Abstract
A novel strain of Halomonas (Tc-202), which has the capability of removing Tc(VII) from solid- and aqueous-phase material aerobically, was isolated from the marine environment. Tc-202 removed 55% of the total 99Tc in solutions at 15°C by reducing Tc(VII) to Tc(V), but other Halomonas strains did not.
Technetium-99 (99Tc) is a β-emitting radionuclide (half-life, 2.1 × 105 years). It has been released into the environment from past nuclear weapons tests and is also contained in effluents from nuclear reprocessing plants (1, 3, 15). The pertechnetate anion [Tc(VII)O4−], which is its most stable form, is highly soluble and mobile in the environment (14, 15). This anion is incorporated into plants by sulfate transport mechanisms and is accumulated in animals and plants through the food chain (2, 3, 12). Due to these characteristics of Tc, a technique for the removal of Tc from the environment is clearly required. Reduced Tc species, e.g., Tc(IV) and Tc(V) oxides, show a low aqueous solubility (5). Hence, many recent studies have reported the reduction of technetium by microorganisms (4, 6-10, 13, 16). However, all of these bacteria can reduce Tc only under anaerobic conditions. The objectives of this study were to isolate aerobic bacteria accumulating Tc aerobically and to characterize their removal of Tc from solution.
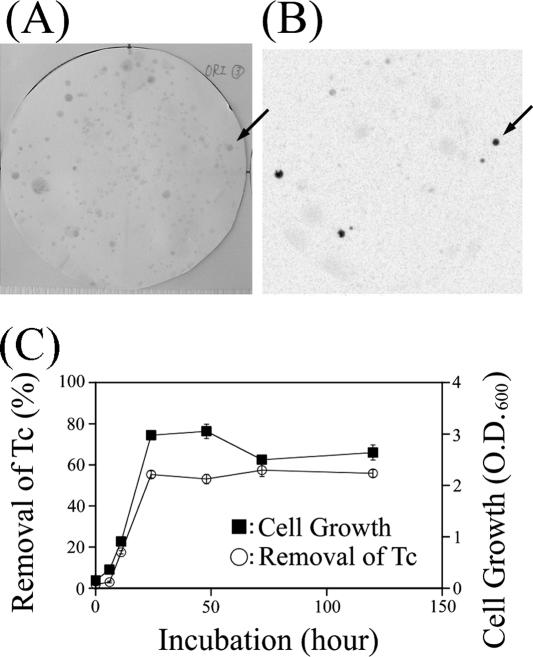
We attempted to isolate Tc-accumulating bacteria from marine samples (seawater, sediments, seaweeds, plankton, annelids, crustaceans, and fish) collected by the research vessel Soyo-maru of the National Research Institute of Fisheries Science. One hundred microliters of sample suspended in sterilized seawater was spread on modified PPES-II medium plates supplemented with 4.8 pmol of 99Tc as NH499TcO4 (Eckert & Ziegler, California) (11). Figure 1 shows an example of sterilized filter paper replicated from the 99Tc-supplemented PPES plates and the image of a storage phosphor screen (SPS) (Amersham Biosciences) exposed by the filter paper. The SPS was scanned with Storm860 (Amersham Biosciences) and analyzed by using ImageJ 1.33u software (NIH). Colonies accumulating 99Tc were detected as black spots on the image of SPS (Fig. 1B). About 5% of all colonies which grew on 99Tc spread plates had the ability to accumulate Tc. Five strains out of 231 colonies were able to remove Tc from the liquid culture medium. One typical strain (Tc-202), which displayed satisfactory growth and removal, was selected and used for the following studies. Tc-202 was isolated from the surface of drifting brown seaweed. Though the biochemical features of Tc-202 differed from those of the other Halomonas strains (see Table S1 in the supplemental material), the molecular phylogenetic analysis using the 16S rRNA gene sequences assigned Tc-202 to the genus Halomonas (see Fig. S1 in the supplemental material). Figure 1C shows the time course of cell growth for Tc-202 and the amount of Tc removed from solution. The optical density at 600 nm of the culture of Tc-202 increased from 0.1 (at the initial time) to 3.0 within 24 h (Fig. 1C, right axis). The amount of Tc precipitated with Tc-202 cells by centrifugation increased with cell growth and reached 55% of the total Tc added to the culture medium (Fig. 1C, left axis, at 72 h of incubation) in stationary phase. Although the status of Tc-202 shifted to extinction phase after 72 h, the amount of Tc removed from the medium hardly changed.
FIG. 1.
Screening of Tc-accumulating microorganisms. (A) Filter paper with colonies transferred from a plate supplemented with 4.8 pmol of 99Tc at 15°C for 48 h. (B) Autoradiogram of the filter paper exposed to SPS for 48 h in a lead shield. Arrows indicate the colony of Tc-202. (C) Time course of growth and Tc removal by Tc-202. A cell suspension (optical density at 600 nm [O.D.600] = 0.1) was prepared with the medium containing 0.16 μM of NH499TcO4 and incubated at 15°C with shaking at 120 rpm. At each period, cell density was measured and 99Tc radioactivity of the supernatant after centrifugation at 12,000 × g for 5 min was measured with a liquid scintillation counter. Values are means ± standard deviations for four independent experiments.
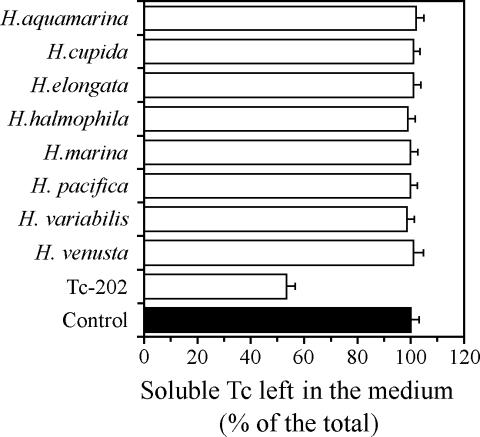
In order to investigate whether the capability for removing Tc from the solution is restricted to Tc-202, eight type strains of different Halomonas species were used for comparison. Though the previous report indicated that another Halomonas strain was able to remove 40% of the total amount of Tc under anaerobic conditions (13), all of the eight type strains were grown satisfactorily but did not show the capability of removing Tc from the solution under aerobic conditions (Fig. 2). The Tc precipitated with Tc-202 cells was not released by washing with saline (data not shown). Tc(VII) would not have been adsorbed on the cell surface without its reduction, because it is hardly attached to particles by itself (1). Previous studies have reported that reduced Tc is localized mainly at peripheries of the cells (7-10, 16). Combination analysis by transmission electron microscopy and X-ray absorption spectroscopy would be necessary to determine the exact location and amount of Tc in Tc-202 cells.
FIG. 2.
Removal of Tc from solution by Tc-202 and other strains of Halomonas. Cell density was adjusted at 0.1 at the start of the experiment with modified PPES medium containing 0.16 μM of 99Tc. After incubation at 15°C for 24 h, cells and insoluble Tc were removed from solution by centrifugation prior to measurement of the percentage of Tc remaining in the supernatant with a liquid scintillation counter. The control was incubation without bacteria. Values are means ± standard deviations for four independent experiments.
Tc-202 can perform the removal of Tc from culture medium prepared at pHs 4.5 to 10.5, while it cannot do so at less than pH 4.0 (see Fig. S2A in the supplemental material). The amount of Tc precipitated with Tc-202 cells did not depend on the pH value in the medium. Though alkali metal ions (Li+, Na+, K+, Rb+, Cs+) and Fe3+ and Hg2+ ions (final concentration, <5 mM) had no influence on the removal of Tc by Tc-202, the presence of Cr2+, Mn2+, Co2+, Ni2+, Cu2+, Zn2+, Sr2+, and Cd2+ facilitated the removal of Tc by 20 to 25% (see Fig. S2B in the supplemental material).
Paper chromatography analysis indicated the Tc(V) band (Rf value = 0.0) was detected in the precipitate fraction of Tc-202 (Fig. 3, lane 1). However, no band representing other reduced Tc species, such as Tc(IV) (Rf = 0.9), was detected. No band was detected in the fractions of the control without bacteria or in those of the type strains of Halomonas (data not shown).
FIG. 3.
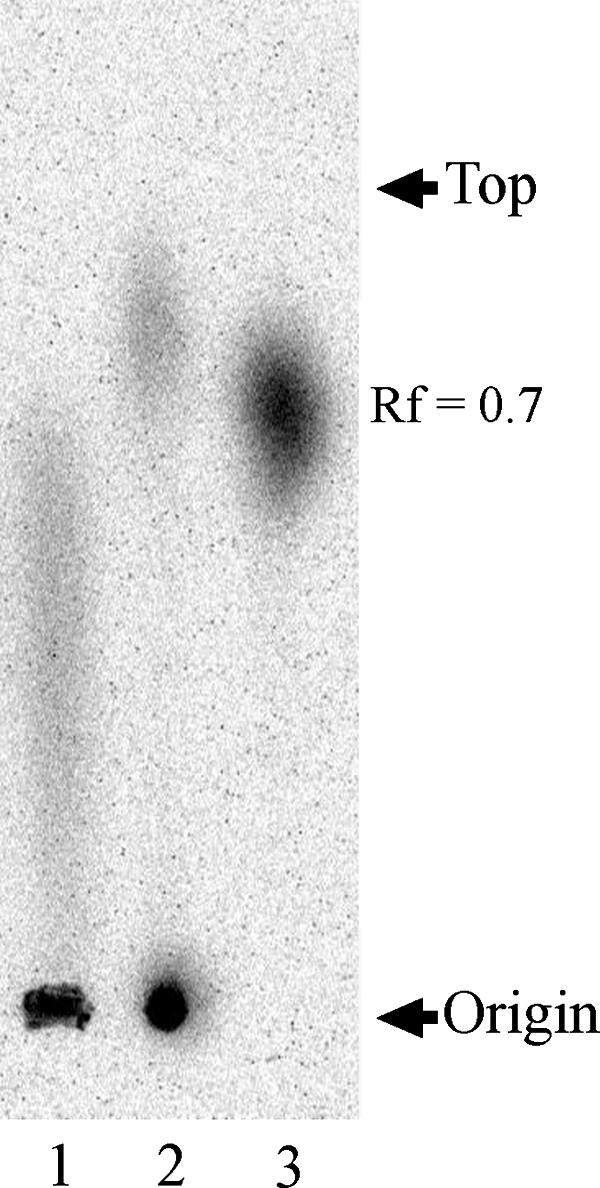
Chromatogram of Tc removed by Tc-202 cultured in medium containing 1.6 μM of 99Tc. After incubation at 15°C for 24 h, cells and insoluble Tc were collected from solution by centrifugation at 12,000 × g for 5 min. The cell pellet was spotted on 3MM filter paper and developed using 1 N HCl. The filter paper was exposed to SPS for 48 h. Lane 1, Tc-202 incubated with 99Tc(VII); lane 2, Tc(V) standard; lane 3, Tc(VII) standard. Tc(V) was prepared by reduction of Tc(VII). Tc(V) and Tc(VII) represent Rf values of 0.0 and 0.7, respectively.
Therefore, Tc-202 was thought to reduce Tc(VII) to Tc(V) in the removal process. However, we cannot exclude the possibility that this solvent system failed to separate Tc from the cell.
Boiled cells of Tc-202 failed to remove Tc from the culture medium (data not shown). Therefore, it was predicted that some proteins are involved in the process of Tc removal by Tc-202. Previous studies have revealed that reduction of Tc by Escherichia coli and Desulfovibrio fructosovorans is mediated by hydrogenase (4, 8). Tc reduction by these bacteria was completely inhibited by 0.5 mM Cu(II) ion. In contrast, Tc removal by Tc-202 was stimulated by the presence of the Cu(II) ion (see Fig. S2B in the supplemental material). Thus, the removal process of Tc-202 is considered to be carried out by a different kind of hydrogenase or to progress through a different mechanism from that of E. coli and D. fructosovorans.
In conclusion, Tc-202, identified as a Halomonas sp., has the ability to remove Tc from solutions over a broad pH range under aerobic conditions. The removal ability of Tc-202 is less than those of microorganisms previously described (4, 6-10, 13, 16). However, considering the various kinds of waste and environmental conditions, aerobic and broad-pH-range conditions for removal by the Tc-202 strain are considered to be beneficial for practical use. The Tc-202 strain is anticipated to be able to play an important role in development of new methods for removal of Tc from contaminated areas and wastes.
Supplementary Material
Footnotes
Published ahead of print on 20 October 2006.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Beasley, T. M., and H. V. Lorz. 1986. A review of the biological and geochemical behavior of technetium in the marine environment. J. Environ. Radioact. 3:1-22. [Google Scholar]
- 2.Cataldo, D. A., T. R. Garland, R. E. Wildung, and R. J. Fellows. 1989. Comparative metabolic behaviour and interrelationship of Tc and S in soybean plants. Health Phys. 57:281-288. [DOI] [PubMed] [Google Scholar]
- 3.Copplestone, D., D. Jackson, R. G. Hartnoll, M. S. Johnson, P. McDonald, and N. Wood. 2004. Seasonal variations in activity concentrations of 99Tc and 137Cs in the edible meat fraction of crabs and lobsters from the central Irish Sea. J. Environ. Radioact. 73:29-48. [DOI] [PubMed] [Google Scholar]
- 4.De Luca, G., P. de Philip, Z. Dermoun, M. Rousset, and A. Verméglio. 2001. Reduction of technetium(VII) by Desulfovibrio fructosovorans is mediated by the nickel-iron hydrogenase. Appl. Environ. Microbiol. 67:4583-4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotegov, K. V., O. N. Pavlov, and V. P. Shvendov. 1968. Technetium, p. 1-90. In H. J. Emelius and A. G. Sharpe (ed.), Advances in inorganic chemistry and radiochemistry. Academic Press, New York, N.Y.
- 6.Lloyd, J. R., and L. E. Macaskie. 1996. A novel phosphorimager-based technique for monitoring the microbial reduction of technetium. Appl. Environ. Microbiol. 62:578-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd, J. R., H.-F. Nolting, V. A. Sole, K. Bosecker, and L. E. Macaskie. 1998. Technetium reduction and precipitation by sulfate-reducing bacteria. Geomicrobiol. J. 15:45-58. [Google Scholar]
- 8.Lloyd, J. R., J. A. Cole, and L. E. Macaskie. 1997. Reduction and removal of heptavalent technetium from solution by Escherichia coli. J. Bacteriol. 179:2014-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd, J. R., J. Ridley, T. Khizniak, N. N. Lyalikova, and L. E. Macaskie. 1999. Reduction of technetium by Desulfovibrio desulfuricans: biocatalyst characterization and use in a flowthrough bioreactor. Appl. Environ. Microbiol. 65:2691-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lloyd, J. R., V. A. Sole, C. V. G. Van Praagh, and D. R. Lovely. 2000. Direct and Fe(II)-mediated reduction of technetium by Fe(III)-reducing bacteria. Appl. Environ. Microbiol. 66:3743-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maeda, T., Y. Matsuo, M. Furushita, and T. Shiba. 2003. Seasonal dynamics in a coastal Vibrio community examined by a rapid clustering method based on 16S rDNA. Fish. Sci. 69:385-394. [Google Scholar]
- 12.Nawakowski, C., M. D. Nicholson, P. J. Kershaw, and K. S. Leonard. 2004. Modelling 99Tc concentrations in Fucus vesiculosus from the north-east Irish Sea. J. Environ. Radioact. 77:159-173. [DOI] [PubMed] [Google Scholar]
- 13.Tatiana, V. K., N. M. Natalia, and S. Monique. 2003. Reduction of pertechnetate by haloalkaliphilic strains of Halomonas. FEMS Microbiol. Ecol. 44:109-115. [DOI] [PubMed] [Google Scholar]
- 14.Trabalka, J. R., and C. T. Garten, Jr. 1983. Behavior of the long-lived synthetic elements and their natural analogues in food chains, p. 39-104. In J. T. Lett, U. K. Ehman, and A. B. Cox (ed.), Advances in radiation biology, vol. 10. Academic Press, Inc., London, United Kingdom. [Google Scholar]
- 15.Wildung, R. E., K. M. McFadden, and T. R. Garland. 1979. Technetium sources and behaviour in the environment. J. Environ. Qual. 8:156-161. [Google Scholar]
- 16.Wildung, R. E., Y. A. Gorby, K. M. Krupka, N. J. Hess, S. W. Li, A. E. Plymale, J. P. McKinley, and J. K. Fredrickson. 2000. Effect of electron donor and solution chemistry on products of dissimilatory reduction of technetium by Shewanella putrefaciens. Appl. Environ. Microbiol. 66:2451-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.