Abstract
MICs of six broad-spectrum biocides and two specific metabolic inhibitors and fractional inhibitory concentration indexes (FICIs) for controlling a sulfide-producing consortium were determined. Nitrite was synergistic (FICI < 1) with all but one biocide due to its specific inhibition of dissimilatory sulfite reductase. Hence, combining nitrite with biocides allows more efficient and cost-effective control of sulfate-reducing bacteria.
Sulfate-reducing bacteria (SRB) contribute to souring, the production of sulfide in oil and gas fields. Sulfide is corrosive and toxic (14) and causes reservoir plugging (7). Control of biogenic sulfide production decreases operating costs and can be achieved through the application of biocides (18, 24), nitrate (31), or nitrite (29). Environmental regulations and development of oil reservoirs in environmentally sensitive areas have spurred the development of easily degradable “green” biocides that are less toxic to higher, nontarget organisms, like fish (10). Nitrite, a specific metabolic inhibitor of SRB (13, 15), is also relatively nontoxic and inexpensive and has been successfully used to inhibit sulfide production in oil field settings (6, 31).
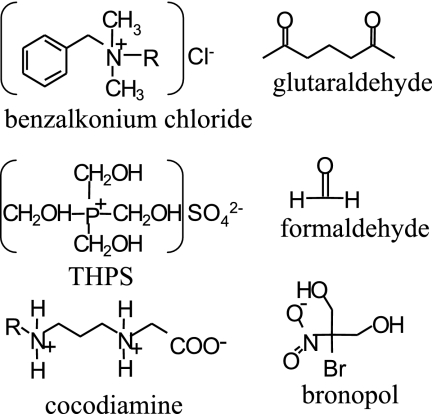
The structures of the biocides used are shown in Fig. 1. Their chemical natures and supposed mechanisms of action are summarized in Table 1. The metabolic inhibitors nitrite and molybdate specifically inhibit SRB metabolism. Nitrite inhibits dissimilatory sulfite reductase, which catalyzes the reduction of sulfite to sulfide in all SRB (13), blocking sulfate respiration. The nitrite dose required for inhibition depends on the concentration of the available electron donor, on the biomass concentration, and on the presence of nitrite reductase, which prevents inhibition of SRB by nitrite (13, 15). ATP sulfurylase activates sulfate with ATP to adenosine phosphosulfate. Use of molybdate in this reaction gives adenosine phosphomolybdate, which is unstable and hydrolyzes spontaneously to AMP and molybdate, depleting ATP reserves (30, 32).
FIG. 1.
Structures of the six biocides used in this study.
TABLE 1.
Chemical classes and mechanisms of action of biocides and metabolic inhibitors used to kill or inhibit SRB
| Metabolic inhibitor or biocide | Chemical nature and mechanism of action | Classa | Reference(s) | MICb |
|---|---|---|---|---|
| Metabolic inhibitors | ||||
| Nitrite | Sulfite analog; inhibitor of dissimilatory sulfite reductase | S | 13 | 5 mM |
| Molybdate | Sulfate analog; converted to adenosine phosphomolybdate by ATP sulfurylase; hydrolysis of adenosine phosphomolybdate depletes cellular ATP reserves | S | 30, 32 | 3 mM |
| Biocides | ||||
| Bronopol | Alcohol; inactivates sulfhydryl group-containing proteins | T | 3, 19, 28 | 4 mM |
| Formaldehyde | Aldehyde; cross-links amino groups of proteins and nucleic acids | X | 4, 5, 19 | 6 mM |
| Glutaraldehyde | Aldehyde; cross-links amino and sulfhydryl groups of proteins and nucleic acids | X | 3, 5, 19 | 5 mM |
| Benzalkonium chloride | Quaternary ammonium cationic surfactant; solubilizes cell membrane and may improve uptake of other antimicrobials | C | 3, 27 | 50 mg/liter |
| Cocodiaminec | Same as benzalkonium chloride | C | 3, 27 | 0.003% (vol/vol) |
| THPS | Quaternary phosphonium; mechanism of action unknown; unlikely to work as a surfactant in view of the short side chains | U | 9, 10 | 0.1 mM |
S, metabolic inhibitor of sulfate reduction; X, cross-linking agent; C, cationic surfactant; T, thiol inactivator; U, unknown.
Determined in this study. The MIC was the minimum concentration that prevented sulfide production by the SRB consortium for 1 month under conditions described in the text.
Cocodiamine is the trivial name for 1-alkyl (C6-C18)-1,3 propane diamine acetate.
It is clear from Table 1 that biocides have much broader activities than nitrite or molybdate. Use of compounds with two different mechanisms of action may lead to synergy and increased time for microbial resistance to develop, resulting in decreased costs and environmental toxicity (16). Although synergistic combinations of biocides, such as combinations of glutaraldehyde with quaternary ammonium compounds, have been used in the oil and gas industry (1, 11, 12, 20, 26), the potential of synergy between metabolic inhibitors and biocides has not been explored previously. We report here that combining biocides with nitrite results in considerable synergy in SRB control, allowing significantly lower biocide concentrations to be used.
Bacterial strains, media, chemicals, and analyses.
A Desulfovibrio species-containing SRB consortium was obtained by inoculating saline Postgate's medium C (22, 25) with produced water from the Coleville oil field in Saskatchewan, Canada, and subsequently transferring the culture monthly (13). Inhibition experiments were done in modified CSB medium (22). Anaerobic modified CSB medium (100 ml in an 158-ml serum bottle) was inoculated with 2 ml of the SRB consortium and incubated at 30°C in the dark. Biocides and/or metabolic inhibitors were added at mid-log phase (tML), when approximately 5 mM sulfide had been produced. Incubation was continued for 1 month following this addition. Cultures in which sulfide production did not recover during this time were considered to be permanently inhibited. Glutaraldehyde, bronopol, and tetrakishydroxymethylphosphonium sulfate (THPS) were obtained from Sigma (St. Louis, MO). Benzalkonium chloride was obtained from ICN (Aurora, OH). The cocodiamine biocide used, designated T-397, was generously provided by Brenntag Canada (Etobicoke, Ontario, Canada). Formaldehyde (37% solution in 15% methanol with the balance water) was obtained from Fisher (Fair Lawn, NJ). All other chemicals were laboratory reagent grade and were obtained from standard chemical supply companies. Sulfide, sulfate, and nitrite concentrations were determined as described elsewhere (8, 22). Molybdate caused the medium to become yellow-brown, which interfered with sulfide analysis. Hence, possible resumption of sulfide production was demonstrated by sulfate removal only. Some biocides caused precipitates to form in the medium. These precipitates were briefly centrifuged out of samples before analyses to prevent interference with the spectrophotometric methods.
Determination of the MIC and the FICI.
The MIC, defined as the concentration of a compound that inhibited sulfide production by the SRB consortium for 1 month when it was added at mid-log phase, was determined for the six biocides and two metabolic inhibitors. The results are summarized in Table 1. Because solid benzalkonium chloride and liquid cocodiamine consist of multiple, related compounds having various molecular weights, their concentrations were not expressed in mM. For mixtures, concentrations were varied in the ranges 0 < [A] < MICA and 0 < [B] < MICB using a checkerboard approach, where MICA is the MIC of compound A and MICB is the MIC of compound B. The fractional inhibitory concentration index (FICI) for combinations of a biocide(s) and/or a metabolic inhibitor(s) that caused inhibition was calculated as follows (16): FICI = [A]/MICA + [B]/MICB.
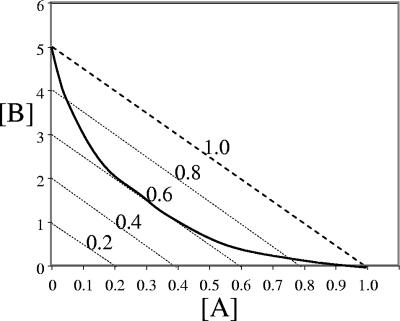
For a synergistic mixture (FICI, <1) the MIC (MICAB) combinations may be related by the solid line in Fig. 2. The lowest FICI obtained for all inhibitory combinations on the checkerboard was considered the FICI for the pair. For example, when MICA was 1 mM, MICB was 5 mM, and a mixture of 0.5 mM compound A and 0.5 mM compound B was inhibitory, then FICI was 0.5/1 + 0.5/5 or 0.6. If this was the lowest value for the pair, then all MICAB combinations for [A] and [B] could be represented by the curved solid line in Fig. 2, which has the equation given above with FICI equal to 0.6 as the tangent. Hence, although the curved solid line crosses a range of FICI values (Fig. 2), only the lowest value is relevant and is reported here.
FIG. 2.
MIC (MICAB) of a mixture of two biocides or metabolic inhibitors A and B as a function of concentration (solid line). The line is theoretical. Mixture compositions above the line are not inhibitory, whereas compositions below the line are inhibitory. The dotted lines represent the equation given in the text, using an MICA of 1 mM, an MICB of 5 mM, and FICI values of 0.2, 0.4, 0.6, 0.8, and 1.0, as shown.
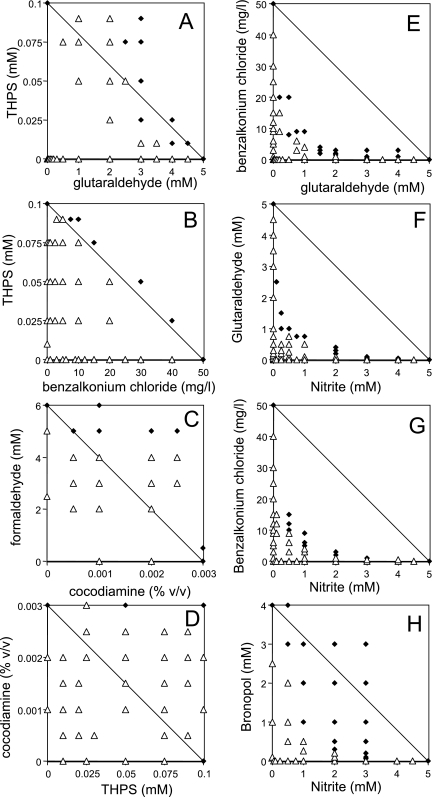
Examples of the checkerboard approach used to determine the FICI values for each combination of antimicrobial compounds are shown in Fig. 3. Combinations of cocodiamine and THPS were effective only when the MIC of both compounds were added (Fig. 3D). Hence, the checkerboard filled with open triangles (representing no inhibition of sulfide production) and a maximal FICI value of 2 was estimated, indicating that these biocides were antagonistic. In the case of nitrite and bronopol most combinations were inhibitory (Fig. 3H). The lowest FICI was estimated to be 0.28, indicating that this combination was synergistic. Combinations of THPS and benzalkonium chloride appeared to be indifferent, with an FICI of 1 (Fig. 3B). The estimated FICI for each pair of antimicrobial agents tested is shown in Table 2. In the case of THPS and glutaraldehyde both antagonistic and synergistic mixtures were observed (Fig. 3A). These two compounds were considered indifferent based on the complete data set. Glutaraldehyde and benzalkonium chloride (Fig. 3E and Table 2) (FICI, 0.35) are known to be synergistic and are considered a standard for industrially available biocide combinations. However, the following five other combinations proved to have FICI values that were comparable to or lower than the value for this standard pair (Table 2): nitrite and glutaraldehyde (Fig. 3F), nitrite and benzalkonium chloride (Fig. 3G), and nitrite and bronopol (Fig. 3H), as well as benzalkonium chloride and bronopol, and glutaraldehyde and bronopol. Of the 28 combinations tested, 16 were synergistic, 2 were synergistic or indifferent, 7 were indifferent, and 3 were antagonistic. Antagonistic reactions were observed with THPS and formaldehyde, cocodiamine, or bronopol (Table 2). The number of synergistic combinations and their average FICI were 6 and 0.44, respectively, for nitrite; 6 and 0.48, respectively, for benzalkonium chloride; 5 and 0.36, respectively, for glutaraldehyde; 4 and 0.32, respectively, for bronopol; 4 and 0.54, respectively, for molybdate; 4 and 0.58, respectively, for formaldehyde; and 3 and 0.52, respectively, for cocodiamine. THPS was indifferent or antagonistic in all combinations tested. Hence, adding the metabolic inhibitor nitrite together with a biocide was beneficial in all but one case.
FIG. 3.
Inhibition (⧫) or lack of inhibition (▵) of sulfide production by an SRB consortium with mixtures of antimicrobial compounds. (A) Glutaraldehyde plus THPS. (B) Benzalkonium chloride plus THPS. (C) Cocodiamine plus formaldehyde. (D) Cocodiamine plus THPS. (E) Glutaraldehyde plus benzalkonium chloride. (F) Nitrite plus glutaraldehyde. (G) Nitrite plus benzalkonium chloride. (H) Nitrite plus bronopol. The FICI values for these mixtures derived from the data shown, as well as the FICI values for all other mixtures tested, are listed in Table 2. The criterion for inhibition was no sulfide production for 1 month for addition at mid-log phase.
TABLE 2.
Estimated FICI values for combinations of antimicrobial agents
| Antimicrobial agent A
|
Antimicrobial agent B
|
FICI | Ratingb | ||
|---|---|---|---|---|---|
| Compound | Classa | Compound | Classa | ||
| Nitrite | S | Bronopol | T | 0.28 | Synergistic |
| Glutaraldehyde | X | Bronopol | T | 0.28 | Synergistic |
| Nitrite | S | Glutaraldehyde | X | 0.30 | Synergistic |
| Nitrite | S | Benzalkonium chloride | C | 0.30 | Synergistic |
| Benzalkonium chloride | C | Bronopol | T | 0.33 | Synergistic |
| Glutaraldehyde | X | Benzalkonium chloride | C | 0.35 | Synergistic |
| Glutaraldehyde | X | Molybdate | S | 0.37 | Synergistic |
| Cocodiamine | C | Bronopol | T | 0.39 | Synergistic |
| Glutaraldehyde | X | Formaldehyde | X | 0.48 | Synergistic |
| Molybdate | S | Formaldehyde | X | 0.50 | Synergistic |
| Nitrite | S | Cocodiamine | C | 0.53 | Synergistic |
| Benzalkonium chloride | C | Formaldehyde | X | 0.57 | Synergistic |
| Nitrite | S | Molybdate | S | 0.59 | Synergistic |
| Benzalkonium chloride | C | Cocodiamine | C | 0.63 | Synergistic |
| Nitrite | S | Formaldehyde | X | 0.67 | Synergistic |
| Benzalkonium chloride | C | Molybdate | S | 0.69 | Synergistic |
| Bronopol | T | Formaldehyde | X | 0.80 | Synergistic/indifferent |
| Molybdate | S | Bronopol | T | 0.80 | Synergistic/indifferent |
| Cocodiamine | C | Molybdate | S | 0.83 | Indifferent |
| Glutaraldehyde | X | THPS | U | 0.90 | Indifferent |
| Cocodiamine | C | Formaldehyde | X | 1.00 | Indifferent |
| Nitrite | S | THPS | U | 1.05 | Indifferent |
| Benzalkonium chloride | C | THPS | U | 1.05 | Indifferent |
| Glutaraldehyde | X | Cocodiamine | C | 1.06 | Indifferent |
| THPS | U | Molybdate | S | 1.17 | Indifferent |
| THPS | U | Formaldehyde | X | 1.57 | Antagonistic |
| Cocodiamine | C | THPS | U | 1.50 | Antagonistic |
| THPS | U | Bronopol | T | 2.00 | Antagonistic |
S, metabolic inhibitor of sulfate reduction; X, cross-linking agent; C, cationic surfactant; T, thiol inactivator; U, unknown.
Mixtures were considered synergistic when the lowest FICI was <0.8, indifferent when 0.8 < FICI < 1.2, and antagonistic when the highest FICI was >1.2.
Biocidal activity was evaluated by determining the most probable numbers (MPN) (cells ml−1) of viable cells remaining 24 h after the MIC of glutaraldehyde, benzalkonium chloride, or nitrite was added at tML. Duplicate samples from treated and control cultures were taken at tML and at tML plus 24 h. MPNs, determined by standard three-tube MPN assays (2), decreased most when the biocides glutaraldehyde and benzalkonium chloride were added (no viable cells remaining) and least when nitrite was added (5% viable cells remaining) (results not shown).
Synergy of the metabolic inhibitors nitrite and molybdate in inhibiting sulfide production by oil field SRB and in inhibiting abiotic corrosion (21, 23), as well as synergy among biocides in inhibiting SRB (17), has been demonstrated previously. Glutaraldehyde and quaternary ammonium compound blends have also been found to be more effective in killing SRB and general aerobic bacteria than the individual compounds (1, 11, 12, 20, 26). Although insufficient combinations of concentrations were tested to clearly demonstrate synergy, the improved killing by the combinations of compounds suggested that synergy did occur with these blends. However, the usefulness of combining biocides (Fig. 1) and the metabolic inhibitors nitrite and molybdate, as suggested here, has not been reported previously.
The combinations of biocides and/or metabolic inhibitors used are ranked on the basis of their FICI values in Table 2. The synergistic mixtures included glutaraldehyde and formaldehyde (FICI, 0.48), both of which are classified as cross-linking aldehydes, indicating that their modes of action may not be the same despite this similar classification. Similarly, the cationic detergents cocodiamine and benzalkonium chloride appeared to be somewhat synergistic (FICI, 0.63). THPS did not exhibit synergy in any of the combinations tested; three of the seven combinations tested were in fact antagonistic (Table 2), suggesting that the structure of THPS or its mode of action was not compatible with the other compounds.
Bronopol, glutaraldehyde, and, to a lesser extent, benzalkonium chloride interacted synergistically with most other compounds. Bronopol has a different structure and mechanism of action than the other biocides tested, which may have resulted in its excellent capacity for synergy. Glutaraldehyde has been demonstrated to be more effective than formaldehyde (4, 12, 20), explaining the generally lower FICI values for mixtures including glutaraldehyde (Table 2).
Strong synergy was observed between nitrite and the biocides glutaraldehyde, benzalkonium chloride, and bronopol (0.28 < FICI < 0.30). The very specific mode of action of nitrite as an inhibitor of dissimilatory sulfite reductase (13, 15) can apparently be favorably combined with broad-spectrum biocidal activities to achieve synergy. Nitrite is reduced to either nitrogen or ammonia, products that cause little environmental harm. Although both nitrite and molybdate are specific metabolic inhibitors of the SRB sulfate reduction pathway, we found that molybdate was less suitable than nitrite (0.37 < FICI < 1.17) (Table 2). Because it is not transformed to similarly harmless products and is more expensive than nitrite, molybdate is inferior in metabolic inhibitor-biocide combinations. Hence, from a practical viewpoint, use of synergistic combinations of nitrite and other biocides to inhibit souring is most suitable for decreasing bioicide use, which decreases toxicity and results in potential cost savings in oil and gas recovery operations where sulfide control is needed.
Acknowledgments
This research was funded by Natural Sciences and Engineering Research Council of Canada (NSERC) strategic grant STPGP 234833-00 entitled “Sulfide removal with nitrate-reducing, sulfide-oxidizing bacteria” and by ConocoPhillips. E.A.G. was supported by an NSERC postdoctoral fellowship.
Footnotes
Published ahead of print on 22 September 2006.
REFERENCES
- 1.Al-Hashem, A., J. Carew, and M. Salman. 1998. Control of microbially influenced corrosion in an effluent water injection system in west Kuwait, paper no. 293. In Corrosion/98. NACE International, Houston, TX.
- 2.American Public Health Association. 1975. Standard methods for the examination of water and wastewater, 14th ed. American Public Health Association, American Water Works Association, Water Pollution Control Federation, Washington, DC.
- 3.Boivin, J. 1995. Oil industry biocides. Mater. Perform. 34:65-68. [Google Scholar]
- 4.Cheung. C. W. S., and I. B. Beech. 1996. The use of biocides to control sulphate-reducing bacteria in biofilms on mild steel surfaces. Biofouling 9:231-249. [Google Scholar]
- 5.Cheung, C. W. S., I. B. Beech, A. Campbell, J. Satherley, and D. J. Schiffrin. 1994. The effect of industrial biocides on sulphate-reducing bacteria under high pressure. Int. Biodeterior. Biodegrad. 33:299-310. [Google Scholar]
- 6.Ciaraldi, S. W., H. H. Ghazal, T. H. Abou Shadey, H. A. El-Leil, and S. M. El-Raghy. 1999. Progress in combating microbiologically induced corrosion in oil production, paper no. 181. In Corrosion/99. NACE International, Houston, TX.
- 7.Cord-Ruwisch, R., W. Kleinitz, and F. Widdel. 1987. Sulfate-reducing bacteria and their activities in oil production. J. Petrol. Technol. 1:97-106. [Google Scholar]
- 8.Cord-Ruwisch, R. 1985. A quick method for determination of dissolved and precipitated sulfides in cultures of sulfate-reducing bacteria. J. Microbiol. Methods 4:33-36. [Google Scholar]
- 9.Downward, B. L., R. E. Talbot, and T. K. Haack. 1997. Tetrakishydroxymethylphosphonium sulfate (THPS), a new industrial biocide with low environmental toxicity, paper no. 401. In Corrosion/97. NACE International, Houston, TX.
- 10.Frey, R. 1998. Award-winning biocides are lean, mean and green. Today's Chem. Work 7:34-35, 37-38. [Google Scholar]
- 11.Georgie, W. J., P. I. Nice, and S. Maxwell. 1991. Selection, optimization and monitoring of biocide efficacy in the Statfjord water injection systems, p. 1-35. In Proceedings of the Institute for Corrosion UK Corrosion 91 Conference, vol. 1. Institute for Corrosion UK, Manchester, United Kingdom. [Google Scholar]
- 12.Grab, L. A., and A. B. Theis. 1992. Comparative biocidal efficacy vs sulfate-reducing bacteria, paper no. 184. In Corrosion/92. NACE International, Houston, TX.
- 13.Greene, E. A., C. Hubert, M. Nemati, G. E. Jenneman, and G. Voordouw. 2003. Nitrite reductase activity of sulfate-reducing bacteria prevents their inhibition by nitrate-reducing, sulfide-oxidizing bacteria. Environ. Microbiol. 5:607-617. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton, W. A., and W. Lee. 1995. Biocorrosion, p. 242-264. In L. L. Barton (ed.), Sulfate-reducing bacteria. Plenum Press, New York, NY.
- 15.Haveman, S. A., E. A. Greene, C. P. Stilwell, J. K. Voordouw, and G. Voordouw. 2004. Physiological and gene expression analysis of inhibition of Desulfovibrio vulgaris Hildenborough by nitrite. J. Bacteriol. 186:7944-7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodges, N. A., and G. W. Hanlon. 1991. Detection and measurement of combined biocide action, p. 297-310. In S. P. Denyer and W. B. Hugo (ed.), Mechanisms of action of chemical biocides, their study and exploitation. Blackwell Scientific Publications, London, United Kingdom.
- 17.Hongfang, L., X. Liming, Z. Jiashen, and L. Jing. 2000. New bactericide for biocide-resistant sulfate-reducing bacteria. Mater. Perform. 39:52-55. [Google Scholar]
- 18.Jack, T. R., and D. W. S. Westlake. 1995. Control in industrial settings, p. 265-292. In L. L. Barton (ed.), Sulfate-reducing bacteria. Plenum Press, New York, NY.
- 19.Maillard, U.-Y. 2002. Bacterial target sites for biocide action. J. Appl. Microbiol. Symp. Suppl. 92:16S-27S. [PubMed] [Google Scholar]
- 20.McIlwaine, D. B., and A. B. Theis. 1994. Biocide usage in pipelines, p. 1-20. In Proceedings of the 1994 Prevention of Pipeline Corrosion Conference. Gulf Publishing Company and Scientific Surveys Ltd., Houston, Tex.
- 21.Mustafa, C. M., A. K. M. Obaydur Rahman, and D. A. Begum. 1996. Effects of time and temperature on he mild steel corrosion inhibition by molybdate and nitrite. Indian J. Chem. Technol. 3:44-48. [Google Scholar]
- 22.Nemati, M., G. E. Jenneman, and G. Voordouw. 2001. A mechanistic study on microbial control of souring in oil reservoirs. Biotechnol. Bioeng. 74:424-434. [DOI] [PubMed] [Google Scholar]
- 23.Nemati, M., T. Mazutinec, G. E. Jenneman, and G. Voordouw. 2001. Control of biogenic H2S production by nitrite and molybdate. J. Ind. Microbiol. Biotechnol. 26:350-355. [DOI] [PubMed] [Google Scholar]
- 24.Okabe, S., W. L. Jones, W. Lee, and W. G. Characklis. 1994. Anaerobic SRB biofilms in industrial water systems: a process analysis, p. 189-204. In G. G. Geesy, Z. Lewandowsky, and H. C. Flemming (ed.), Biofouling and biocorrosion in industrial water systems. Lewis Publishers, Boca Raton, FL.
- 25.Postgate, J. R. 1984. The sulfate-reducing bacteria, p. 30-34. Cambridge University Press, Cambridge, United Kingdom.
- 26.Prasad, R. 1994. Biocide comparison: aldehyde versus mixture of aldehyde and quaternary amine, paper no. 273. In Corrosion/94. NACE International, Houston, TX.
- 27.Schaeufele, P. J. 1984. Advances in quaternary ammonium biocides. J. Am. Oil Chem. Soc. 61:387-389. [Google Scholar]
- 28.Shepherd, J. A., R. D. Waigh, and P. Gilbert. 1988. Antibacterial action of 2-bromo-2-nitropropane-1,3-diol (Bronopol). Antimicrob. Agents Chemother. 32:1693-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sturman, P. J., and D. M. Goeres. 1999. Control of hydrogen sulfide in oil and gas wells with nitrite injection, p. 357-363. In SPE Technical Conference and Exhibition (Production Operations and Engineering). SPE publication 56772. Society of Petroleum Engineers, Houston, TX.
- 30.Taylor, B. F., and R. S. Oremland. 1979. Depletion of adenosine triphosphate in Desulfovibrio by oxyanions of group VI elements. Curr. Microbiol. 3:101-103. [Google Scholar]
- 31.Telang, A. J., S. Ebert, J. M. Foght, D. W. S. Westlake, G. E. Jenneman, D. Gevertz, and G. Voordouw. 1997. Effect of nitrate injection on the microbial community in an oil field as monitored by reverse sample genome probing. Appl. Environ. Microbiol. 63:1785-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson, L. G., and R. S. Bandurski. 1958. Enzymatic reactions involving sulfate, sulfite, selenite and molybdate. J. Biol. Chem. 233:975-981. [PubMed] [Google Scholar]