Abstract
In vitro coculture fermentations of Bifidobacterium longum BB536 and two acetate-converting, butyrate-producing colon bacteria, Anaerostipes caccae DSM 14662 and Roseburia intestinalis DSM 14610, with oligofructose as the sole energy source, were performed to study interspecies interactions. Two clearly distinct types of cross-feeding were identified. A. caccae DSM 14662 was not able to degrade oligofructose but could grow on the fructose released by B. longum BB536 during oligofructose breakdown. R. intestinalis DSM 14610 could degrade oligofructose, but only after acetate was added to the medium. Detailed kinetic analyses of oligofructose breakdown by the last strain revealed simultaneous degradation of the different chain length fractions, in contrast with the preferential degradation of shorter fractions by B. longum BB536. In a coculture of both strains, initial oligofructose degradation and acetate production by B. longum BB536 took place, which in turn also allowed oligofructose breakdown by R. intestinalis DSM 14610. These and similar cross-feeding mechanisms could play a role in the colon ecosystem and contribute to the combined bifidogenic/butyrogenic effect observed after addition of inulin-type fructans to the diet.
Since the introduction of the concept of prebiotics more than a decade ago (22), various food components have been screened for their ability to beneficially alter the composition and/or the activity of the colon microbiota (21). However, inulin-type fructans, such as oligofructose and inulin, largely remain the most studied and well-established prebiotics (43). The vast amount of research performed on their health-promoting properties led to their acceptance as model prebiotics (44).
After their ingestion as a part of the human diet, inulin and oligofructose largely escape digestion in the upper gastrointestinal tract and reach the colon virtually intact (20, 39), where they are fermented by some members of the resident microbiota (1, 39). Their stimulating effect on the colonic bifidobacterial population has been demonstrated extensively by in vitro and in vivo animal and human trials (21, 30, 45). Also, it has been shown that inulin-type fructans have a regulatory effect on bowel functions (9, 40), increase calcium (23, 51) and magnesium (50) absorption, and reduce triglyceridemia in slightly hyperlipidemic individuals (13, 32). The influence of oligofructose and inulin on bone health (12, 58), cholesterolemia (6, 13), the immune system (57), and the development of inflammatory bowel disease (24) and colon cancer (41) remains under investigation.
Many of the health-promoting effects attributed to oligofructose and inulin are at least partially due to their influence on the production of short-chain fatty acids (SCFA) by the colon microbiota (13, 24, 40, 41, 58). In humans, colonic fermentation of inulin-type fructans generally does not lead to a significant increase of fecal concentrations of SCFA or to a change in molar proportions of acetate, propionate, and butyrate (40), which can be explained by their very efficient colonic absorption (34, 35). However, in vitro and animal studies show an enhancement of SCFA production by inulin and oligofructose (14, 56). In particular, fermentation of inulin-type fructans by the colon microbiota seems to cause an increase in butyrate formation, the so-called butyrogenic effect (8, 31, 52). Butyrate is of key importance for gut health: it is not only the preferred energy source for the colonic epithelium (34) but also has important effects on the development of and the gene expression in intestinal cells (37, 47). In addition, it is generally thought to play a protective role against colorectal cancer and colitis (2, 10, 25).
The link between consumption of inulin-type fructans, the bifidogenic effect, and the increase of butyrate production in the colon remains unclear up to now. Bifidobacteria produce lactate, acetate, formate, ethanol, and even minor amounts of succinate (3, 53, 54, 59) but have never been reported to produce butyrate (L. Makras, G. Falony, R. Van der Meulen, and L. De Vuyst, Letter, J. Appl. Microbiol. 100:1388-1389, 2006). Furthermore, evidence of direct degradation of inulin-type fructans by butyrate-producing colon bacteria is scarce (19). Cross-feeding between different members of the colon microbiota has been suggested as a possible mechanism responsible for colonic butyrate production (5, 7, 18, 29, 42). However, research attempting to unravel the exact nature of this cross-feeding has until now been limited to small-scale experiments under uncontrolled conditions, not allowing kinetic analysis (5, 18, 29).
Recently, the use of more-complex culture media has allowed the isolation of some previously unknown butyrate-producing colon bacteria (4), belonging to clostridial cluster XIVa (11, 17, 48), one of the most abundant bacterial groups in human feces (26, 49). Species such as Anaerostipes caccae and Roseburia intestinalis have been shown to be efficient lactate and/or acetate converters (16, 18). Bacteria related to these species have been reported to compose up to 3% of the colon microbiota (27). Cross-feeding between these microorganisms and lactate- and/or acetate-producing inulin degraders, such as bifidobacteria, might be a key aspect of the gut ecosystem, with important consequences for human health. The aim of this work was to investigate kinetically some of the mechanisms behind this type of cross-feeding, using intermediate-scale, in vitro, mono- and coculture batch fermentation techniques under strictly controlled conditions.
MATERIALS AND METHODS
Microorganisms and media.
The commercialized probiotic strain Bifidobacterium longum BB536 was kindly provided by Morinaga Industry Co., Ltd. (Tokyo, Japan). Anaerostipes caccae DSM 14662 (48) and Roseburia intestinalis DSM 14610 (17), two recently described butyrate-producing colon bacteria, were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSM; Göttingen, Germany). All strains were stored at −80°C in reinforced clostridial medium (RCM; Oxoid Ltd., Basingstoke, United Kingdom), supplemented with 25% (vol/vol) glycerol as a cryoprotectant.
Fermentation experiments were performed in a medium for colon bacteria (MCB), developed by Van der Meulen et al. (55) to support growth of various human colon bacteria when supplemented with an adequate energy source. The medium was composed of the following (concentrations in g liter−1): bacteriological peptone (Oxoid), 6.5; soy peptone (Oxoid), 5.0; yeast extract (VWR International, Darmstadt, Germany), 3.0; tryptone (Oxoid), 2.5; NaCl, 4.5; KCl, 2.0; MgSO4 · 7H2O, 0.5; CaCl2 · 2H2O, 0.45; cysteine-HCl · H2O, 0.4; NaHCO3, 0.2; MnSO4 · H2O, 0.2; FeSO4 · 7H2O, 0.005; ZnSO4 · 7H2O, 0.005; hemin, 0.005; menadione, 0.005. It also contained H3PO4 (0.5 ml liter−1) and Tween 80 (2 ml liter−1). When proven indispensable for bacterial growth, modified MCB containing 6.8 g liter−1 of NaCH3COOH · 3H2O was used. The pH was adjusted to 5.8, and the medium was autoclaved at 210 kPa and 120°C for 20 min. After sterilization, fructose (VWR International) or oligofructose (RaftiloseP95; Orafti NV, Tienen, Belgium) was added aseptically as the sole energy source at a concentration of 9 g liter−1. Fructose was autoclaved under the same conditions as the MCB; oligofructose was sterilized through membrane filtration using Minisart filters (pore size, 0.2 μm; Sartorius AG, Göttingen, Germany). RaftiloseP95 is a commercial powder obtained through enzymatic hydrolysis of chicory inulin. It consists mainly of oligofructose (≥93.2%, wt/wt) but contains also some minor amounts of glucose, fructose, and sucrose (<6.8%, wt/wt). The degree of polymerization (DP) of the oligofructose fractions varies between 2 and 8, with an average of 4.
Solid RCM was prepared by adding 1.5% (wt/vol) agar (Oxoid) to RCM.
Fermentation experiments.
Mono- and coculture fermentations were carried out in 1.5-liter Biostat B-DCU fermentors (Sartorius AG). Inocula were prepared as follows: strains were transferred from −80°C to RCM and incubated anaerobically at 37°C for 24 h in a modular atmosphere-controlled system (MG anaerobic work station; Don Withley Scientific, West Yorkshire, United Kingdom) that was continuously sparged with a mixture of 80% nitrogen, 10% carbon dioxide, and 10% hydrogen (Air Liquide, Paris, France). Subsequently, the strains were propagated twice in MCB with fructose (and acetate, when proven indispensable) as the sole energy source(s) and finally added to the fermentor. During inoculum buildup, the transferred volume was always 5% (vol/vol). Anaerobic conditions were assured during fermentation experiments by continuously sparging the medium with a mixture of 90% nitrogen and 10% carbon dioxide (Air Liquide). Fermentation temperature was maintained constant at 37°C. A linear pH profile, mimicking colonic pH and transit by raising pH from 5.8 to 6.8 in 48 h, was imposed and controlled automatically using 1.5 M solutions of NaOH and H3PO4. To keep the medium homogeneous, gentle stirring (100 rpm) was applied. Temperature, pH, and agitation speed were controlled online (MFCS/win 2.1; Sartorious AG). Fermentations were followed up for 48 h; samples for further analysis were taken at regular time intervals. All fermentations were carried out in duplicate; the results and figures presented are representative for both fermentations.
Analysis of growth.
Growth was followed throughout all fermentations by both plating and biomass determination. Bacterial biomass was determined as cell dry mass (CDM) through membrane filtration of 15 ml of sample using cellulose nitrate filters (pore size, 0.45 μm; Sartorius AG). The filters were dried at 105°C for 48 h and weighed. Samples were plated on RCM agar and incubated for 24 h under anaerobic conditions (modular atmosphere-controlled system). However, as A. caccae DSM 14662 and R. intestinalis DSM 14610 grew poorly on solid culture media, results of enumerations are not reported.
Analysis of fructose and oligofructose breakdown.
Residual concentrations of glucose, fructose and oligofructose (expressed as mM fructose equivalents [FE]) were determined by high-performance liquid chromatography with a chromatograph (Waters Corp., Milford, MA) equipped with a 2414 differential refractometer, a 600S controller, a column oven, and a 717plus autosampler. An ICSep ICE ORH-801 column (Interchim, Montluçon, France) was used with 10 mM of H2SO4 as the mobile phase at a flow rate of 0.4 ml min−1. The column temperature was kept constant at 35°C. Samples were centrifuged (21,036 × g for 20 min), and an equal volume of 20% (wt/vol) trichloroacetic acid was added for protein removal. After centrifugation (21,036 × g, 20 min) the supernatant was filtered (pore size, 0.2 μm; Minisart RC4 filters; Sartorius AG) before injection. All samples were analyzed in triplicate.
Detailed analysis of the breakdown of the different oligofructose fractions of RaftiloseP95 was performed by gas chromatography using an HRGC 5300-HT Mega (Carlo Erba, Rodina, Italy) as described previously (28, 54). The gas chromatograph was equipped with an SGE Aluminum Clad-5 capillary column (Achrom NV, Zulte, Belgium), a cool on-column autoinjector AS-550, and a flame ionization detector (detector temperature of 447°C). The oven temperature varied linearly from 105 to 440°C at 10°C min−1. Samples were derivatized following a procedure involving oximation and silylation (28). The oxime-trimethylsilyl sugar derivatives were extracted using iso-octane; the resulting iso-octane phase was injected into the gas chromatograph. The same procedure was carried out for reference samples containing reference oligofructose (RaftiloseP95X; Orafti NV), glucose, fructose, and sucrose as external standards.
Analysis of metabolite production.
The concentrations of lactate, acetate, butyrate, and formate were determined through high-performance liquid chromatography as described above.
Succinate was determined using a 2695 high-performance liquid chromatograph (Waters) coupled to a Quattro Micro mass spectrometer (Waters). The column (Atlantis; Waters) was kept at 35°C. The mobile phase, at a flow rate of 0.2 ml min−1, was composed of ultrapure water (eluent A), acetonitrile (eluent B), and 10 mM of ammonium acetate (pH 6.5; eluent C). The gradient (vol/vol) used was as follows: 0.0 min, 85% eluent A, 5% eluent B, and 10% eluent C; 15.0 min, 40% A, 50% B, and 10% C; 15.1 min, 10% A, 80% B, and 10% C; 23.0 min, 10% A, 80% B, and 10% C; 23.1 min, 85% A, 5% B, and 10% C; 30.0 min, 85% A, 5% B, and 10% C. Samples were centrifuged (21,036 × g for 20 min), and 100 μl of internal standard (3,4-dihydroxybenzoic acid) was added to 500 μl of supernatant. Afterwards, 600 μl of acetonitrile was added, and the samples were again centrifuged (21,036 × g for 20 min). The supernatant was filtered (Minisart RC4) and injected. All samples were analyzed in triplicate.
Ethanol was measured on a 6890 gas chromatograph (Agilent Technologies, Palo Alto, CA) equipped with a programmable temperature vaporization injector, coupled to a 5973N mass spectrometer (Agilent Technologies). A capillary column (DB-WAXetr; Agilent Technologies) was used with the following oven temperature program: 0.0 min, 40°C; 5.0 min, 40°C; 9.29 min, 100°C; 10.37 min, 230°C; 15 min, 230°C. Helium (Air Liquide) was used as carrier gas at a flow rate of 1.1 ml min−1. The samples were centrifuged (21,036 × g for 20 min), and 100 μl of methanol (0.5% [wt/vol] in ultrapure water) was added as an internal standard to 500 μl of supernatant. After mixing for 15 s, 750 μl of chloroform was added to the sample and mixed thoroughly (30 min). Subsequently, the organic phase was transferred into a vial. The extraction procedure with chloroform was performed twice, after which the samples were injected. Analyses were performed in triplicate.
Carbon recovery.
The carbon recovery (CR; in percent) was calculated by dividing the total amount of carbon recovered in the sugar metabolites by the total amount of carbon present in the added energy source(s). In the case of fructose or oligofructose (RaftiloseP95) degradation by A. caccae DSM 14662 or R. intestinalis DSM 14610, production of 2 moles of carbon dioxide for every mole of fructose (or FE) consumed, as suggested by Duncan et al. (16, 18), was taken into account.
RESULTS
Growth of B. longum BB536, A. caccae DSM 14662, and R. intestinalis DSM 14610 in MCB supplemented with fructose or fructose and acetate.
B. longum BB536 reached a maximal biomass of 2.0 g CDM liter−1 after 15 h of fermentation. Acetate, formate, ethanol, and lactate, as well as minor concentrations of succinate, were produced (Table 1). The calculated CR was 99.4%.
TABLE 1.
Growth characteristics of Bifidobacterium longum BB536, Anaerostipes caccae DSM 14662, and Roseburia intestinalis DSM 14610 in MCB supplemented with 50 mM of fructose or 50 mM of fructose and 50 mM of acetate
| Strain | Mean consumption ± SD (mM) of substrate:
|
Mean production ± SD (mM) of metabolite:
|
CR (%) | Fructose depletion time (h) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fructose | Acetate | Lactate | Acetate | Butyrate | Formate | Ethanol | Succinate | CO2a | |||
| B. longum BB536 | 49.8 ± 0.4 | 10.7 ± 0.4 | 93.8 ± 1.4 | 39.0 ± 0.5 | 18.2 ± 2.0 | 0.60 ± 0.02 | 99.4 | 15 | |||
| A. caccae DSM 14662 | 50.2 ± 0.2 | 40.1 ± 0.5 | 100.4 | 86.6 | 48 | ||||||
| 49.3 ± 0.1 | 28.8 ± 0.3 | 56.1 ± 0.4 | 98.6 | 91.4 | 30 | ||||||
| R. intestinalis DSM 14610 | 47.9 ± 0.2 | 19.7 ± 0.2 | 55.6 ± 0.4 | 95.8 | 97.5 | 30 | |||||
A. caccae DSM 14662 was able to degrade fructose without the addition of acetate to the fermentation medium. Maximal biomass levels of 1.4 g CDM liter−1 after 30 h and 1.6 g CDM liter−1 after 48 h of fermentation were reached in the presence and absence of acetate, respectively. In both cases, butyrate and gases were the only metabolites produced (Table 1). CR values were calculated as 91.4% and 86.6%, respectively. In the presence of acetate, fructose and acetate consumption took place simultaneously. After fructose depletion, acetate consumption stopped.
Growth of R. intestinalis DSM 14610 was detected only in MCB supplemented with both fructose and acetate. Biomass reached a maximum of 1.3 g CDM liter−1 after 24 h of fermentation. Acetate was consumed only during fructose degradation. Again, butyrate and gases were the only metabolites produced (Table 1). CR was calculated as 97.5%.
Growth of B. longum BB536, A. caccae DSM 14662, and R. intestinalis DSM 14610 in MCB supplemented with oligofructose or oligofructose and acetate.
With B. longum BB536, a slower fermentation took place compared with growth on fructose. Maximal biomass of 1.4 g CDM liter−1 was measured after 48 h. Again, acetate, formate, ethanol, lactate, and succinate were produced (Table 2). The calculated CR was 94.6%.
TABLE 2.
Growth characteristics of Bifidobacterium longum BB536 and Roseburia intestinalis DSM 14610 in MCB supplemented with 50 mM FE of oligofructose or 50 mM FE of oligofructose and 50 mM of acetate
| Strain | Mean consumption ± SD (mM) of substrate:
|
Mean production ± SD (mM) of metabolite:
|
CR (%) | Fructose depletion time (h) | Free fructose concn in medium during fermentation (mM) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oligofructose | Acetate | Lactate | Acetate | Butyrate | Formate | Ethanol | Succinate | CO2a | ||||
| B. longum BB536 | 38.8 ± 0.2 | 2.6 ± 0.1 | 78.9 ± 0.6 | 29.1 ± 0.2 | 12.8 ± 1.9 | 0.80 ± 0.04 | 94.6 | >48 | 0.57-1.27 | |||
| R. intestinalis DSM 14610 | 48.2 ± 0.2 | 29.3 ± 0.3 | 61.5 ± 0.5 | 96.4 | 98.5 | >48 | 1.71-1.12 | |||||
Theoretical CO2 production by R. intestinalis DSM 14610, according to the pathway proposed by Duncan et al. (16).
Addition of acetate to the MCB had no influence on the ability of A. caccae DSM 14662 to degrade oligofructose: when RaftiloseP95 was used as an energy source, only minor growth on the monosaccharides present in the preparation was observed (results not shown). Again, butyrate and gases were produced.
R. intestinalis DSM 14610 was not able to degrade oligofructose in MCB without acetate. In MCB supplemented with acetate, butyrate and gases were the only metabolites produced (Table 2). A maximal biomass of 1.6 g CDM liter−1 was measured after 48 h of fermentation. CR was calculated as 98.5%.
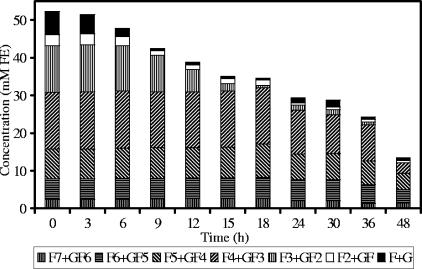
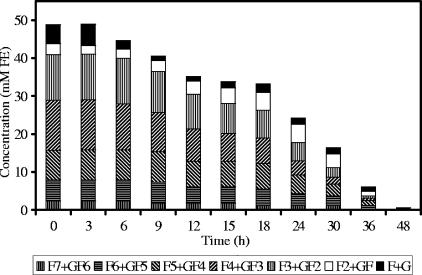
Detailed analysis of fructan degradation by B. longum BB536 (Fig. 1) and R. intestinalis DSM 14610 (Fig. 2) revealed a remarkable difference between the oligofructose breakdown patterns of both strains. B. longum BB536 showed preferential degradation of short-chain oligofructose fractions (DP ≤ 3), degrading longer fractions only after nearly complete depletion of the shorter ones. R. intestinalis DSM 14610 expressed simultaneous breakdown of different chain length fractions (DP 3 and 4). In both cases, low concentrations of free fructose were detected in the medium during the entire course of the fermentations.
FIG. 1.
Oligofructose degradation by Bifidobacterium longum BB536 in MCB supplemented with 50 mM FE of oligofructose (RaftiloseP95).
FIG. 2.
Oligofructose degradation by Roseburia intestinalis DSM 14610 in MCB supplemented with 50 mM FE of oligofructose (RaftiloseP95) and 50 mM of acetate.
Coculture of B. longum BB536 and A. caccae DSM 14662 in MCB supplemented with oligofructose.
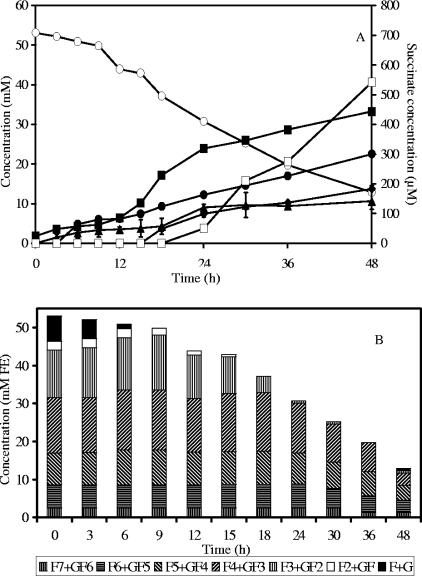
In a coculture of B. longum BB536 and A. caccae DSM 14610, maximal biomass was 1.7 g CDM liter−1 after 48 h of fermentation. Acetate, butyrate, formate, ethanol, and traces of succinate were produced (Fig. 3A). Gas production was observed.
FIG. 3.
Substrate consumption and metabolite production (A) and oligofructose degradation (B) by a coculture of Bifidobacterium longum BB536 and Anaerostipes caccae DSM 14662 in MCB supplemented with 50 mM FE of oligofructose (RaftiloseP95). ○, oligofructose (FE); ▪, acetate; •, butyrate; ⧫, formate; ▴, ethanol; □, succinate; F, fructose; G, glucose.
Detailed analysis of fructan degradation (Fig. 3B) revealed a similar breakdown profile as in B. longum BB536 monoculture fermentations. However, almost no free fructose was detected in the medium during oligofructose breakdown, indicating fructose consumption by A. caccae DSM 14662. Considering that the metabolism of A. caccae DSM 14662 in the coculture was comparable with the one observed in the monoculture in MCB supplemented with fructose and acetate (see above), it can be calculated that the strain would have needed 19.8 mM of fructose to produce 22.5 mM of butyrate, which is nearly one-half of the total concentration of FE consumed during the fermentation. This clearly shows intense cross-feeding between both strains. The previous considerations allow the calculation of the CR in this coculture as 96.6%, taking into account CO2 production by A. caccae DSM 14662.
Coculture of B. longum BB536 and R. intestinalis DSM 14610 in MCB supplemented with oligofructose.
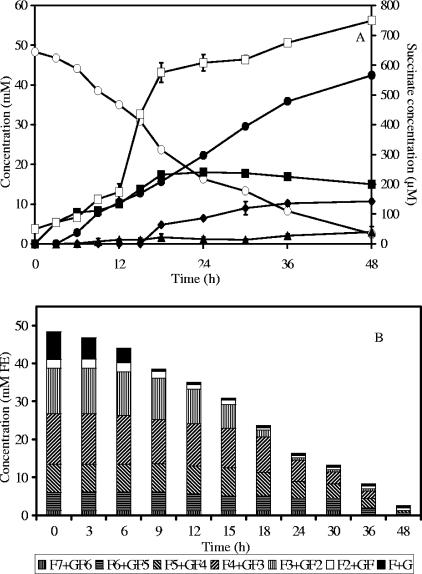
In a coculture of B. longum BB536 and R. intestinalis DSM 14610, biomass reached a maximum of 2.2 g CDM liter−1 after 18 h of fermentation. Besides gases, butyrate, acetate, formate, ethanol, and traces of succinate were the metabolites produced (Fig. 4A).
FIG. 4.
Substrate consumption and metabolite production (A) and oligofructose degradation (B) by a coculture of Bifidobacterium longum BB536 and Roseburia intestinalis DSM 14610 in MCB supplemented with 50 mM FE of oligofructose (RaftiloseP95). ○, oligofructose (FE); ▪, acetate; •, butyrate; ⧫, formate; ▴, ethanol; □, succinate; F, fructose; G, glucose.
Kinetic analysis of the degradation of the different oligofructose fractions showed preferential breakdown of shorter (DP ≤ 3) fructan components (Fig. 4B). However, degradation of longer fractions started before depletion of the shorter ones, and the overall consumption rate was higher than in B. longum BB536 monoculture fermentations. During oligofructose degradation, concentrations of free fructose varied between 0.57 and 0.59 mM.
Considering that R. intestinalis DSM 14610 as well as B. longum BB536 behaved similarly in mono- and coculture fermentations (see above), it can be calculated that R. intestinalis DSM 14610 needed 33.2 mM FE of oligofructose to produce 42.4 mM of butyrate. This estimation allows the calculation of the CR in the coculture as 102.9%, taking into account CO2 production by R. intestinalis DSM 14610.
DISCUSSION
In vitro fermentation studies using monocultures and cocultures certainly have their limitations regarding their in vivo significance, as they by no means take into account the complexity of the human colon ecosystem. These inherent limitations make them, however, an excellent tool for the study of individual bacterial metabolism and interspecies interactions (5, 55). Hence, they can provide a valuable contribution to the further exploration of the gut ecosystem, together with in vitro studies using fecal slurry and in vivo animal or human trials.
Compared with the results of an earlier study with B. longum BB536 carried out by Van der Meulen et al. (55), the monoculture fermentations performed with this strain in the present study confirmed its preferential degradation of shorter oligofructose fractions, a characteristic that seems to be common among bifidobacteria, and its mixed acid fermentation depending on its growth rate (53-55). Growth on low concentrations of oligofructose led to an even more marked metabolic shift towards mixed-acid fermentation than reported earlier (55), indicating that both sugar consumption rate and substrate concentration determine the metabolic profile expressed by this strain. Indeed, when growing on RaftiloseP95, B. longum BB536 produced more formate, ethanol, and acetate, at the expense of lactate, to generate more ATP.
The present kinetic study showed that A. caccae DSM 14662 was able to grow on fructose without addition of acetate to the fermentation medium. This might imply a high flexibility in the metabolism of this strain, allowing it to produce enough endogenous acetate to compensate for the lack of exogenous acetate necessary for butyrate production (16, 18). However, optimal growth occurred only after addition of acetate to the medium. Indeed, growth of A. caccae DSM 14662 in a complex medium showed that it was a net converter of glucose or lactate in combination with acetate (5, 18). This led to the suggestion that it produced butyrate through a butyryl-coenzyme A (CoA):acetate-CoA transferase pathway, with use of (partly) exogenous acetate. Louis et al. (33) showed that this pathway is most common among butyrate-producing members of the colon microbiota, instead of the butyrate kinase pathway, as was thought earlier (38). A. caccae DSM 14662 was not able to degrade oligofructose, in spite of earlier reports of acid production on fructo-oligosaccharides (48), stressing the necessity to report on the exact composition of the inulin-type fructans used and detailed kinetic analyses of substrate degradation.
Enzymatic studies showed that R. intestinalis DSM 14610 possessed acetate kinase and butyryl-CoA:acetate-CoA transferase activity, but no butyrate kinase was detected (16). The results of this study confirmed these findings by showing the absolute need for acetate of this strain to grow on fructose and oligofructose. Regarding growth of R. intestinalis DSM 14610 on inulin-type fructans, conflicting results have been published in the past (17, 19). Moreover, it has recently been shown that the related strain Roseburia sp. strain A2-183, belonging to the newly proposed species Roseburia hominis (15), is not able to grow significantly on oligofructose (5). The present study revealed for the first time the kinetics of the degradation of oligofructose by R. intestinalis DSM 14610 in an acetate-containing fermentation medium. Nonpreferential degradation of the different oligofructose fractions was observed, a phenomenon that has also been reported for other colon bacteria belonging to the genera Bacteroides (55) and Lactobacillus (36), but so far not for Bifidobacterium (54, 55). It has been suggested that simultaneous breakdown of oligofructose fractions with different chain lengths indicates extracellular degradation (55), which also explains the higher concentrations of free fructose found in the fermentation medium in those cases. Most bifidobacteria degrade oligofructose intracellularly (46), which might contribute to their selective stimulation by inulin-type fructans in the gut (55).
The present study of coculture fermentations led to the identification of two clearly distinct types of cross-feeding between B. longum BB536 and butyrate-producing colon bacteria. In the case of the cocultures with A. caccae DSM 14662, butyrate production could be attributed only to the latter strain. As this strain was not able to grow on oligofructose, its only available energy sources were the fructose released and the acetate produced by B. longum BB536 during oligofructose degradation. This was reflected by the fact that the breakdown profile in the coculture matched the one of the B. longum BB536 monoculture, except for the fact that no free fructose was detected. Recently, a study focusing on cross-feeding between A. caccae DSM 14662 and a Bifidobacterium adolescentis strain in a rich medium containing starch as an energy source pointed out that lactate was the key substrate in the described cross-feeding mechanism (5). The results presented in the present paper reveal the flexibility of cross-feeding in the function of the bacterial partner and the available substrate.
Butyrate in the coculture of B. longum BB536 and R. intestinalis DSM 14610 could be produced only by the latter strain. Detailed kinetic analyses of oligofructose degradation showed a profile in between simultaneous and preferential degradation of different oligofructose fractions (Fig. 4B). R. intestinalis DSM 14610, capable of simultaneous breakdown, had an absolute requirement for acetate to degrade oligofructose. Initially, no acetate was present in the fermentation medium; it appeared only as a metabolic end product of bifidobacterial growth. Subsequently, oligofructose was further degraded as the result of the combined efforts of both strains. A recently published, small-scale cross-feeding study performed by Belenguer et al. (5) on a coculture of B. adolescentis L2-32 and R. hominis A2-183 did not reveal oligofructose degradation by the latter strain. The cross-feeding mechanism described in the present study for A. caccae DSM 14662 is probably comparable to the one observed previously for R. hominis A2-183, where butyrate production by this strain was attributed to cross-feeding of the partially degraded carbohydrate substrate, although degradation kinetics were not studied in detail (5). Finally, other Bifidobacterium strains, and to a lesser extent Lactobacillus species, are reported to produce lactate as well as acetate on oligofructose or on starch (5, 30, 36, 53, 54). Therefore, the types of cross-feeding may be strain dependent.
In conclusion, the results presented in this paper are, to our knowledge, the first that kinetically describe cross-feeding between a Bifidobacterium sp. and butyrate-producing colon bacteria growing on a prebiotic substrate under controlled conditions. This study confirms the earlier reported shift of in vitro bifidobacterial metabolism towards less lactate production during growth on oligofructose (54, 55). However, during oligofructose degradation, B. longum BB536 released substantial amounts of free fructose into the extracellular environment, enough to support growth of A. caccae DSM 14662, a strain that was not able to degrade the substrate itself. This study is also the first detailed kinetic analysis of oligofructose degradation by R. intestinalis DSM 14610. To do so, the strain showed an absolute requirement for acetate, which is sufficiently present in the gut too (34) and, as was shown in this study, can be provided by bifidobacteria. These and similar cross-feeding mechanisms could play a role in the colon ecosystem and contribute to the combined bifidogenic/butyrogenic effect observed after addition of inulin-type fructans to the diet. However, the physiological relevance of oligofructose degradation by R. intestinalis DSM 14610 has to be studied further through in vitro colon simulation models and in vivo trials. Also, additional in vitro studies of the metabolism of butyrate-producing colon bacteria, combined with in vivo trials with genetic tools to monitor the effect of addition of oligofructose to the human diet on the colonic population of butyrate producers (27), are needed.
Acknowledgments
This research was funded by the Research Council of the Vrije Universiteit Brussel, the Fund for Scientific Research—Flanders, and the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen; GBOU project IWT-010054, “Development of a fast, noninvasive technological tool to investigate the functionality and effectiveness of pro- and prebiotics in normal healthy humans: the use of a labeled biomarker”). Gwen Falony was the recipient of a Ph.D. grant from the IWT-Vlaanderen.
We thank the people of the analytical laboratory of Orafti NV for their support.
Footnotes
Published ahead of print on 20 October 2006.
REFERENCES
- 1.Alles, M. S., J. G. A. Hautvast, F. M. Nagengast, R. Hartemink, K. M. J. van Laere, and J. M. B. J. Jansen. 1996. Fate of fructooligosaccharides in the human intestine. Br. J. Nutr. 76:211-221. [DOI] [PubMed] [Google Scholar]
- 2.Archer, S., S. F. Meng, J. Wu, J. Johnson, R. Tang, and R. Hodin. 1998. Butyrate inhibits colon carcinoma cell growth through two distinct pathways. Surgery 124:248-253. [PubMed] [Google Scholar]
- 3.Ballongue, J. 1998. Bifidobacteria and probiotic action, p. 519-587. In S. Salminen and A. von Wright (ed.), Lactic acid bacteria: microbiology and functional aspects. Marcel Dekker Inc., New York, N.Y.
- 4.Barcenilla, A., S. E. Pryde, J. C. Martin, S. H. Duncan, C. S. Stewart, C. Henderson, and H. J. Flint. 2000. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 66:1654-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belenguer, A., S. H. Duncan, G. Calder, G. Holtrop, P. Louis, G. E. Lobley, and H. J. Flint. 2006. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl. Environ. Microbiol. 72:3593-3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beylot, M. 2005. Effects of inulin-type fructans on lipid metabolism in man and in animal models. Br. J. Nutr. 93:S163-S168. [DOI] [PubMed] [Google Scholar]
- 7.Bourriaud, C., R. J. Robins, L. Martin, F. Kozlowski, E. Tenailleau, C. Cherbut, and C. Michel. 2005. Lactate is mainly fermented to butyrate by human intestinal microfloras but inter-individual variation is evident. J. Appl. Microbiol. 99:201-212. [DOI] [PubMed] [Google Scholar]
- 8.Campbell, J. M., G. C. Fahey, and B. W. Wolf. 1997. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J. Nutr. 127:130-136. [DOI] [PubMed] [Google Scholar]
- 9.Cherbut, C. 2002. Inulin and oligofructose in the dietary fibre concept. Br. J. Nutr. 87:S159-S162. [DOI] [PubMed] [Google Scholar]
- 10.Christl, S. U., H. D. Eisner, G. Dusel, H. Kasper, and W. Scheppach. 1996. Antagonistic effects of sulfide and butyrate on proliferation of colonic mucosa—a potential role for these agents in the pathogenesis of ulcerative colitis. Dig. Dis. Sci. 41:2477-2481. [DOI] [PubMed] [Google Scholar]
- 11.Collins, M. D., P. A. Lawson, A. Willems, J. J. Cordoba, J. Fernandez-Garayzabal, P. Garcia, J. Cai, H. Hippe, and J. A. E. Farrow. 1994. The phylogeny of the genus Clostridium: proposal for five new genera and eleven new species combinations. Int. J. Syst. Bacteriol. 44:812-826. [DOI] [PubMed] [Google Scholar]
- 12.Coxam, V. 2005. Inulin-type fructans and bone health: state of the art and perspectives in the management of osteoporosis. Br. J. Nutr. 93:S111-S123. [DOI] [PubMed] [Google Scholar]
- 13.Delzenne, N. M., C. Daubioul, A. Neyrinck, M. Lasa, and H. S. Taper. 2002. Inulin and oligofructose modulate lipid metabolism in animals: review of biochemical events and future prospects. Br. J. Nutr. 87:S255-S259. [DOI] [PubMed] [Google Scholar]
- 14.Djouzi, Z., and C. Andrieux. 1997. Compared effects of three oligosaccharides on metabolism of intestinal microflora in rats inoculated with a human faecal flora. Br. J. Nutr. 78:313-324. [DOI] [PubMed] [Google Scholar]
- 15.Duncan, S. H., R. I. Aminov, K. P. Scott, P. Louis, T. B. Stanton, and H. J. Flint. 2006. Proposal of Roseburia faecis sp. nov., Roseburia hominis sp. nov., and Roseburia inulinovorans sp. nov., based on isolates from human faeces. Int. J. Syst. Evol. Microbiol. 56:2437-2441. [DOI] [PubMed] [Google Scholar]
- 16.Duncan, S. H., A. Barcenilla, C. S. Stewart, S. E. Pryde, and H. J. Flint. 2002. Acetate utilization and butyryl-coenzyme A (CoA):acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl. Environ. Microbiol. 68:5186-5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duncan, S. H., G. L. Hold, A. Barcenilla, C. S. Stewart, and H. J. Flint. 2002. Roseburia intestinalis sp. nov., a novel saccharolytic, butyrate-producing bacterium from human feces. Int. J. Syst. Evol. Microbiol. 52:1615-1620. [DOI] [PubMed] [Google Scholar]
- 18.Duncan, S. H., P. Louis, and H. J. Flint. 2004. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 70:5810-5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duncan, S. H., K. P. Scott, A. G. Ramsay, H. J. M. Harmsen, G. W. Welling, C. S. Stewart, and H. J. Flint. 2003. Effects of alternative dietary substrates on competition between human colonic bacteria in an anaerobic fermentor system. Appl. Environ. Microbiol. 69:1136-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellegård, L., H. Andersson, and I. Bosaeus. 1997. Inulin and oligofructose do not influence the absorption of cholesterol, or the excretion of cholesterol, Ca, Mg, Zn, Fe, or bile acids but increase energy excretion in ileostomy subjects. Eur. J. Clin. Nutr. 51:1-5. [DOI] [PubMed] [Google Scholar]
- 21.Gibson, G. R., H. M. Probert, J. A. E. Van Loo, R. A. Rastall, and M. B. Roberfroid. 2004. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr. Res. Rev. 17:259-275. [DOI] [PubMed] [Google Scholar]
- 22.Gibson, G. R., and M. B. Roberfroid. 1995. Dietary modulation of the human colonic microbiota—introducing the concept of prebiotics. J. Nutr. 125:1401-1412. [DOI] [PubMed] [Google Scholar]
- 23.Griffin, I. J., P. M. D. Hicks, R. P. Heaney, and S. A. Abrams. 2003. Enriched chicory inulin increases calcium absorption mainly in girls with lower calcium absorption. Nutr. Res. 23:901-909. [Google Scholar]
- 24.Guarner, F. 2005. Inulin and oligofructose: impact on intestinal diseases and disorders. Br. J. Nutr. 93:S61-S65. [DOI] [PubMed] [Google Scholar]
- 25.Hague, A., A. J. Butt, and C. Paraskeva. 1996. The role of butyrate in human colonic epithelial cells: an energy source or inducer of differentiation and apoptosis? Proc. Nutr. Soc. 55:937-943. [DOI] [PubMed] [Google Scholar]
- 26.Hold, G. L., S. E. Pryde, V. J. Russell, E. Furrie, and H. J. Flint. 2002. Assessment of microbial diversity in human colonic samples by 16S rDNA sequence analysis. FEMS Microbiol. Ecol. 39:33-39. [DOI] [PubMed] [Google Scholar]
- 27.Hold, G. L., A. Schwiertz, R. I. Aminov, M. Blaut, and H. J. Flint. 2003. Oligonucleotide probes that detect quantitatively significant groups of butyrate-producing bacteria in human feces. Appl. Environ. Microbiol. 69:4320-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joye, D., and H. Hoebregs. 2000. Determination of oligofructose, a soluble dietary fiber, by high-temperature capillary gas chromatography. J. AOAC Int. 83:1020-1025. [PubMed] [Google Scholar]
- 29.Kanauchi, O., Y. Fujiyama, K. Mitsuyama, Y. Araki, T. Ishii, T. Nakamura, Y. Hitomi, K. Agata, T. Saiki, A. Andoh, A. Toyonaga, and T. Bamba. 1999. Increased growth of Bifidobacterium and Eubacterium by germinated barley foodstuff, accompanied by enhanced butyrate production in healthy volunteers. Int. J. Mol. Med. 3:175-179. [DOI] [PubMed] [Google Scholar]
- 30.Kolida, S., K. Tuohy, and G. R. Gibson. 2002. Prebiotic effects of inulin and oligofructose. Br. J. Nutr. 87:S193-S197. [DOI] [PubMed] [Google Scholar]
- 31.Le Blay, G., C. Michel, H. M. Blottiere, and C. Cherbut. 1999. Prolonged intake of fructooligosaccharides induces a short-term elevation of lactic acid-producing bacteria and a persistent increase in cecal butyrate in rats. J. Nutr. 129:2231-2235. [DOI] [PubMed] [Google Scholar]
- 32.Letexier, D., F. Diraison, and M. Beylot. 2003. Addition of inulin to a moderately high-carbohydrate diet reduces hepatic lipogenesis and plasma triacylglycerol concentrations in humans. Am. J. Clin. Nutr. 77:559-564. [DOI] [PubMed] [Google Scholar]
- 33.Louis, P., S. H. Duncan, S. I. McCrae, J. Millar, M. S. Jackson, and H. J. Flint. 2004. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J. Bacteriol. 186:2099-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macfarlane, G. T., and J. H. Cummings. 1991. The colonic flora, fermentation and large bowel digestive function, p. 51-92. In S. F. Phillips, J. H. Pemberton, and R. G. Shorter (ed.), The large intestine: physiology, pathophysiology and disease. Raven Press Ltd., New York, N.Y.
- 35.Macfarlane, S., and G. T. Macfarlane. 2003. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 62:67-72. [DOI] [PubMed] [Google Scholar]
- 36.Makras, L., G. Van Acker, and L. De Vuyst. 2005. Lactobacillus paracasei subsp. paracasei 8700:2 degrades inulin-type fructans exhibiting different degrees of polymerization. Appl. Environ. Microbiol. 71:6531-6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mariadason, J. M., K. L. Rickard, D. H. Barkla, L. H. Augenlicht, and P. R. Gibson. 2000. Divergent phenotypic patterns and commitment to apoptosis of Caco-2 cells during spontaneous and butyrate-induced differentiation. J. Cell. Physiol. 183:347-354. [DOI] [PubMed] [Google Scholar]
- 38.Miller, T. L., and M. J. Wolin. 1979. Fermentations by saccharolytic intestinal bacteria. Am. J. Clin. Nutr. 32:164-172. [DOI] [PubMed] [Google Scholar]
- 39.Molis, C., B. Flourie, F. Ouarne, M. F. Gailing, S. Lartigue, A. Guibert, F. Bornet, and J. P. Galmiche. 1996. Digestion, excretion, and energy value of fructooligosaccharides in healthy humans. Am. J. Clin. Nutr. 64:324-328. [DOI] [PubMed] [Google Scholar]
- 40.Nyman, M. 2002. Fermentation and bulking capacity of indigestible carbohydrates: the case of inulin and oligofructose. Br. J. Nutr. 87:S163-S168. [DOI] [PubMed] [Google Scholar]
- 41.Pool-Zobel, B. L. 2005. Inulin-type fructans and reduction in colon cancer risk: review of experimental and human data. Br. J. Nutr. 93:S73-S90. [DOI] [PubMed] [Google Scholar]
- 42.Pryde, S. E., S. H. Duncan, G. L. Hold, C. S. Stewart, and H. J. Flint. 2002. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 217:133-139. [DOI] [PubMed] [Google Scholar]
- 43.Roberfroid, M. B. 2005. Introducing inulin-type fructans. Br. J. Nutr. 93:S13-S25. [DOI] [PubMed] [Google Scholar]
- 44.Roberfroid, M. B. 2005. Inulin-type fructans and the modulation of the intestinal microflora, p. 151-181. In M. B. Roberfroid and I. Wolinsky (ed.), Inulin-type fructans: functional food ingredients. CRC Press LCC, Boca Raton, Fla.
- 45.Roberfroid, M. B., J. A. E. Van Loo, and G. R. Gibson. 1998. The bifidogenic nature of chicory inulin and its hydrolysis products. J. Nutr. 128:11-19. [DOI] [PubMed] [Google Scholar]
- 46.Rossi, M., C. Corradini, A. Amaretti, M. Nicolini, A. Pompei, S. Zanoni, and D. Matteuzzi. 2005. Fermentation of fructooligosaccharides and inulin by bifidobacteria: a comparative study of pure and fecal cultures. Appl. Environ. Microbiol. 71:6150-6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheppach, W., and F. Weiler. 2004. The butyrate story: old wine in new bottles? Curr. Opin. Clin. Nutr. Metab. Care 7:563-567. [DOI] [PubMed] [Google Scholar]
- 48.Schwiertz, A., G. L. Hold, S. H. Duncan, B. Gruhl, M. D. Collins, P. A. Lawson, H. J. Flint, and M. Blaut. 2002. Anaerostipes caccae gen. nov., sp. nov., a new saccharolytic, acetate-utilizing, butyrate-producing bacterium from human feces. Syst. Appl. Microbiol. 25:46-51. [DOI] [PubMed] [Google Scholar]
- 49.Suau, A., R. Bonnet, M. Sutren, J. J. Godon, G. R. Gibson, M. D. Collins, and J. Doré. 1999. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl. Environ. Microbiol. 65:4799-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tahiri, M., J. C. Tressol, J. Arnaud, F. Bornet, C. Bouteloup-Demange, C. Feillet-Coudray, V. Ducros, D. Pepin, F. Brouns, A. M. Roussel, Y. Rayssiguier, and C. Coudray. 2001. Five-week intake of short-chain fructooligosaccharides increases intestinal absorption and status of magnesium in postmenopausal women. J. Bone Miner. Res. 16:2152-2160. [DOI] [PubMed] [Google Scholar]
- 51.Tahiri, M., J. C. Tressol, Y. Arnaud, F. R. J. Bornet, C. Bouteloup-Demange, C. Feillet-Coudray, M. Brandolini, V. Ducros, D. Pepin, F. Brouns, A. M. Roussel, Y. Rayssiguier, and C. Coudray. 2003. Effect of short-chain fructooligosaccharides on intestinal calcium absorption and calcium status in postmenopausal women: a stable-isotope study. Am. J. Clin. Nutr. 77:449-457. [DOI] [PubMed] [Google Scholar]
- 52.Tsukahara, T., Y. Iwasaki, K. Nakayama, and K. Ushida. 2003. Stimulation of butyrate production in the large intestine of weaning piglets by dietary fructooligosaccharides and its influence on the histological variables of the large intestinal mucosa. J. Nutr. Sci. Vitaminol. 49:414-421. [DOI] [PubMed] [Google Scholar]
- 53.Van der Meulen, R., T. Adriany, K. Verbrugghe, and L. De Vuyst. 2006. Kinetic analysis of bifidobacterial metabolism reveals a minor role for succinic acid in the regeneration of NAD+ through its growth-associated production. Appl. Environ. Microbiol. 72:5204-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van der Meulen, R., L. Avonts, and L. De Vuyst. 2004. Short fractions of oligofructose are preferentially metabolized by Bifidobacterium animalis DN-173 010. Appl. Environ. Microbiol. 70:1923-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van der Meulen, R., L. Makras, K. Verbrugghe, T. Adriany, and L. De Vuyst. 2006. In vitro kinetic analysis of oligofructose consumption by Bacteroides and Bifidobacterium spp. indicates different degradation mechanisms. Appl. Environ. Microbiol. 72:1006-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, X., and G. R. Gibson. 1993. Effects of the in vitro fermentation of oligofructose and inulin by bacteria growing in the human large intestine. J. Appl. Bacteriol. 75:373-380. [DOI] [PubMed] [Google Scholar]
- 57.Watzl, B., S. Girrbach, and M. Roller. 2005. Inulin, oligofructose and immunomodulation. Br. J. Nutr. 93:S49-S55. [DOI] [PubMed] [Google Scholar]
- 58.Weaver, C. M. 2005. Inulin, oligofructose and bone health: experimental approaches and mechanisms. Br. J. Nutr. 93:S99-S103.15877902 [Google Scholar]
- 59.Wolin, M. J., Y. C. Zhang, S. Bank, S. Yerry, and T. L. Miller. 1998. NMR detection of (CH3COOH)-C-13-C-13 from 3-C-13-glucose: a signature for Bifidobacterium fermentation in the intestinal tract. J. Nutr. 128:91-96. [DOI] [PubMed] [Google Scholar]