Abstract
The Hg-methylating ability of dissimilatory iron-reducing bacteria in the genera Geobacter, Desulfuromonas, and Shewanella was examined. All of the Geobacter and Desulfuromonas strains tested methylated mercury while reducing Fe(III), nitrate, or fumarate. In contrast, none of the Shewanella strains produced methylmercury at higher levels than abiotic controls under similar culture conditions. Geobacter and Desulfuromonas are closely related to known Hg-methylating sulfate-reducing bacteria within the Deltaproteobacteria.
Methylmercury (MeHg) concentrations in most sediments are controlled by in situ net microbial methylation (1, 13). Environmental mercury methylation is an anaerobic microbial process generally driven by dissimilatory sulfate-reducing bacteria (DSRB) (1). However, recent research suggests that dissimilatory iron-reducing bacteria (DIRB) may play a role in environmental methylation (10, 27). Further, Fleming et al. (10) demonstrated Hg methylation by a Geobacter strain isolated from Clear Lake, CA. Consequently, we designed an experiment to screen a phylogenetically diverse group of DIRB cultures for Hg-methylating capability in order to develop insight into in situ biological methylation controls and to further investigate the phylogenetic distribution of methylating bacteria.
Two studies have demonstrated net MeHg production in sediments where iron was the dominant terminal electron acceptor (10, 27), but another demonstrated inhibition of methylation by iron (23). Iron could potentially influence Hg methylation rates either through changes in DIRB activity or via the impact of iron on Hg complexation and bioavailability. In a study of estuarine wetland sediment slurries from San Francisco Bay, CA, Mehrotra and Sedlak (23) observed decreases in Hg methylation rates with the addition of 30 mM Fe(III), and they suggested that this effect was caused by decreases in dissolved Hg and sulfide due to complexation with iron. However, Warner et al. (27) found measurable methylation in sediments where iron reduction was the dominant terminal electron acceptor, although rates of methylation were lower than those observed in sulfate-reducing or methanogenic sediments. Similarly, in sediments from Clear Lake, CA (10), where microbial Fe(III) reduction was apparent, chemical inhibition of sulfate reduction did not result in complete inhibition of Hg methylation. This decoupling of Hg methylation from sulfate reduction suggests that another process (i.e., iron reduction) may be responsible for some amount of in situ Hg methylation. Mercury methylation by a Geobacter strain isolated from Clear Lake (10) further supports this hypothesis.
Phylogenetic relationships between members of the Geobacteraceae and the Hg-methylating DSRB also suggest a possible role for DIRB in environmental Hg methylation. The Geobacteraceae are found in the Deltaproteobacteria, branching phylogenetically between the orders Desulfovibrionales and Desulfobacterales (15), both of which contain DSRB with methylating capability (2, 3, 9, 16). A wide variety of bacteria and archaea are capable of dissimilatory Fe(III) reduction (19, 20, 22), including Shewanella, which is in the γ subclass of Proteobacteria. This phylogenic distribution of DIRB implicates Geobacteraceae as possible Hg methylators and provides strains that are phylogenetically distant from the DSRB, which may give insight into the phylogenetic distribution of Hg methylation.
To assess the role of DIRB in Hg methylation, pure cultures of Desulfuromonas palmitatis SDBY-1 (8), Geobacter hydrogenophilus (7), Geobacter metallireducens GS-15 (21), Geobacter sulfurreducens (6), Shewanella alga BrY (5), Shewanella oneidensis MR-1 (26), and Shewanella putrefaciens CN-32 (18) were tested for the ability to methylate inorganic Hg while growing on a variety of electron donors and acceptors, including Fe(III), nitrate, and organic substrates (Table 1). Cultures were grown in media modified from the work of Bond and Lovley (4) with electron donors and acceptors as described in Table 1, using previously described trace elements and vitamins (17).
TABLE 1.
Electron donors and acceptors used in Hg methylation tests
| Culture | Electron donor | Electron acceptor(s) |
|---|---|---|
| D. palmitatis | 20 mM acetate | 55 mM Fe(III) |
| G. hydrogenophilus | 20 mM acetate | 55 mM Fe(III) |
| G. sulfurreducens | 20 mM acetate | 55 mM Fe(III), 40 mM fumarate |
| G. metallireducens | 20 mM acetate | 55 mM Fe(III), 30 mM nitrate |
| S. alga | 30 mM lactate | 55 mM Fe(III), 30 mM nitrate |
| S. oneidensis | 30 mM lactate | 55 mM Fe(III), 30 mM nitrate |
| S. putrefaciens | 30 mM lactate | 55 mM Fe(III), 30 mM nitrate |
MeHg production was assayed by measuring the amount of MeHg produced from an inorganic Hg spike during batch culture growth through stationary phase. All Hg methylation assays were conducted in 20-ml Hungate tubes with butyl-rubber stoppers under strictly anaerobic conditions at 30°C and pH 7.0. Assays using G. metallireducens, G. sulfurreducens, S. putrefaciens, and S. oneidensis were conducted using an enriched stable Hg isotope, added as 201HgCl2, at a final concentration of 10 ng/ml. Assays using D. palmitatis, G. hydrogenophilus, and S. alga were conducted with natural isotopic abundance HgCl2 at the same concentration. For each strain and growth condition, triplicate assays and abiotic controls were prepared. Abiotic controls were composed of autoclaved medium spiked with inorganic HgCl2.
Analysis of total MeHg was performed via distillation/ethylation (11)/cold vapor atomic fluorescence (CVAF), using a Tekran 2500 atomic fluorescence detector. For CVAF analysis, the method detection limit was determined by the method blank, which was generally <20 pg/sample. For analysis of a 20-ml culture sample at 10 ng Hg/liter, this yields a blank equivalent to roughly 0.01% methylation. Analysis of Me201Hg was performed by distillation/ethylation/ICP-MS (inductively coupled plasma mass spectometry), with isotope dilution (14), using a Perkin Elmer ELAN 6100 DRCII. Me200Hg (96.4% purity) was used as the isotope dilution standard. The concentration of Me200Hg was determined by reverse isotope dilution analysis against certified standards. Me200Hg was synthesized from 200HgCl2 using an aqueous methylcobalamine method (14). All enriched isotopes were purchased from Oak Ridge National Labs as HgO. Method detection limits using isotope dilution-ICP-MS were generally <1 pg Me201Hg/sample, or <0.001% methylation.
Phylogenetics of DIRB Hg-methylating capability.
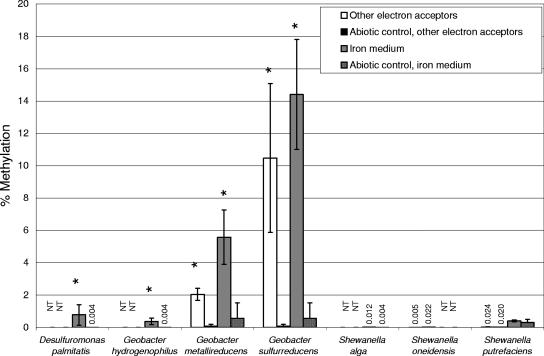
Methylation of inorganic mercury significantly above that in uninoculated controls (t tests, P < 0.05, two-tailed, unequal variances) was observed on Fe-reducing medium in G. metallireducens, G. sulfurreducens, G. hydrogenophilus, and D. palmitatis but not S. alga or S. putrefaciens (Fig. 1). While growing with electron acceptors other than Fe(III), both G. metallireducens and G. sulfurreducens produced MeHg above abiotic control levels, while S. oneidensis and S. putrefaciens did not. The small percentages of methylation observed in abiotic controls are attributed to abiotic formation of MeHg in the experiment or during analysis (12).
FIG. 1.
Observed MeHg production by pure cultures of DIRB, expressed as the percentage of added inorganic Hg methylated. “Other electron acceptors” refers to cultures that were grown with either nitrate or fumarate as an electron acceptor; “iron medium” refers to cultures grown with Fe(III) citrate as an electron acceptor (see Table 1). Significant differences (P < 0.05) in MeHg production in cultures relative to matched abiotic controls are indicated by stars. Error bars represent the standard deviations for three separately prepared tubes for each sample. Culture and growth conditions that were not assayed for MeHg production are labeled “NT” (not tested).
These results, in combination with the observation by Fleming et al. (10) of methylation by a Geobacter isolate, suggest that the ability to methylate Hg may be common among the Geobacteraceae. However, the observed lack of methylating capability among the Shewanella strains tested (all Gammaproteobacteria) shows that the ability to methylate Hg is not ubiquitous among Fe(III)-reducing bacteria. To date, essentially all strains for which Hg methylation has been demonstrated fall in the Deltaproteobacteria (2, 9, 16, 24). These include DSRB from the orders Desulfovibrionales and Desulfobacterales. However, it is important to note that the ability to produce MeHg is not ubiquitous among DSRB in these families. Further studies are needed to ascertain whether the Hg-methylating capability is randomly distributed among Proteobacteria or related to phylogeny. Improved understanding of the phylogenetic distribution of Hg methylation capability may provide insight into the biochemical process of MeHg production within cells.
It is important to note that the Geobacter strains tested produced MeHg during growth with Fe(III) and with other electron acceptors (nitrate and fumarate). This indicates that active Fe(III)-reducing electron-transport chains are not necessary for Hg methylation in these strains. However, this experiment was not designed to quantify the effect of electron acceptors and donors on methylation rates. Further studies would be needed to quantify these effects.
Environmental significance of MeHg production by DIRB.
The observation of Hg methylation by DIRB has implications for the prediction of in situ MeHg production. Due to the importance of DSRB as methylators, current models for methylation are based on relationships between methylation and sulfate reduction (1). However, the finding that DIRB can produce MeHg suggests that Hg methylation may be important in sediments and soils where these organisms are dominant, e.g., iron-rich sediments with low concentrations of sulfate (25). Iron can affect methylation by altering the chemistry of Hg (and hence its bioavailability) or by changing the activity of DIRB versus other groups of organisms, particularly DSRB (10, 23, 25, 27). The influence of iron on both Hg complexation and microbial activity will need to be considered in order to resolve how Hg methylation by DIRB will change the paradigm for in situ MeHg production.
Acknowledgments
This work was supported through National Science Foundation Division of Environmental Biology grant 0451345, Division of Ocean Sciences grants 0351050 and 0550547, and the Smithsonian Institution. E. J. Kerin was supported by a Chesapeake Biological Laboratory Graduate Student Fellowship. J. D. Coates was supported through grant DE-FG02-98ER63592 from the Department of Energy, Natural and Accelerated Bioremediation Program.
We thank Tyler Bell, Georgia Riedel, and Sarah Werner for lab assistance at SERC, Christopher Conaway for editorial assistance, and Mandy Ward and Chad Saltikov for providing Shewanella cultures.
Footnotes
Published ahead of print on 20 October 2006.
REFERENCES
- 1.Benoit, J. M., C. C. Gilmour, A. Heyes, R. P. Mason, and C. L. Miller. 2003. Geochemical and biological controls over methylmercury production and degradation in aquatic ecosystems. Am. Chem. Soc. Symp. Ser. 835:262-297. [Google Scholar]
- 2.Benoit, J. M., C. C. Gilmour, and R. P. Mason. 2001. Aspects of bioavailability of mercury for methylation in pure cultures of Desulfobulbus propionicus (1pr3). Appl. Environ. Microbiol. 67:51-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benoit, J. M., C. C. Gilmour, and R. P. Mason. 2001. The influence of sulfide on solid phase mercury bioavailability for methylation by pure cultures of Desulfobulbus propionicus (1pr3). Environ. Sci. Technol. 35:127-132. [DOI] [PubMed] [Google Scholar]
- 4.Bond, D. R., and D. R. Lovley. 2003. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 69:1548-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caccavo, F., R. P. Blakemore, and D. R. Lovley. 1992. A hydrogen-oxidizing, Fe(III)-reducing microorganism from the Great Bay Estuary, New Hampshire. Appl. Environ. Microbiol. 58:3211-3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caccavo, F., D. J. Lonergan, D. R. Lovley, M. Davis, J. F. Stolz, and M. J. McInerney. 1994. Geobacter sulfurreducens sp. nov., a hydrogen-oxidizing and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl. Environ. Microbiol. 60:3752-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coates, J. D., V. K. Bhupathiraju, L. A. Achenbach, M. J. McInerney, and D. R. Lovley. 2001. Geobacter hydrogenophilus, Geobacter chapellei, and Geobacter grbiciae, three new, strictly anaerobic, dissimilatory Fe(III)-reducers. Int. J. Syst. Evol. Microbiol. 51:581-588. [DOI] [PubMed] [Google Scholar]
- 8.Coates, J. D., D. J. Lonergan, E. J. P. Philips, H. Jenter, and D. R. Lovley. 1995. Desulfuromonas palmitatis sp nov., a marine dissimilatory Fe(III) reducer that can oxidize long-chain fatty acids. Arch. Microbiol. 164:406-413. [PubMed] [Google Scholar]
- 9.Compeau, G. C., and R. Bartha. 1985. Sulfate-reducing bacteria: principal methylators of mercury in anoxic estuarine sediment. Appl. Environ. Microbiol. 50:498-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming, E. J., E. E. Mack, P. G. Green, and D. C. Nelson. 2006. Mercury methylation from unexpected sources: molybdate-inhibited freshwater sediments and an iron-reducing bacterium. Appl. Environ. Microbiol. 72:457-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilmour, C. C., G. S. Riedel, M. C. Ederington, J. T. Bell, J. M. Benoit, G. A. Gill, and M. C. Stordal. 1998. Methylmercury concentrations and production rates across a trophic gradient in the northern Everglades. Biogeochemistry 40:327-345. [Google Scholar]
- 12.Hammerschmidt, C. R., and W. F. Fitzgerald. 2001. Formation of artifact methylmercury during extraction from a sediment reference material. Anal. Chem. 73:5930-5936. [DOI] [PubMed] [Google Scholar]
- 13.Hammerschmidt, C. R., and W. F. Fitzgerald. 2004. Geochemical controls on the production and distribution of methylmercury in near-shore marine sediments. Environ. Sci. Technol. 38:1487-1495. [DOI] [PubMed] [Google Scholar]
- 14.Hintelmann, H., and N. Ogrinc. 2003. Determination of stable mercury isotopes by ICP/MS and their application in environmental studies. Am. Chem. Soc. Symp. Ser. 835:321-338. [Google Scholar]
- 15.Holmes, D. E., K. P. Nevin, and D. R. Lovley. 2004. Comparison of 16S rRNA, nifD, recA, gyrB, rpoB and fusA genes within the family Geobacteraceae fam. nov. Int. J. Syst. Evol. Microbiol. 54:1591-1599. [DOI] [PubMed] [Google Scholar]
- 16.King, J. K., J. E. Kostka, M. E. Frischer, and F. M. Saunders. 2000. Sulfate-reducing bacteria methylate mercury at variable rates in pure culture and in marine sediments. Appl. Environ. Microbiol. 66:2430-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laanbroek, H. J., and N. Pfennig. 1981. Oxidation of short-chain fatty acids by sulfate-reducing bacteria in freshwater and marine sediments. Arch. Microbiol. 128:330-335. [DOI] [PubMed] [Google Scholar]
- 18.Liu, C. G., J. M. Zachara, Y. A. Gorby, J. E. Szecsody, and C. F. Brown. 2001. Microbial reduction of Fe(III) and sorption/precipitation of Fe(II) on Shewanella putrefaciens strain CN32. Environ. Sci. Technol. 35:1385-1393. [DOI] [PubMed] [Google Scholar]
- 19.Lonergan, D. J., H. L. Jenter, J. D. Coates, E. J. P. Phillips, T. M. Schmidt, and D. R. Lovley. 1996. Phylogenetic analysis of dissimilatory Fe(III)-reducing bacteria. J. Bacteriol. 178:2402-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovley D. R., D. E. Holmes, and K. P. Nevin. 2004. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 49:219-286. [DOI] [PubMed] [Google Scholar]
- 21.Lovley, D. R., S. J. Giovannoni, D. C. White, J. E. Champine, E. J. P. Phillips, Y. A. Gorby, and S. Goodwin. 1993. Geobacter metallireducens gen. nov., sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch. Microbiol. 159:336-344. [DOI] [PubMed] [Google Scholar]
- 22.Lovley, D. R., E. E. Roden, E. J. P. Phillips, and J. C. Woodward. 1993. Enzymatic iron and uranium reduction by sulfate-reducing bacteria. Mar. Geol. 113:41-53. [Google Scholar]
- 23.Mehrotra, A. S., and D. L. Sedlak. 2005. Decrease in net mercury methylation rates following iron amendment to anoxic wetland sediment slurries. Environ. Sci. Technol. 39:2564-2570. [DOI] [PubMed] [Google Scholar]
- 24.Pak, K. R., and R. Bartha. 1998. Mercury methylation and demethylation in anoxic lake sediments and by strictly anaerobic bacteria. Appl. Environ. Microbiol. 64:1013-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roden, E. E., and R. G. Wetzel. 1996. Organic carbon oxidation and suppression of methane production by microbial Fe(III) oxide reduction in vegetated and unvegetated freshwater wetland sediments. Limnol. Oceanogr. 41:1733-1748. [Google Scholar]
- 26.Venkateswaran, K., D. P. Moser, M. E. Dollhopf, D. P. Lies, D. A. Saffarini, B. J. MacGregor, D. B. Ringelberg, D. C. White, M. Nishijima, H. Sano, J. Burghardt, E. Stackebrandt, and K. H. Nealson. 1999. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int. J. Syst. Bacteriol. 49:705-724. [DOI] [PubMed] [Google Scholar]
- 27.Warner, K. A., E. E. Roden, and J. C. Bonzongo. 2003. Microbial mercury transformation in anoxic freshwater sediments under iron-reducing and other electron-accepting conditions. Environ. Sci. Technol. 37:2159-2165. [DOI] [PubMed] [Google Scholar]