Abstract
The nucleotide sequence of a 17.2-kb chromosomal DNA fragment containing the odc gene encoding ornithine decarboxylase has been determined in the putrescine producer Oenococcus oeni RM83. This DNA fragment contains 13 open reading frames, including genes coding for five transposases and two phage proteins. This description might represent the first evidence of a horizontal gene transfer event as the origin of a biogenic amine biosynthetic locus.
Wines are highly selective media and support the growth of only a few species of lactic acid bacteria, mainly, Oenococcus oeni and several lactobacilli. O. oeni is often responsible for wine malolactic fermentation and is frequently utilized as a starter culture to promote malolactic conversion.
In acidic media like wine, decarboxylation of amino acids to their corresponding amines is thought to provide energy through electrogenic transport as well as assist in maintaining an optimal internal pH (11). Some of these amines are considered “biogenic” and may cause intoxication when consumed. The biogenic amine putrescine, which can potentiate the action of histamine, is the most prevalent amine in wine and is found in almost all analyzed wines (12, 15).
Biogenic amines are formed primarily by decarboxylation of the corresponding amino acids by microorganisms through substrate-specific decarboxylases. The capability of biogenic amine production appears to be strain dependent rather than species specific. Previously, we reported the identification of the odc gene in the putrescine producer O. oeni RM83 (formerly O. oeni BIFI-83) for the first time (14). The odc gene encodes a deduced 745-amino-acid putative ornithine decarboxylase (ODC) (EC 4.1.1.17) which catalyzes the conversion of ornithine to putrescine. The odc gene is seldom present in the O. oeni genome, as it has not been detected in a screen of 42 O. oeni strains tested to date (14). Moreover, in silico analysis of the draft O. oeni PSU-1 genome did not reveal the presence of any odc homologs (16).
Recently, Lucas et al. described that the potential for producing histamine in Lactobacillus hilgardii 0006 is encoded on an unstable 80-kb plasmid (13); the authors further suggested that it is very likely that the histamine producer Tetragenococcus muriaticus and O. oeni 9204 harbor the same plasmid (13). However, the localization of the odc gene in O. oeni RM83 remains unknown.
This study was undertaken to gain deeper insight into the origin of putrescine production in O. oeni RM83. Additionally, O. oeni RM83 ODC was expressed in Escherichia coli and biochemically characterized.
Genetic location of the odc locus in O. oeni RM83.
The putrescine producer O. oeni RM83, formerly O. oeni BIFI-83, was previously isolated from lees of a Spanish red wine (14). Putrescine production by O. oeni RM83 was maintained without ornithine pressure, suggesting that the odc locus was stable. To determine if O. oeni RM83 harbored any plasmids, total DNA was extracted and analyzed by standard agarose gel electrophoresis (19). This assay revealed the absence of small plasmids in O. oeni RM83 (data not shown). Subsequently, native total DNA was embedded in agarose plugs and analyzed by pulsed-field gel electrophoresis as described previously (1). Again, plasmids were not detected. Moreover, Southern hybridization with a 1.4-kb DNA probe targeted to an internal odc fragment (14) yielded a positive signal only in the chromosomal DNA (data not shown). Therefore, it was concluded that odc in O. oeni RM83 is located on the chromosome.
Characterization of the odc region in O. oeni RM83.
Since (i) in silico analysis of the O. oeni PSU-1 draft genome did not reveal the presence of an odc gene (16), (ii) the presence of the odc gene appears to be infrequent in the O. oeni genome (14), and (iii) the odc gene is chromosomally located in O. oeni RM83, we decided to identify the chromosomal DNA region involved in putrescine production in O. oeni RM83. The 17.2-kb sequence flanking the previously described 2.3-kb odc region was determined earlier (14). This sequence was ascertained by creating a phage library of O. oeni RM83 genomic DNA and by several inverse PCR experiments.
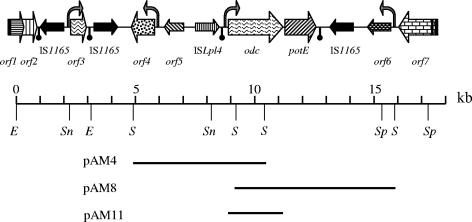
To construct the O. oeni RM83 DNA library, chromosomal DNA was partially digested with Sau3AI restriction enzyme and ligated to the ZAP Express vector (Stratagene, La Jolla, CA) digested with BamHI. The screening of the library using the 1.4-kb internal odc DNA fragment as a probe yielded five positive clones. Since the inserts of three of them were included in pAM4 and pAM8, only these plasmids were sequenced (Fig. 1). A 10,891-bp O. oeni RM83 DNA fragment was sequenced from the pAM4 and pAM8 plasmids. Two successive reverse PCR experiments, utilizing SnaBI and EcoRV, allowed us to sequence the 5′ end of the fragment (Fig. 1). Similarly, a reverse PCR experiment using SpeI allowed for the sequencing of the total 17,270-bp EcoRV-SpeI chromosomal DNA fragment surrounding the O. oeni RM83 odc gene (Fig. 1).
FIG. 1.
Genetic organization of the O. oeni RM83 17.2-kb DNA region containing the odc gene. Thick and thin arrows represent complete and interrupted ORFs, respectively. The locations of putative promoters (vertical bent arrow) and predicted transcriptional terminator regions (ball and stick) are indicated. Some of the plasmids used in this study are indicated, as are relevant restriction sites: E, EcoRV; S, Sau3AI; Sp, SpeI; Sn, SnaBI. Only some of the corresponding restriction sites present in this fragment are represented.
Sequence analysis of this DNA fragment showed the presence of 11 complete (albeit some interrupted) and 2 partial open reading frames (ORFs) in the odc region (Fig. 1; Table 1). Two interesting features were observed: the presence of two putative phage proteins and the presence of five transposase-coding genes. The first incomplete ORF (orf1) is predicted to code for a protein showing the highest similarity (>30% identity) to Streptococcus thermophilus bacteriophage proteins. Notably, it has been reported previously that the genomes of currently characterized S. thermophilus phages exhibit homology to each other in a modular fashion (2). Furthermore, orf2 is predicted to encode a protein similar to a DNA replication protein from an Enterococcus faecalis prophage. The O. oeni PSU-1 draft genome does not contain any intact temperate bacteriophage or larger tracts of obvious bacteriophage origin, although several prophage integration sites have been found previously (16).
TABLE 1.
odc region-encoded proteins: properties and similarities to proteins in the databases
| Gene or IS | Location in nucleotide sequence | G+C (%) | Predicted protein (aa/kDa)a | Similar polypeptide(s) (aa)a | Proposed function | Accession no. | % Identitya | Organism |
|---|---|---|---|---|---|---|---|---|
| orf1 | 0-237 | 36.3 | Str0776 (286) | Replication protein, phage or plasmid associated | Q5M098 | 32.5 (in 81-aa overlap) | S. thermophilus CNRZ 1066 | |
| ORF12 (269) | Conserved protein in S. thermophilus phages | O34043 | 30.1 (in 63-aa overlap) | S. thermophilus temperate bacteriophage ϕ O1205 | ||||
| ORF35 (271) | Hypothetical protein | Q9XJC8 | 30.5 (in 59-aa overlap) | S. thermophilus lytic bacteriophage DT1 | ||||
| orf2 | 230-784 | 32.8 | 184/20.5 | EF1279 | DNA replication protein | Q835U3 | 27.6 | Enterococcus faecalis V583 putative prophage 02 |
| IS1165 | 1175-2236/c | 48.1 | 353/39.5 | IS1165 transposase | Transposase | Q48788 | 98.8 (in 335-aa overlap) | Leuconostoc mesenteroides subsp. cremoris |
| orf3 | 2502-3095/c | 35.2 | 197/21.6 | EfaeDRAFT_2583 | Hypothetical protein | Q3Y3B9 | 55.7 | Enterococcus faecium DO |
| Spr0580 | Hypothetical protein | Q8DQN6 | 55.6 | Streptococcus pneumoniae R6 | ||||
| IS1165 | 3258-4319 | 48.1 | 353/39.5 | IS1165 transposase | Transposase | Q48788 | 98.8 (in 335-aa overlap) | L. mesenteroides subsp. cremoris |
| orf4 | 4767-5837/c | 35.2 | 356/39.5 | LJ1779 | Major facilitator superfamily permease | Q74HG7 | 50.4 | Lactobacillus johnsonii NCC533 |
| orf5 | 6541-6927/c | 46.0 | 128/13.6 | lp_3570 | Transposase | Q88S70 | 58.5 | Lactobacillus plantarum WCFS1 |
| ISLpl4 | 7828-7953 | 43.2 | 41/4.7 | ISLpl4 transposase | Transposase | CAI93853.1 | 97 (in 34-aa overlap) | L. plantarum CECT4645 |
| odc | 8866-11103 | 36.3 | 745/81 | OdcI | Ornithine decarboxylase | P43099 | 67.1 | Lactobacillus sp. strain 30a |
| potE | 11184-12509 | 39.2 | 441/47.6 | PotE | Putrescine-ornithine antiporter | P44768 | 67.3 | Haemophilus influenzae Rd |
| IS1165 | 13235-14295/c | 48.1 | 353/39.5 | IS1165 transposase | Transposase | Q48788 | 98.8 (in 335-aa overlap) | L. mesenteroides subsp. cremoris |
| orf6 | 15240-15800/c | 30.4 | 186/22.0 | BCE3325150 | Probable membrane protein | Q630E9 | 36.8 (in 148-aa overlap) | Bacillus cereus ZK |
| orf7 | 15966+/c | 40.2 | Ooen02001060 | Hypothetical protein | ZP_00319317 | 78.2 (in 307-aa overlap) | O. oeni PSU-1 |
aa, amino acids.
Contiguous to and divergently transcribed from orf2, we found a variant copy of the insertion sequence (IS) IS1165 (99% nucleotide identity) (10). The existence of two additional copies of IS1165, at positions 3121 to 4675 and 12879 to 14433/c (“/c” indicates that the sequence corresponds to the strand complementary to that included in the EMBL/GenBank database), was observed. All three of the IS1165 copies are identical and contain the canonical terminal inverted repeats. Although IS1165 was originally described for Leuconostoc mesenteroides subsp. cremoris, copies of this IS have been described for other lactic acid bacteria, such as Leuconostoc lactis, O. oeni, Pediococcus sp., Lactobacillus helveticus, and Lactobacillus casei (10).
Upstream of orf4, there is an 876-bp region that might correspond to an insertion sequence-like element on the basis of sequence similarity (58.5% nucleotide identity to a Lactobacillus plantarum transposase). Almost-perfect 17-bp inverted repeats were found at positions 6124 to 6140 and 6984 to 7000. Another IS, a copy of ISLpl4, is found 722 nucleotides further upstream (4).
The odc gene is located downstream of ISLpl4. The ODC protein is predicted to possess 745 amino acid residues, including conserved residues involved in enzymatic activity as well as the consensus sequence containing the pyridoxal-5-phosphate binding domain (14). The highest sequence identity (67%) was found between O. oeni and Lactobacillus sp. strain 30a (Table 1). Surprisingly, O. oeni RM83 ODC showed lower identity with similar proteins found in other members of the lactic acid bacteria (49% with Lactobacillus johnsonii and Lactobacillus acidophilus) than with enzymes from unrelated microorganisms, such as Haemophilus influenzae (64%), Pasteurella multocida (64%), and E. coli (58%) (14).
The next identified ORF is a putative potE gene. It encodes a 441-amino-acid, 47.6-kDa protein showing 67% identity to the putrescine-ornithine antiporter (PotE) from Haemophilus influenzae and 66% to PotE proteins from some Enterobacteriaceae. Unexpectedly, O. oeni PotE only shows a 14% identity to amino acid transporters from lactic acid bacteria (data not shown). PotE can catalyze both the uptake and the excretion of putrescine (8).
Functional expression of the odc gene from O. oeni RM83.
To confirm that the odc gene from O. oeni RM83 encodes a functional ODC, we expressed this gene in E. coli HT414 (CGSC strain 6856), as this strain is defective for ODC activity (20). First, the gene was PCR amplified from O. oeni RM83 DNA by using Pfu DNA polymerase (Stratagene, La Jolla, CA) and oligonucleotides PIN-ODC-up (5′-GGAACTCTAGAGGGTATTAATAATGGATAGCGAAATAAATGATGA TTC) and PIN-ODC-down (5′-CGCATTGCGTTCACGTCGTTGCTCAATTATCATCTTTTTTCTTCATCTTTTGAC). The purified PCR fragment was inserted into pIN-III(lppp-5)A3 (9) by using a strategy described by Geiser et al. (7).
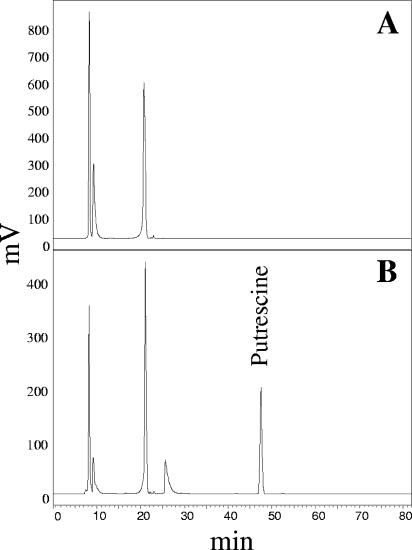
Cell extracts for the ODC enzymatic assay were obtained from E. coli HT414 cells harboring the control plasmid pIN-III(lppp-5)A3 or the recombinant plasmid pAM11 as previously described (18). The ODC assay was performed in 50 mM sodium phosphate buffer (pH 6.5) in the presence of 3.6 mM ornithine and 0.4 mM pyridoxal-5-phosphate. The reaction mixture was incubated at 37°C for 1 h. Subsequently, the putrescine formed in the reaction mixture was derivatized and detected by thin-layer chromatography (6) and by reverse-phase high-pressure liquid chromatography (14). Extracts from the strains harboring pAM11 were able to decarboxylate the supplied ornithine to putrescine, whereas extracts prepared from control cells containing the vector plasmid alone did not (Fig. 2). Therefore, we have provided experimental evidence that the odc gene encodes a functional ODC.
FIG. 2.
Putrescine production by soluble cell extracts of IPTG (isopropyl-β-d-thiogalactopyranoside)-induced cultures of E. coli HT414 harboring pAM11. The putrescine produced during the enzymatic reaction was subjected to an automatic precolumn derivatization with o-ophthaldialdehyde prior to injection. Putrescine was determined by reverse-phase high-pressure liquid chromatography as previously described (14). (A) Results from reaction with E. coli HT414 bearing the control pIN-III(lppp-5)A3 plasmid. (B) Results from reaction with E. coli HT414 bearing the recombinant pAM11 plasmid.
Recombination as origin of the odc region in O. oeni RM83.
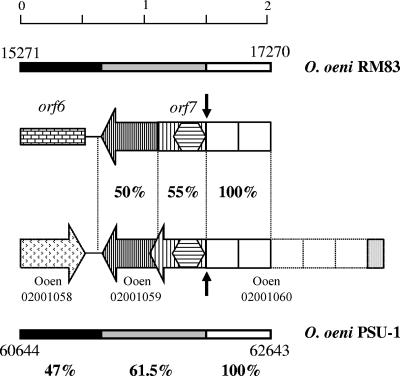
A careful comparison of nucleotide positions 15271 to 17270 (2 kb) of the sequenced O. oeni RM83 DNA fragment with positions 60644 to 62643 of the draft genome sequence of O. oeni PSU-1, GenBank accession number NZ_AABJ03000005, reveals three distinct regions based on their nucleotide sequence identity (Fig. 3). The leftmost 649 nucleotides of both sequences showed 47% nucleotide identity, the next 827 nucleotides were 61.5% identical between RM83 and PSU-1, and the rightmost 524 nucleotide residues exhibited perfect identity between both strains.
FIG. 3.
Schematic overview of the sequence conservation between O. oeni PSU-1 and O. oeni RM83 chromosomal regions containing the proposed recombination site. Genes are represented by arrows. The rectangle corresponds to the interrupted gene. The genes present in these regions are indicated: orf6 and orf7 in O. oeni RM83 and Ooen02001058 (encoding a putative transcriptional terminator), Ooen02001059 (encoding the carbamoylphosphate synthase large subunit), and Ooen02001060 (coding for a hypothetical protein, ZP_00319317) in O. oeni PSU-1. The complete ORF coding for the hypothetical protein in O. oeni PSU-1 is also represented. Open squares and hexagons represent GW domains and MucBP domains, respectively. ORF regions with identical shading correspond to regions having the same degree of sequence identity. The degrees of amino acid identity between the protein fragments encoded by these ORFs are also shown. The colors of the upper and lower bars indicate the degrees of nucleotide identity between the 2-kb DNA regions: black, 47% identity; gray, 61.5%; and white, 100%. Two black arrows indicate the recombination site. The nucleotide positions corresponding to both sequences are also indicated. The O. oeni PSU-1 nucleotide sequence appears in the GenBank database under accession number NZ_AABJ03000005.
The proteins encoded by these 2-kb sequences are remarkable as well. From nucleotide position 61293, O. oeni PSU-1 encodes a 156-residue protein, Ooen02001059, annotated as a carbamoyl phosphate synthase, and Ooen02001060, a 631-amino-acid hypothetical protein (Fig. 3). This hypothetical protein contains an N-terminal putative signal peptide extending to amino acid 33, followed by five GW domains and a 130-amino-acid C-terminal end containing a MucBP (mucin-binding protein) domain (Fig. 3). GW and MucBP domains are found in bacterial cell wall bound proteins. Some putative surface proteins bind the bacterial surface by way of noncovalent interactions mediated by their C-terminal GW domains. These ∼80-amino-acid-long domains contain the dipeptide Gly-Trp (GW modules). The MucBP domains consist of sequences of around 50 residues in length found in bacterial peptidoglycan bound proteins.
It is noteworthy that orf7 in O. oeni RM83 appears to be a chimeric protein originating from the fusion of a gene encoding a protein 50% identical to the O. oeni PSU-1 putative carbamoyl phosphate synthase (Ooen02002059) and a gene encoding a protein 55% identical on its MucBP domain to the PSU-1 hypothetical protein (Ooen02002060). Taking into account that GW and MucBP domains are found in a variety of bacterial proteins, it is possible that the unknown donor protein could have domains encoded by DNA regions showing high nucleotide similarity with the corresponding O. oeni regions. This similarity could facilitate the crossover between this unknown donor DNA and O. oeni chromosomal DNA. Upon examination of regions of maximal identity, the crossover point appears to reside at nucleotide position 16747 of the O. oeni RM83 sequence described in this work and position 62120 of O. oeni PSU-1 (GenBank accession number NZ_AABJ03000005). This recombination site is located in the junction of the MucBP domain and the first GW domain. Interestingly, in O. oeni PSU-1 the gene coding for the hypothetical protein Ooen02001060 is found less than 5 kb downstream of the gene recP, coding for a transketolase. Recently, de las Rivas et al. described for the recP locus a possible example of a recombinatorial event from an unknown source (3). The description of two recombinatorial events in the same DNA region indicates a region of great flexibility in the O. oeni chromosome, as described recently for L. plantarum (17).
It is now understood that horizontal gene transfer provides an important mechanism for generating genotypic and phenotypic diversity in bacteria. This phenomenon has been studied extensively in relation to bacterial adaptability or fitness under certain growth conditions. Accordingly, it has been reported widely that adaptability traits can be encoded by mobile genetic elements. Genomic islands (GI) are clusters of chromosomal genes that have been described as horizontally acquired DNA regions (5). They often possess genes (or pseudogenes) coding for mobility-related elements, such as phage genes, insertion sequences, transposases, and origins of replication. A typical GI carries genes encoding traits that may increase bacterial adaptability under certain growth conditions. All of these observations taken together suggest that the 16.7-kb O. oeni RM83-specific DNA may be a fragment of a GI transferred by horizontal gene transfer.
Nucleotide sequence accession number.
The DNA sequence of the O. oeni RM83 odc region has been deposited in the EMBL/GenBank database under accession number AJ746165.
Acknowledgments
This work was supported by grants AGL2005-00470 (CICYT), RM03-002 (INIA), and S-0505/AGR/000153 (CAM).
We thank E. García and D. Llull for their help with the pulsed-field gel electrophoresis experiments. We thank the E. coli Genetic Stock Center (http://cgsc.biology.yale.edu) for generously providing the E. coli HT414 strain. We also thank D. Sela for correcting the English version of the manuscript. The technical assistance of M. V. Santamaría and A. Gómez is greatly appreciated.
Footnotes
Published ahead of print on 20 October 2006.
REFERENCES
- 1.Arrecubieta, C., R. López, and E. García. 1994. Molecular characterization of cap3A, a gene from the operon required for the synthesis of the capsule of Streptococcus pneumoniae type 3: sequencing of mutations responsible for the unencapsulated phenotype and localization of the capsular cluster on the pneumococcal chromosome. J. Bacteriol. 176:6375-6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brüssow, H., A. Probst, M. Frémont, and J. Sidoti. 1994. Distinct Streptococcus thermophilus bacteriophages share an extremely conserved DNA fragment. Virology 200:854-857. [DOI] [PubMed] [Google Scholar]
- 3.de las Rivas, B., A. Marcobal, and R. Muñoz. 2004. Allelic diversity and population structure in Oenococcus oeni as determined from sequence analysis of housekeeping genes. Appl. Environ. Microbiol. 70:7210-7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de las Rivas, B., A. Marcobal, A. Gómez, and R. Muñoz. 2005. Characterization of ISLpl4, a functional insertion sequence in Lactobacillus plantarum. Gene 363:202-210. [DOI] [PubMed] [Google Scholar]
- 5.Dobrindt, U., B. Hochnut, U. Hentschel, and J. Hacker. 2004. Genomic islands in pathogenic and environmental microorganisms. Nat. Rev. Microbiol. 2:414-424. [DOI] [PubMed] [Google Scholar]
- 6.García-Moruno, E., A. V. Carrascosa, and R. Muñoz. 2005. A rapid and inexpensive method for the determination of biogenic amines from bacterial cultures by thin-layer chromatography. J. Food Prot. 68:625-629. [DOI] [PubMed] [Google Scholar]
- 7.Geiser, M., R. Cèbe, D. Drewello, and R. Schmitz. 2001. Integration of PCR fragments at any specific site within cloning vectors without the use of restriction enzymes and DNA ligase. BioTechniques 31:88-92. [DOI] [PubMed] [Google Scholar]
- 8.Igarashi, K., and K. Kashiwagi. 1999. Polyamine transport in bacteria and yeast. Biochem. J. 344:633-642. [PMC free article] [PubMed] [Google Scholar]
- 9.Inouye, S., and M. Inouye. 1985. Up-promoter mutations in the lpp gene of Escherichia coli. Nucleic Acids Res. 13:3101-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansen, E., and A. Kibenich. 1992. Isolation and characterization of IS1165, an insertion sequence of Leuconostoc mesenteroides subsp. cremoris and other lactic acid bacteria. Plasmid 27:200-206. [DOI] [PubMed] [Google Scholar]
- 11.Konings, W. N. 2002. The cell membrane and the struggle for life of lactic acid bacteria. Antonie Leeuwenhoek 82:3-27. [PubMed] [Google Scholar]
- 12.Landete, J. M., S. Ferrer, L. Polo, and I. Pardo. 2005. Biogenic amines in wine from three Spanish regions. J. Agric. Food Chem. 53:1119-1124. [DOI] [PubMed] [Google Scholar]
- 13.Lucas, P. M., W. A. M. Wolken, O. Claisse, J. S. Lolkema, and A. Lonvaud-Funel. 2005. Histamine-producing pathway encoded on an unstable plasmid in Lactobacillus hilgardii 0006. Appl. Environ. Microbiol. 71:1417-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcobal, A., B. de las Rivas, M. V. Moreno-Arribas, and R. Muñoz. 2004. Identification of the ornithine decarboxylase gene in the putrescine-producer Oenococcus oeni BIFI-83. FEMS Microbiol. Lett. 239:213-220. [DOI] [PubMed] [Google Scholar]
- 15.Marcobal, A., P. J. Martín-Alvarez, M. C. Polo, R. Muñoz, and M. V. Moreno-Arribas. 2006. Formation of biogenic amines during red wine manufacture. J. Food Prot. 70:157-164. [DOI] [PubMed] [Google Scholar]
- 16.Mills, D. A., H. Rawsthorne, C. Parker, D. Tamir, and K. Makarova. 2005. Genomic analysis of Oenococcus oeni PSU-1 and its relevance to winemaking. FEMS Microbiol. Rev. 29:465-475. [DOI] [PubMed] [Google Scholar]
- 17.Molenaar, D., F. Bringel, F. H. Schuren, W. M. de Vos, R. J. Siezen, and M. Kleerebezem. 2005. Exploring Lactobacillus plantarum genome diversity by using microarrays. J. Bacteriol. 187:6119-6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muñoz., R., R. López, M. de Frutos, and E. García. 1999. First molecular characterization of a uridine diphosphate galacturonate 4-epimerase: an enzyme required for capsular biosynthesis in Streptococcus pneumoniae type 1. Mol. Microbiol. 31:703-713. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Tabor, H., C. W. Tabor, M. S. Cohn, and E. W. Hafner. 1981. Streptomycin resistance (rpsL) produces an absolute requirement for polyamines for growth of an Escherichia coli strain unable to synthesize putrescine and spermidine [Δ(speA-speB) ΔspeC]. J. Bacteriol. 147:702-704. [DOI] [PMC free article] [PubMed] [Google Scholar]