Abstract
A novel Escherichia coli outer membrane protein A (OmpA) was discovered through a proteomic investigation of cell surface proteins. DNA polymorphisms were localized to regions encoding the protein's surface-exposed loops which are known phage receptor sites. Bacteriophage sensitivity testing indicated an association between bacteriophage resistance and isolates having the novel ompA allele.
Outer membrane protein A (OmpA) is a major, two-domain, heat-modifiable membrane protein in bacteria. The N-terminal domain is comprised of antiparallel β-strands that cross the membrane eight times, producing four large surface-exposed hydrophilic loops and three short periplasmic turns. The C terminus, located in the periplasm, is connected to the outer membrane via interactions with peptidoglycan (6). It has been proposed that OmpA is involved in the structural integrity of the outer membrane (1, 2). OmpA also acts as a phage and colicin receptor (1, 3, 7), and a number of ompA mutants with alterations near residues 25, 70, and 110 have been found to be resistant to bacteriophage (6, 7). The residues involved in phage resistance occur in the large surface-exposed loops of the protein, the same loops that act as phage receptors.
Outer membrane proteins similar to OmpA have been identified in 17 species of gram-negative bacteria (1). Similarities in the structure of OmpA and the high degree of similarity within the nucleotide and amino acid sequences of several enteric species indicate a high degree of evolutionary conservation. Further, a comparison of five closely related genera have shown that the β-strands are highly conserved, whereas the surface-exposed loops are highly variable (9).
An investigation to identify differences in the outer membrane proteins of Escherichia coli from different animal sources resulted in the identification of a novel ompA allele (ompA2). Here we describe the genetic characteristics of the novel ompA allele, its frequency in isolates from a range of vertebrate hosts, and an evolutionary advantage of organisms possessing the novel allele.
Outer membrane protein identification and characterization.
Outer membrane proteins from three vertebrate E. coli isolates (H474 from a human, TA024 from a Tasmanian devil, and B194 from a varied honeyeater) were isolated using a carbonate extraction method combined with two-dimensional gel electrophoresis (8). Differential display of protein profiles from each of the three isolates showed a distinct shift in the isoelectric point of an integral outer membrane protein for isolate B194. Matrix-assisted laser desorption ionization-time of flight mass spectrometry was used to obtain mass fingerprints for each protein spot (8). Peptide analysis using appropriate databases (Profound and TrEMBL) indicated that the proteins were most similar to the E. coli OmpA protein.
Nucleotide analysis of the ompA allele from isolates characterized using proteomics revealed two sequences (ompA1 and ompA2). A BLASTN search identified ompA1 as being the ompA sequence of E. coli (GenBank accession no. OMPAECOLI). The ompA2 allele was distinct from previously described ompA genes, although it was most similar (approximately 97%) to Shigella flexneri (GenBank accession no. AY305875). Sequencing across the variable regions of a further 14 E. coli isolates indicated the novel ompA2 allele to be present in human and marsupial isolates (Table 1).
TABLE 1.
Type of ompA allele present in E. coli isolates from humans and Australian vertebrates for which sequence data were obtained
| Isolate | Source | E. coli ompA type |
|---|---|---|
| H22 | Human | ompA1 |
| H55 | Human | ompA2 |
| H137 | Human | ompA1 |
| H312 | Human | ompA1 |
| H322 | Human | ompA2 |
| H474a | Human | ompA1 |
| H562 | Human | ompA1 |
| H753 | Human | ompA1 |
| AH1 | Human | ompA1 |
| AH2 | Human | ompA1 |
| TA024a | Tasmanian devil (Sarcophilus harrisii) | ompA1 |
| TA298 | Mountain possum (Trichosuris caninus) | ompA1 |
| TA165 | Mountain possum (Trichosuris caninus) | ompA1 |
| TA411 | Brushtail possum (Trichosuris vulpecula) | ompA1 |
| TA252 | Brushtail possum (Trichosuris vulpecula) | ompA1 |
| TA421 | Eastern gray kangaroo (Macropus giganteus) | ompA2 |
| B194a | Varied honeyeater (Lichenostomus versicolor) | ompA2 |
E. coli isolates used for proteomic analysis.
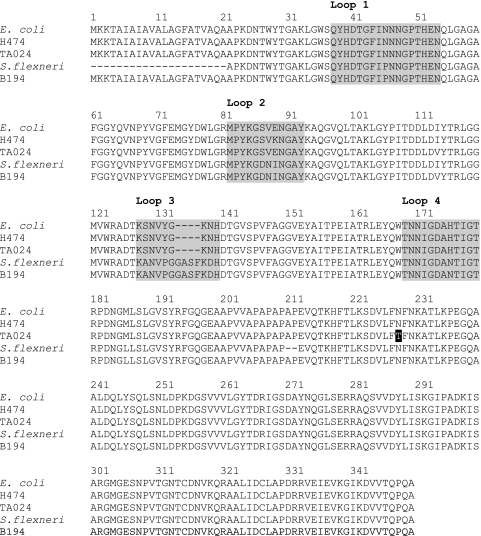
Translation and alignment of the OmpA amino acid sequences showed that the surface-exposed loops 2 and 3 were the most variable, and single-amino-acid changes were identified in loops 1 and 4 (Fig. 1). The percentages of identity of the E. coli ompA2 allele to the described sequences for E. coli and S. flexneri were 96.9% and 99.6%, respectively, and a single amino acid change between the E. coli ompA2 allele and S. flexneri within β-strand 5 at residue 93 was observed.
FIG. 1.
Translation of the E. coli ompA1 and ompA2 alleles from three E. coli isolates H474 (human), TA024 (Tasmanian devil), and B194 (bird) and alignment to the E. coli and S. flexneri ompA sequences (GenBank accession no. ECOMPA and AY305875, respectively). Variations occur within the exposed loops of the protein (shaded gray). A single amino acid change was identified external to a loop region in isolate TA024 (shaded black).
Frequency of the OmpA variant in vertebrate hosts.
The frequency of the ompA2 allele was determined by screening 524 E. coli isolates selected from a collection of greater than 1,300 isolates sampled from a variety of sources throughout Australia. Human clinical and fecal isolates were chosen, in addition to fecal isolates from nondomesticated Australian mammals. These strains were previously screened for mitomycin C-inducible colicin production and lysogeny (4). PCR primers were designed to specifically amplify a region of the ompA2 sequence (OmpAVF1 [5′-GGCTAACGTACCTGGTGGCGCA-3′] and OmpAVR1 [5′-CGACGATCCGGAGCCAGGCA-3′]). E. coli isolates were identified as having the ompA2 allele by the presence of a 550-bp product using electrophoresis.
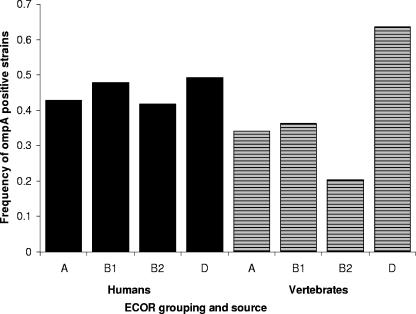
The novel ompA2 allele was identified in 43% of E. coli strains screened using the ompA2-specific PCR. The frequency of the ompA2 variant was dependent on the source of the strain (human versus animal) and the strain's E. coli Reference Collection (ECOR) group membership (2) (nominal logistic regression, likelihood ratio test, source, χ21 = 2.39, P = 0.122; ECOR group, χ23 = 18.7, P = 0.003; source × ECOR interaction, χ23 = 9.9, P = 0.02). The frequency of the ompA2 allele was 45% in the human isolates and did not vary significantly among the four ECOR groups. In fecal isolates from nondomesticated mammals, the frequency was 38%, and significant differences were observed in the frequencies of the ompA2 variant among the four ECOR groups (Fig. 2). Further, of the 524 strains examined, those strains with the ompA2 allele were significantly less likely to be lysogenic (3.2%) than strains with the ompA1 allele (16.8%) (likelihood ratio test, χ21 = 19.1, P = <0.001).
FIG. 2.
Frequency of Escherichia coli strains carrying the ompA2 allele with respect to source of strain and ECOR group membership of the strains.
Phage sensitivity of the novel allele.
We hypothesized that the high frequency of the ompA2 allele in E. coli isolates conferred a selective advantage to the organism. The majority of sequence variation in the ompA2 allele occurs within loops 2 and 3, the same regions in which laboratory-engineered mutations induced resistance to bacteriophage (6).
To determine whether the ompA2 allele reduced sensitivity to bacteriophage, 52 ompA1 and 52 ompA2 isolates (13 from each of the four ECOR groups) were randomly selected (5). Lawns of the selected isolates were spotted with phage extracts (25 μl) prepared from 24 bacteriophage-positive E. coli strains (4). Natural E. coli populations having the ompA2 allele were less sensitive to lysis by bacteriophage than strains with the ompA1 allele were. This screening indicated that 19.2% of the ompA2 strains were sensitive to one or more of the 24 phages, while 39.2% of ompA1 strains were sensitive (likelihood ratio test, χ21 = 5.05, P = 0.025). Although the phages used in this study were not identified, the use of 24 different phages from infected E. coli isolates would have increased the chances of screening multiple phage types.
Past studies have demonstrated that the exposed-loop regions of the protein function as phage receptors and that induced mutations in these regions alter sensitivity to bacteriophage (6, 7). We have identified a naturally occurring ompA allele that is associated with increased resistance to bacteriophage over typical E. coli OmpA expression. To confirm this hypothesis, the sensitivity of E. coli carrying the ompA2 allele to bacteriophage known to use OmpA as the receptor needs to be tested. Further investigations will aid in understanding the functions of the exposed loops of the OmpA protein.
Nucleotide sequence accession numbers.
Sequences from this study have been entered in GenBank under accession numbers AY682204, AY682205, and AY682206.
Acknowledgments
Funding for this work was provided by Sydney Water Corporation, a Macquarie University Collaborative Research grant, and the National Capital Authority.
We acknowledge Raj Shanker for assistance in instigating this research. The proteomics aspect of this work was conducted through direct access to the Australian Proteome Analysis Facility (APAF), Sydney, Australia. Thank you to the APAF staff for their assistance, especially Martin Larsen for mass spectrometry analysis. We thank Peter Cox and Mark Angles from the Sydney Water Corporation for assistance during the project and review of the manuscript.
Footnotes
Published ahead of print on 15 September 2006.
REFERENCES
- 1.Beher, M. G., C. A. Schnaitman, and A. P. Pugsley. 1980. Major heat-modifiable outer membrane protein in gram-negative bacteria: comparison with the OmpA protein of Escherichia coli. J. Bacteriol. 143:906-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foulds, J., and C. Barrett. 1973. Characterization of Escherichia coli mutants tolerant to bacteriocin JF246: two new classes of tolerant mutants. J. Bacteriol. 116:885-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon, D. M., M. A. Riley, and T. Pinou. 1998. Temporal changes in the frequency of colicinogeny in Escherichia coli from house mice. Microbiology 144:2233-2244. [DOI] [PubMed] [Google Scholar]
- 5.Herzer, P. J., S. Nouye, M. Nouye, and T. S. Whittam. 1990. Specific and sensitive two-step polymerase reaction assay for the detection of the Salmonella species. Clin. Microbiol. Infect. Dis. 15:603-607. [DOI] [PubMed] [Google Scholar]
- 6.Koebnik, R. 1999. Structural and functional roles of the surface-exposed loops of the B-barrel membrane protein OmpA from Escherichia coli. J. Bacteriol. 181:3688-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morona, R., C. Kramer, and U. Henning. 1985. Bacteriophage receptor area of outer membrane protein OmpA of Escherichia coli K-12. J. Bacteriol. 164:539-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nouwens, A., S. J. Cordwell, M. R. Larsen, M. P. Molloy, M. Gillings, M. D. P. Willcox, and B. J. Walsh. 2000. Complementing genomics with proteomics: the membrane subproteome of Pseudomonas aeruginosa PAO1. Electrophoresis 21:3797-3809. [DOI] [PubMed] [Google Scholar]
- 9.Pautsch, A., and G. E. Schulz. 1998. Structure of the outer membrane protein A transmembrane domain. Nat. Struct. Biol. 5:1013-1017. [DOI] [PubMed] [Google Scholar]