9 days, respectively (6a). However, more studies need to be developed in order to evaluate, in different conditions, the stability of the animal adenoviruses and polyomaviruses proposed as tools for the identification of sources of environmental contamination.
To the best of our knowledge, there are no data reporting on the prevalence and shedding rates of animal adenoviruses and polyomaviruses. The only available information on the rates of excretion of adenoviruses in farm animals was obtained in a preliminary study limited to the area of Catalonia (Spain) (27). In this study, pools of feces collected from two different farms were analyzed for the presence of BAdV and PAdV. BAdV have been detected in 50% of the bovine feces pools analyzed, while PAdV were present in 70% of the porcine feces pools. Information on the prevalence of PAdV and BPyV infections and excretion rates in farms in widely distributed geographical areas are required to assess the applicability of the proposed parameters as source-tracking tools.
In this study, a preliminary analysis of the prevalence in the environment and the potential usefulness of the molecular detection of human and animal adenoviruses and polyomaviruses has been developed.
Twenty-two slaughterhouse sewage samples were collected in the area of Catalonia, in the northeast of Spain, and none of them was shown to be positive for HAdV, as we expected due to the low amount of human fecal contamination supposed to be present. These results, together with the fact that approximately 100% of urban sewage samples from a wide diversity of geographical areas have been found to be positive for HAdV and JCPyV, even when these samples consist of only a few milliliters, support the idea of using HAdV as a tracer of environmental contamination of human origin, which may be also complemented by the detection of JCPyV, a human virus commonly excreted in urine which has no animal hosts identified to date.
PAdV were detected in all the samples collected from the slaughterhouse processing swine. This prevalence is higher than the one previously reported for fecal samples, as is common for viruses that are more frequently detected in sewage than in individual fecal samples (27). Analysis of the hexon sequences showed isolates similar (98%) to the PAdV3 IAF strain (GenBank accession no. AY28887) and closely similar (98%) to other strains identified in swine fecal samples collected in this area. Other sequences were similar to that of PAdV5 (98%), and the amplification of a fragment of the protease gene confirmed this identity, as it had high similarity, 99%, to strains previously described in data banks (GenBank accession no. AF289262). PAdV5 was isolated for the first time in Japan from the respiratory tract of pigs, and the two reference strains (HNF-61 and HNF-70) were isolated from the same herd (19). To the best of our knowledge, this is the first time that PAdV5 has been identified in environmental samples and also as a common and probably subclinical infection of pigs in our area. PAdVs were also detected in seven out of nine river water samples, in which only serotype 5 was identified.
The high frequency of detection of PAdV and BPyV strains in the river water samples studied that have been collected far away from the point of the discharges and in different periods of the year strongly support the hypothesis that these viruses are frequently excreted and relatively resistant to natural inactivation processes. Ter River samples were collected during a period beginning in December and ending at the end of April, while Llobregat River samples were collected from July to the end of November. Therefore, PAdV and BPyV have been detected in samples collected over the entire year.
BAdV were detected in only 1 out of the 10 samples studied, even though both slaughterhouses analyzed processed cattle. The comparison with the hexon sequence obtained showed an identity of 97% with BAdV type 6 (GenBank accession no. AF207659). This virus is a member of the previously known BAdV subgroup II and, together with OAdV-287 and EDS, has been classified in the recently accepted new genus Atadenovirus. Members of this genus typically possess greater amounts of adenine and thymine and lack the genus-specific complement antigen common to Mastadenovirus members (2). Some of them have been observed to induce acute and fatal enterohemorrhagic diseases in cattle (21). In order to confirm its identity, we amplified and sequence a fragment of the protease gene. It was compared with and observed to share 84% similarity to the BAdV type 4 sequence (accession no. AY288820), the only available data for the protease sequence from atadenoviruses. The amino acid alignment of the sequence (data not shown) in the protease shows the conserved features described for other adenoviruses as the catalytic triad described for all the adenoviral proteases sharing the same active residues His51-Glu71-Cys122 (10). Likewise, the amino acid Cys103, which is believed to be the target of the cellular activator pVI peptide, is present (28). Its presence both in the alignment and in the phylogenetic analysis based on the hexon amplicon reinforces the identification and its classification in the cluster together with BAdV subgroup II serotypes, such BAdV type 4 and BAdV type 7, and other atadenovirus members.
The low prevalence of BAdVs in slaughterhouse sewage was not expected, since they were easily detected in a group of six fecal samples studied from two farms in the same region. Members of the family Adenoviridae are known to possess a high level of stability. Even though no survival studies have been developed for the Atadenovirus genus, there are no grounds for suspecting a different behavior. Thus, based on our knowledge, the failure to detect BAdVs in wastewater samples containing bovine fecal contamination could be related to a low concentration and/or prevalence of BAdV in cattle feces or a higher level of difficulty in detecting the viruses excreted in this matrix. In accordance with this, we could explain the higher frequency of positive results for BPyV in the same samples as being due to their probable excretion in urine, which may be more frequent, and/or it could also represent an increase in the efficiency of the detection methods used.
BPyV were first reported by Rangan et al. (36) as a contaminant of stump-tailed macaque (Macaca arctoides) kidney cell cultures and were originally named STMV. Subsequently, other polyoma-like viruses obtained from different monkey kidney cells were reported. BPyV have been found in high concentrations in bovine fetal and calf serum but not in adult bovine plasma or serum (43).
The complete sequence for BPyV was reported in 1990 (37), and since then to our knowledge there has not been any information published on the existence of other BPyV genotypes. In this study, BPyV have been detected in 95% (20/21) of the samples analyzed. This is the first demonstration of the presence of this virus in the environment. Subsequent sequencing and phylogenetic analysis of the amplicons obtained after the application of nested-PCR techniques confirmed the identity of the viruses detected and also revealed heterogeneity between isolates (six differences in 193 nucleotides). The agnoprotein region seems to be more conserved between the different strains detected, suggesting the use of the VP1 region for phylogenetic studies.
BPyV has been proven to be a useful indicator for tracing bovine fecal/urine contamination. The BPyV have then shown to be highly prevalent in the environment, and we could probably hypothesize that these viruses produce subclinical persistent infections, as is the case for the human polyomaviruses, although this still needs to be tested.
The studied rivers receive human fecal waste as well as contamination from animal farms, but in very different proportions. The Llobregat River receives discharges from more than 20 wastewater treatment plants, and the statistical information reported by the Catalan Institute of Statistics during recent years (http://www.idescat.net/) describes approximate numbers of 7,000 swine and 1,600 bovine head of cattle. In the area contributing to the Ter River, on the other hand, smaller cities with significantly lower numbers of inhabitants are registered, while in contrast a very high abundance of farms with numbers higher than 870,000 swine and 77,640 bovine head of cattle have been reported. There is no specific information available for the quantification of the discharges from animal origin that might reach the rivers. The data on land use in the studied areas correlate with the presence of human and animal contamination in the river water samples analyzed and the presence of a low percentage of positive BPyV samples (1/5 positives) from the Llobregat River.
HAdV, PAdV, and human and bovine polyomaviruses are host specific, although it must be kept in mind that sporadic limited infections in a different host are possible for many viruses. However, the significant prevalence of infections and excretion into the environment is limited by the biological cycle of these viruses in the natural host and is reflected in their high prevalence in slaughterhouse wastewater and sludge and in the absence of the animal viruses in urban sewage presenting high concentrations of human viruses. Their detection and identification by nested-PCR testing has proved to be specific and sensitive; however, the possibility of sporadic unspecific amplifications of DNA cannot be ruled out, and a final confirmation by sequencing analysis of the amplified region will provide the definitive identification of the viral strains amplified.
Further studies are needed in order to obtain more information on adenovirus and polyomavirus strains excreted by animals in diverse geographical areas. The information produced using the procedures described supports the use of the developed assays as useful tools for evaluating the presence of viral contamination and for tracking (or determining) the origin of fecal pollution in the environment.
Acknowledgments
During the development of this study, Nestor Albinana-Gimenez and Carlos Maluquer de Motes were fellows of the Generalitat de Catalunya and of the Ministerio de Educación y Ciencia, respectively.
We thank Serveis Científico Tècnics of the University of Barcelona for the sequencing of PCR products. We also thank Susana Calle for providing excellent technical assistance.
Footnotes
Published ahead of print on 13 October 2006.
REFERENCES
- 1.Albinana-Gimenez, N., P. Clemente-Casares, S. Bofill-Mas, A. Hundesa, F. Ribas, and R. Girones. Environ. Sci. Technol., in press. [DOI] [PubMed]
- 1a.Allard, A., B. Albinsson, and G. Wadell. 2001. Rapid typing of human adenoviruses by a general PCR combined with restriction endonuclease analysis. J. Clin. Microbiol. 39:498-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benko, M., P. Elo, K. Ursu, W. Ahne, S. E. LaPatra, D. Thomson, and B. Harrach. 2002. First molecular evidence for the existence of distinct fish and snake adenoviruses. J. Virol. 76:10056-10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benko, M., and B. Harrach. 1998. A proposal for establishing a new (third) genus within the Adenoviridae family. Arch. Virol. 143:829-837. [DOI] [PubMed] [Google Scholar]
- 4.Bofill-Mas, S., S. Pina, and R. Girones. 2000. Documenting the epidemiologic patterns of polyomaviruses in human populations by studying their presence in urban sewage. Appl. Environ. Microbiol. 66:238-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bofill-Mas, S., and R. Girones. 2001. Excretion and transmission of JCV in human populations. J. Neurovirol. 7:345-349. [DOI] [PubMed] [Google Scholar]
- 6.Bofill-Mas, S., M. Formiga-Cruz, P. Clemente-Casares, F. Calafell, and R. Girones. 2001. Potential transmission of human polyomaviruses through the gastrointestinal tract after exposure to virions or viral DNA. J. Virol. 75:10290-10299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Bofill-Mas, S., N. Albinana-Gimenez, P. Clemente-Casares, A. Hundesa, J. Rodriguez-Manzano, A. Allard, M. Calvo, and R. Girones. 2006. Quantification and stability of human adenoviruses and polyomavirus JCPyV in wastewater matrices. Appl. Environ. Microbiol. 72:7894-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boom, R., C. J. A. Sol, M. M. M. Salimans, C. L. Jansen, P. M. E. Wertheim-van Dillen, and J. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosch, A., F. Lucena, J. M. Diez, R. Gajardo, M. Blasi, and J. Jofre. 1991. Waterborne viruses associated with hepatitis outbreak. J. Am. Water Works Assoc. 83:80-83. [Google Scholar]
- 9.Davison, A. J., K. M. Wright, and B. Harrach. 2000. DNA sequence of frog adenovirus. J. Gen. Virol. 81:2431-2439. [DOI] [PubMed] [Google Scholar]
- 10.Ding, J. B., R. McGrath, M. Sweet, and W. F. Mangel. 1996. Crystal structure of human adenovirus proteinase with its amino acid cofactor. EMBO J. 15:1778-1783. [PMC free article] [PubMed] [Google Scholar]
- 11.Fong, T., and E. K. Lipp. 2005. Enteric viruses of humans and animals in aquatic environmental: health risks, detection, and potential water quality assessment tools. Microbiol. Mol. Biol. Rev. 69:357-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Formiga-Cruz, M., G. Tofiño-Quesada, S. Bofill-Mas, D. N Lees, K. Henshilwood, A. K. Allard, A. C. Condin-Hansson, B. E. Hernroth, A. Vantarakis, A. Tsibouxi, M. Papapetropoulou, D. Furones, and R. Girones. 2002. Distribution of human virus contamination in shellfish from different growing areas in Greece, Spain, Sweden, and the United Kingdom. Appl. Environ. Microbiol. 68:5990-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Formiga-Cruz, M., A. K. Allard, A. C. Conden-Hansson, K. Hensilwood, B. E. Hernroth, J. Jofre, D. N. Lees, F. Lucena, M. Papapetropoulou, R. E. Rangdale, A. Tsibouxi, A. Vantarakis, and R. Girones. 2003. Evaluation of potential indicators of viral contamination in shellfish and their applicability to diverse geographical areas. Appl. Environ. Microbiol. 69:1556-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerba, C. P., D. M. Gramos, and N. Nwachuku. 2002. Comparative inactivation of enteroviruses and adenovirus 2 by UV light. Appl. Environ. Microbiol. 68:5167-5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerba, C. P., S. M. Goyal, R. L. LaBelle, I. Cech, and G. F. Bodgan. 1979. Failure of indicator bacteria to reflect the occurrence of enteroviruses in marine waters. Am. J. Public Health 69:1116-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerhardt, P., R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.). 1994. Methods for general and molecular bacteriology, p. 257-260. American Society for Microbiology, Washington, D.C.
- 17.Havelaar, A. H., W. M. Pot-Hogeboom, K. Furuse, R. Pot, and M. P. Hormann. 1990. F-specific RNA bacteriophages and sensitive host strains in faeces and wastewater of human and animal origin. J. Appl. Bacteriol. 69:30-37. [DOI] [PubMed] [Google Scholar]
- 18.Hejkdal, T. W., B. Keswick, R. L. LaBelle, C. P. Gerba, Y. Sanchez, G. Dreesman, B. Hafkin, and J. L. Melnick. 1982. Viruses in a community water supply associated with an outbreak of gastroenteritis and infectious hepatitis. J. Am. Water Works Assoc. 150:318-321. [Google Scholar]
- 19.Hirahara, T., H. Yasuhara, O. Matsui, M. Yamanaka, M. Tanaka, and S. Fukuyama. 1990. Isolation of porcine adenovirus from the respiratory tract of pigs in Japan. Jpn. J. Vet. Sci. 52:407-409. [DOI] [PubMed] [Google Scholar]
- 20.Irving, L. G., and F. A. Smith. 1981. One-year survey of enteroviruses, adenoviruses, and reoviruses isolated from effluent at an activated-sludge purification plant. Appl. Environ. Microbiol. 41:51-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishibashi, M., and H. Yasure. 1984. Adenoviruses of animal, p. 497-562. In H. S. Ginsberg (ed.), The adenoviruses. Plenum Press, New York, N.Y.
- 22.Jimenez-Clavero, M. A., C. Fernandez, J. A. Ortiz, J. Por, G. Carbonell, J. V. Tarazona, N. Roblas, and V. Ley. 2003. Teschoviruses as indicators of porcine fecal contamination of surface water. Appl. Environ. Microbiol. 69:6311-6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jofre, J. 2002. Bacteriophages as indicators, p. 354-363. In G. Bitton (ed.), Encyclopedia of environmental microbiology. Wiley and Sons, New York, N.Y.
- 24.Kappeler, A., C. Lutz-Wallace, T. Sapp, and M. Sidhu. 1996. Detection of bovine polyomavirus contamination in fetal bovine sera and modified live viral vaccines using polymerase chain reaction. Biologicals 24:131-135. [DOI] [PubMed] [Google Scholar]
- 25.Ley, V., J. Higgins, and R. Fayer. 2002. Bovine enteroviruses as indicators of fecal contamination. Appl. Environ. Microbiol. 68:3455-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipp, E. K., S. A. Farrah, and J. B. Rose. 2001. Assessment and impact of microbial fecal pollution and human enteric pathogens in a coastal community. Mar. Pollut. Bull. 42:286-293. [DOI] [PubMed] [Google Scholar]
- 27.Maluquer de Motes, C., P. Clemente-Casares, A. Hundesa, M. Martin, and R. Girones. 2004. Detection of bovine and porcine adenoviruses for tracing the source of fecal contamination. Appl. Environ. Microbiol. 70:1448-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangel, W. F., D. L. Toledo, J. Ding, R. M. Sweet, and W. J. McGrath. 1997. Temporal and spatial control of the adenovirus proteinase by both a peptide and the viral DNA. Trends Biochem. Sci. 22:393-398. [DOI] [PubMed] [Google Scholar]
- 29.Meng, X. J., P. S. Paul, P. G. Halbur, and M. Lum. 1996. Characterization of a high-virulence US isolate of porcine reproductive and respiratory syndrome virus in a continuous cell line. ATCC CRL11171. J. Vet. Diagn. Investig. 8:374-381. [DOI] [PubMed] [Google Scholar]
- 30.Noble, R. T., S. M. Allen, A. D. Blackwood, W. Chu, S. C. Jiang, G. L. Lovelace, M. D. Sobsey, J. R. Stewart, and D. A. Wait. 2003. Use of viral pathogens and indicators to differentiate between human and non-human fecal contamination in a microbial source tracking comparison study. J. Water Health 1:195-207. [PubMed] [Google Scholar]
- 31.Parry, J., and S. Gardner. 1986. Human exposure to bovine polyomavirus: a zoonosis? Arch. Virol. 87:287-296. [DOI] [PubMed] [Google Scholar]
- 32.Parry, J. V., M. H. Lucas, J. E. Richmond, and S. D. Gardner. 1983. Evidence for a bovine origin of the polyomavirus detected in fetal rhesus monkey kidney cells, FRhK-4 and -6. Arch. Virol. 78:151-165. [DOI] [PubMed] [Google Scholar]
- 33.Payan, A., J. Ebdon, H. Taylor, C. Gantzer, J. Ottoson, G. T. Papageorgiou, A. R. Blanch, F. Lucena, J. Jofre, and M. Muniesa. 2005. Method for isolation of Bacteroides bacteriophage host strains suitable for tracking sources of fecal pollution in water. Appl. Environ. Microbiol. 71:5659-5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pina, S., M. Puig, F. Lucena, J. Jofre, and R. Girones. 1998. Viral pollution in the environment and in shellfish: human adenovirus detection by PCR as an index of human viruses. Appl. Environ. Microbiol. 64:3376-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puig, M., J. Jofre, F. Lucena, A. K. Allard, G. Wadell, and R. Girones. 1994. Detection of adenoviruses and enteroviruses in polluted waters by nested PCR amplification. Appl. Environ. Microbiol. 60:2963-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rangan, S., R. Lowrie, J. Roberts, P. Johnston, and R. Warrick. 1974. Virus from stump-tailed monkey (Macaca arctoides) kidney cultures. Lab. Anim. Sci. 24:211-217. [PubMed] [Google Scholar]
- 37.Schuurman, R., C. Sol, and J. van der Noordaa. 1990. The complete nucleotide sequence of bovine polyomavirus. J. Gen. Virol. 71:1723-1735. [DOI] [PubMed] [Google Scholar]
- 38.Schuurman, R., B. van Steenis, and C. Sol. 1991. Bovine polyomavirus, a frequent contaminant of calf serum. Biologicals 19:265-270. [DOI] [PubMed] [Google Scholar]
- 39.Schuurman, R., B. van Steenis, A. van Strien, J. van der Noordaa, and C. Sol. 1991. Frequent detection of bovine polyomavirus in commercial batches of calf serum by using the polymerase chain reaction. J. Gen. Virol. 72:2739-2745. [DOI] [PubMed] [Google Scholar]
- 40.Tartera, C., and J. Jofre. 1987. Bacteriophages active against Bacteroides fragilis in sewage-polluted water. Appl. Environ. Microbiol. 53:1632-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thurston-Enriquez, J. A., C. N. Haas, J. Jacangelo, and C. P. Gerba. 2003. Chlorine inactivation of adenovirus type 40 and feline calicivirus. Appl. Environ. Microbiol. 69:3979-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thurston-Enriquez, J. A., C. N. Haas, J. Jacangelo, K. Riley, and C. P. Gerba. 2003. Inactivation of feline calicivirus and adenovirus type 40 by UV radiation. Appl. Environ. Microbiol. 69:577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, J., G. W. Horner, and J. S. O'Keefe. 2005. Detection and molecular characterization of bovine polyomavirus in bovine sera in New Zealand. N. Z. Vet. J. 53:26-30. [DOI] [PubMed] [Google Scholar]
- 44.Wognum, A. W., C. Sol, J. van der Noordaa, G. Steenis, and A. Osterhaus. 1984. Isolation and characterization of a papovavirus from cynomolgus macaque kidney cells. Virology 134:254-257. [DOI] [PubMed] [Google Scholar]