Abstract
We attempted to alter the substrate preference of aminopeptidase from Streptomyces septatus TH-2 (SSAP). Because Asp198 and Phe221 of SSAP are located in the substrate binding site, we screened 2,000 mutant enzymes with D198X/F221X mutations. By carrying out this examination, we obtained two enzymes; one specifically hydrolyzed an arginyl derivative, and the other specifically hydrolyzed a cystinyl derivative (65- and 12.5-fold higher kcat values for hydrolysis of p-nitroanilide derivatives than those of the wild type, respectively).
Chiral amino acids are useful building blocks for the synthesis of analogs of biologically active peptides and versatile chiral starting materials or chiral auxiliaries for other synthetic purposes. In our previous study, we identified an aminopeptidase secreted by Streptomyces septatus TH-2 (SSAP) (EC 3.4.11.10) and succeeded in overproducing it using recombinant Escherichia coli (1). SSAP belongs to the M28 family (MEROPS ID: M28.019), in which peptidases have two cocatalytic zinc atoms in their active site (14, 22, 23, 24). The M28 family includes bacterial aminopeptidases and human proteins, such as the prostate-specific membrane antigen and glutamate carboxypeptidase II (13, 15, 20). Zinc ions in their active site tetrahedrally cocoordinate with three amino acid ligands and activated water (8). The bacterial aminopeptidases of this family are considered to be available for the synthesis of chiral amino acids in industrial applications because of their strict enantioselectivity, high thermal stability, and ability to function as amidases and esterases (2, 3, 7, 26). Although they are stable and easy to handle, their abilities as synthetic catalysts are limited by their substrate preference bias toward hydrophobic amino acid derivatives. Therefore, the alteration of their properties may expand their use in new fields in the synthesis of pharmaceuticals or chiral building blocks.
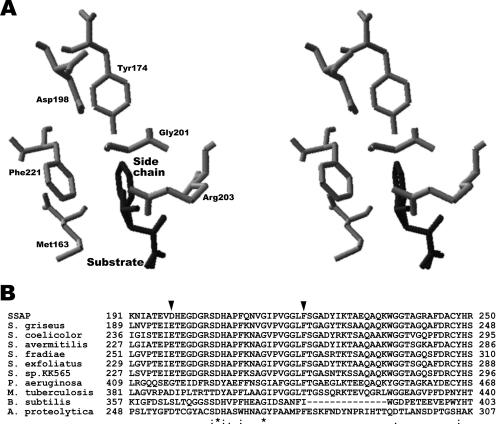
Aminopeptidase from Streptomyces griseus is considered a representative enzyme of the M28 family, and its catalytic mechanism has been extensively studied (4, 9, 10, 11, 12, 19). The sequence of SSAP is 71% identical to that of the enzyme from S. griseus; therefore, SSAP is considered to have a three-dimensional structure similar to that of the enzyme from S. griseus. In the predicted structure of SSAP, there are several residues in direct contact with the side chain of the bound substrate (Fig. 1A). Among these residues, we have shown that two residues of SSAP, namely, Asp198 and Phe221, are associated with substrate specificity (2, 5). Although both residues are conserved, with functionally the same residues among aminopeptidases from Streptomyces, some other bacterial enzymes possess residues with a different function (Fig. 1B). Our previous study demonstrated that both residues are considered to be associated with the environment around the side chain of the bound substrate. In this study, we first examined the substrate preference of wild-type SSAP and 38 SSAP variants with the D198X or F221X mutation toward several aminoacyl-p-nitroanilides (pNAs). Next, we constructed various D198X/F221X mutants to transform SSAP into an aminopeptidase with a different substrate preference.
FIG. 1.
Predicted local three-dimensional structure of SSAP around bound substrates and alignment of SSAP sequence around Asp198 and Phe221 with the corresponding regions of other bacterial aminopeptidases. (A) All residues and substrates are shown using stick models. The residues surrounding the side chain of the substrate are shown in gray and the bound substrates (free Leu and Phe) in dark gray. (B) The two residues mutated in this study are indicated by black arrowheads. *, conserved residues; :, conservative substitutions; ., semiconservative substitutions in all sequences. Proteins: S. griseus, aminopeptidase from Streptomyces griseus; S. coelicolor, putative aminopeptidase from Streptomyces coelicolor; S. avermitilis, putative aminopeptidase from Streptomyces avermitilis; S. fradiae, aminopeptidase from Streptomyces fradiae; S. exfoliatus, aminopeptidase from Streptomyces exfoliatus; S. sp.KK565, aminopeptidase from Streptomyces sp. strain KK565; P. aeruginosa, a secreted aminopeptidase from Pseudomonas aeruginosa; M. tuberculosis, aminopeptidase from Mycobacterium tuberculosis; B. subtilis, extracellular aminopeptidase from Bacillus subtilis; A. proteolytica, leucine aminopeptidase from Aeromonas proteolytica. Multiple sequence alignments were performed with the CLUSTAL algorithm (http://www.ddbj.nig.ac.jp/search/clustalw-j.html).
Activities of wild-type, D198X, and F221X SSAPs toward aminoacyl-pNAs.
The plasmid pET-SSAP:His (4) (ssap gene inserted into NcoI-BamHI gap of pET-KmS2 [16]) was used in expressing wild-type SSAP. The plasmids pET-SSAP:His[D198X] (5) and pET-SSAP:His[F221X] (2) (D198X or F221X mutant ssap gene inserted into NcoI-BamHI gap of pET-KmS2) were used in expressing D198X SSAP and F221X SSAP, respectively. Wild-type and single-mutant SSAPs were purified from the culture supernatant of the E. coli BL21(DE3) strain harboring the constructed plasmid as described by Arima et al. (2, 5). Using purified enzymes, we first examined these SSAP activities toward several aminoacyl-pNAs, including the oxidized form of cysteine-pNA, cystinyl-pNA, to investigate the effect of mutations on substrate preference. The activities toward aminoacyl-pNAs were determined by the increase in absorbance at 405 nm caused by the release of p-nitroaniline per minute at pH 8.0 and 37°C (extinction coefficient of absorbance at 405 nm is 10,600 M−1 cm−1) (18). For cystinyl-pNA hydrolysis, an assay was performed at pH 6.0 because of its poor solubility at pH 8.0.
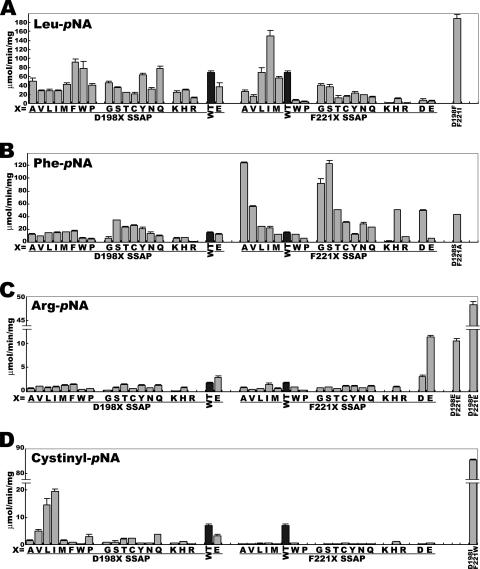
Figure 2 shows the hydrolytic activity of mutants toward several aminoacyl-pNAs. Except for the cystinyl-pNA hydrolytic activities of F221X SSAPs, several single-mutant SSAPs showed a higher activity than the wild-type SSAP. Apparently, the activities were more susceptible to the mutation of Phe221 than to that of Asp198.
FIG. 2.
Hydrolytic activities of wild-type and mutant SSAPs toward Leu-pNA (A), Phe-pNA (B), Arg-pNA (C), and cystinyl-pNA (D). The hydrolytic activities of the wild-type SSAP toward substrates are indicated by black bars and those of mutant enzymes by gray bars. The data are expressed as the means ± standard deviations for three independent experiments. WT, wild type.
Combination of mutations that exhibit largest increases in activity.
We next constructed several D198X F221X double mutants that were expected to have higher activities than the wild-type and single-mutant SSAPs. For example, D198F and F221I SSAPs had the highest Leu-pNA hydrolytic activities in the groups of D198X SSAPs and F221X SSAPs, respectively (Fig. 2A). Therefore, we predicted that a further increase in Leu-pNA hydrolytic activity could be obtained by combining two mutations (D198F and F221I) in the same enzyme.
As shown in Fig. 2A, D198F F221I SSAP exhibits the highest Leu-pNA hydrolytic activity among wild-type and mutant enzymes. However, in the hydrolysis of Phe- and Arg-pNAs, the constructed double mutants, namely, D198S F221A and D198E F221E SSAPs, showed activities lower than that of the single-mutant enzyme, with the highest activity among single mutants (Fig. 2B and C).
Screening for enzymes with high performance.
We further attempted to obtain enzymes with high performance by constructing mutant SSAPs with random combinations of residues at positions 198 and 221. The internal domain of the ssap gene, in which Asp198 was substituted with 19 other residues, and the 3′-end domain, in which Phe221 was substituted with 19 other residues, were amplified by PCR from pET-SSAP:His [D198X] and pET-SSAP:His [F221X], respectively. The former was digested with SacII and PvuI and the latter with PvuI and BamHI. All of the resulting fragments were then mixed and ligated to the SacII and BamHI gap of pET-SSAP:His. The colonies obtained by the transformation of E. coli JM109 using a ligated sample were mixed, and cells were then harvested. Plasmids were prepared from the harvested cells to obtain the pET-SSAP:His[D198X/F221X] mixed plasmid library.
For the screening of enzymes with high performance, E. coli BL21(DE3) transformants harboring pET-SSAP:His[D198X/F221X] were cultivated in 0.5 ml of a synthetic medium (16) using 96-well cultivation plates at 22°C for 36 h with 24 h of induction with 0.5 mM isopropyl-β-thiogalactopyranoside. Then, culture supernatants were used for screening, and harvested cells were stored at −20°C for use in the preparation of plasmids.
When we screened 2,000 supernatant samples of the culture medium, we obtained several double-mutant enzymes with substrate specificities different from those of the wild-type and single-mutant enzymes. The relative activities of the obtained mutants are shown in Table 1. Among the obtained double-mutant enzymes, there were two high-performance mutants, namely, D198P F221E and D198I F221W SSAPs. As shown in Fig. 2C, the D198P F221E mutations resulted in a marked increase in Arg-pNA hydrolytic activity, although the substitution of Asp198 of the wild type with Pro led to a decrease in activity. A similar effect was observed with the D198I F221W mutations, that is, cystinyl-pNA hydrolytic activity was highly increased by this mutation (Fig. 2D), whereas the substitution of Phe221 with Trp resulted in a decrease in activity compared with that of the wild type. These results indicate that there is an ideal combination of the two residues for the hydrolysis of specific substrates.
TABLE 1.
Relative activities of wild-type and obtained mutant enzymes toward aminoacyl-pNAs
| SSAP variant | Relative activity (% of maximum) toward pNA derivativea,d
|
Sp act toward aminoacyl-pNA with highest hydrolytic activity (μmol/min/mg)d | |||||
|---|---|---|---|---|---|---|---|
| Leu | Phe | Lys | Arg | Ala | Cystine | ||
| WTe | 100 | 23 | 24 | 3 | 1 | 10 | 68 ± 1.3 (Leu-pNA) |
| D198A F221T | 21 | 100 | 12 | 8 | 13 | 5 | 22 ± 1.8 (Phe-pNA) |
| D198F F221D | 20 | 81b | 50 | 100 | 28 | <1 | 5.9 ± 0.2 (Arg-pNA) |
| D198H F221N | 23 | 100 | 4 | 6 | 19 | <1 | 5.4 ± 0.4 (Phe-pNA) |
| D198I F221Wc | 15 | 12 | <1 | <1 | <1 | 100 | 82 ± 1.8 (pNA-CysCys-pNA) |
| D198L F221M | 88 | 100 | 70 | 27 | 24 | <1 | 5.2 ± 0.3 (Phe-pNA) |
| D198L F221W | 69 | 83 | 4 | 5 | 7 | 100 | 42 ± 3.5 (pNA-CysCys-pNA) |
| D198L F221T | 11 | 100 | 8 | 4 | 14 | 3 | 18 ± 1.4 (Phe-pNA) |
| D198L F221E | 10 | 19 | 24 | 100 | 17 | 1 | 8.9 ± 0.7 (Arg-pNA) |
| D198M F221A | 26 | 100 | 5 | 5 | 19 | <1 | 49 ± 4.8 (Phe-pNA) |
| D198M F221G | 33 | 100 | 2 | 1 | 3 | 2 | 45 ± 1.3 (Phe-pNA) |
| D198M F221V | 42 | 100 | 13 | 8 | 38 | 3 | 12 ± 0.2 (Phe-pNA) |
| D198M F221W | 100 | 78 | 11 | 6 | 18 | 18 | 22 ± 0.8 (Leu-pNA) |
| D198P F221E | 2 | <1 | 4 | 100 | 3 | 2 | 48 ± 0.5 (Arg-pNA) |
| D198S F221N | 34 | 100 | 10 | 7 | 34 | <1 | 31 ± 1.9 (Phe-pNA) |
| D198S F221E | 52 | 43 | 13 | 100 | 13 | <1 | 17 ± 0.9 (Phe-pNA) |
| D198V F221D | 38 | 89 | 100 | 93 | 67 | 5 | 4.2 ± 0.9 (Lys-pNA) |
| D198V F221N | 53 | 100 | 23 | 15 | 59 | 17 | 5.5 ± 0.2 (Phe-pNA) |
| D198W F221D | 6 | 92 | 35 | 100 | 28 | <1 | 6.8 ± 0.1 (Arg-pNA) |
Relative activity of enzyme toward aminoacyl-pNA; the highest hydrolytic activities are underlined and in bold.
Relative activities of mutant enzymes toward aminoacyl-pNAs exceeding 50% of their highest hydrolytic activity are in bold.
SSAP mutations in bold are those of high-performance double mutants.
The final concentrations of the enzymes and substrates in this assay were 10 μg/ml and 1 mM, respectively. The increase in absorbance at 405 nm caused by the release of p-nitroaniline was monitored continuously using a SUNRISE THERMO microplate reader (TECAN).
WT, wild type.
Substrate preference of obtained double mutants.
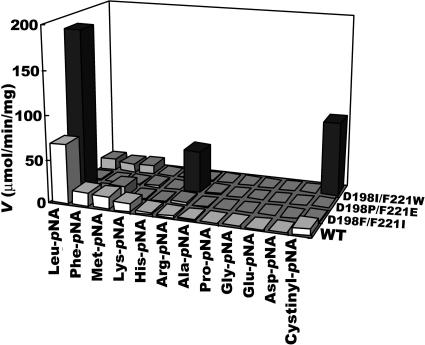
We compared the activities of the obtained high-performance double mutants, namely, D198F F221I, D198P F221E, and D198I F221W SSAPs, toward aminoacyl-pNAs. Interestingly, the substrate preferences of these double mutants were all extremely specific. For D198F F221I SSAP, although its Leu-pNA hydrolytic activity was increased by the double mutation, its activities toward other aminoacyl-pNAs were decreased (Fig. 3). Similarly, the substrate preferences of D198P F221E and D198I F221W SSAPs were also completely switched from Leu-pNA to Arg-pNA and cystinyl-pNA by the mutations, respectively. From these results, the environment around the bound substrate introduced by these mutations is considered to be specifically modified for hydrolyzing these substrates.
FIG. 3.
Substrate specificities of wild-type, D198F F221I, D198P F221E, and D198I F221W SSAPs. The hydrolytic activities of the wild-type SSAP toward the substrates are indicated by white bars (top of the bar is light gray). Those of D198F F221I, D198P F221E, and D198I F221W SSAPs toward Leu-pNA, Arg-pNA, and cystinyl-pNA, respectively, are indicated by dark gray bars, and others are indicated by gray bars. The values are representative of three independent experiments. In all cases, the standard deviation was less than 5% of the mean. WT, wild type.
Kinetic analysis.
We also characterized the kinetics of the mutants, and the results are summarized in Table 2. In the case of D198F F221I SSAP, the Km values for Leu-pNA hydrolysis were higher than that of the wild type, though the activity with Leu-pNA was threefold higher than that of the wild type. Similar results were obtained with D198P F221E SSAP (65-fold higher kcat value and 7.5-fold higher Km value than those of the wild type) (Table 2). In contrast, for D198I F221W SSAP, the kcat and Km values for cystinyl-pNA hydrolysis were 12.5-fold higher and 2-fold lower than those of the wild type, respectively. By this investigation, we found high kcat values to be common among the mutants. This indicates that the mutations positively affect the catalytic cycle rather than the affinity toward substrates.
TABLE 2.
Kinetic parameters for wild-type and high-performance mutant SSAPsa
| Substrate | SSAP variant | kcat (s−1) | Km (mM) | kcat/Km (s−1 · mM−1) |
|---|---|---|---|---|
| Leu-pNA | WT | 41.3 ± 2.6 | 0.57 ± 0.14 | 72.5 |
| D198F F221I | 128 ± 11 | 1.15 ± 0.22 | 111 | |
| Arg-pNA | WT | 1.26 ± 0.2 | 1.00 ± 0.28 | 1.26 |
| D198P F221E | 82.0 ± 5.5 | 7.51 ± 2.3 | 10.9 | |
| Cystinyl-pNA | WT | 3.71 ± 1.2 | 0.11 ± 0.01 | 32.9 |
| D198I F221W | 46.7 ± 8.9 | 0.056 ± 0.01 | 833 |
Km and kcat values were calculated from a nonlinear regression fit to the Michaelis-Menten equation, using initial estimates from double-reciprocal plots. The values are means ± standard deviations for three independent experiments. WT, wild type.
Esterase activities of mutant enzymes.
Our previous study demonstrated that the activities of bacterial aminopeptidases toward aminoacyl derivatives are affected by the flanking moiety (2, 3). For example, the wild-type SSAP exhibited a threefold-lower activity toward Leu-methyl ester (OMe) than toward Leu-pNA (Table 3) (2). To test the effect of the mutations on activities toward other aminoacyl derivatives, we investigated the activities of the wild-type, D198F F221I, D198P F221E, and D198I F221W SSAPs toward Leu-, Arg-, and cystinyl-OMes. In the assay, an enzyme solution (0.1 ml; 0.1 to 1 mg/ml) and a substrate solution (0.1 ml; 100 mM) were added to 0.8 ml of 100 mM Tris-HCl (pH 8.0), and the mixture was incubated at 37°C for 60 to 120 min. For cystinyl-OMe hydrolysis, an assay was performed using 100 mM acetate buffer (pH 6.0) because of its poor solubility at pH 8.0. Specific activities toward aminoacyl-OMes were determined by monitoring the decrease in absorbance at 225, 228, or 230 nm per min caused by the cleavage of methyl ester. The initial activity rate was determined from the linear portion of the optical density profile (extinction coefficients of absorbance at 225, 228, and 230 nm are 79.8, 51.4, and 47.8 M−1 cm−1 for Arg-OMe, cystinyl-OMe, and Leu-OMe, respectively).
TABLE 3.
Hydrolytic activities of wild-type and high-performance mutant SSAPs toward aminoacyl pNA and OMe derivatives
| Substrate | Activity of WT
|
Mutations of high-performance enzyme | Activity of mutant enzyme
|
||
|---|---|---|---|---|---|
| Va (μmol/min/mg) | Relative to that with pNA | V (μmol/min/mg) | Relative to that with pNA | ||
| Leu-pNA | 68 ± 1.3 | 1 | D198F F221I | 188 ± 2.3 | 1 |
| Leu-OMe | 20 ± 0.9 | 0.3 | D198F F221I | 39 ± 2.6 | 0.2 |
| Arg-pNA | 2.0 ± 0.1 | 1 | D198P F221E | 48 ± 0.5 | 1 |
| Arg-OMe | 2.5 ± 0.2 | 1.3 | D198P F221E | 11 ± 0.5 | 0.2 |
| Cystinyl-pNA | 6.2 ± 0.2 | 1 | D198I F221W | 82 ± 1.8 | 1 |
| Cystinyl-OMe | 0.18 ± 0.04 | 0.03 | D198I F221W | 1.9 ± 0.1 | 0.02 |
V, velocity of hydrolytic activity.
As shown in Table 3, similar to the hydrolysis toward aminoacyl-pNA derivatives, the mutant enzymes showed higher activities toward methyl esters than the wild type. In terms of the ratio of the activity toward aminoacyl-pNA to those toward methyl ester derivatives, the activity of D198F F221I SSAP toward Leu-OMe is one-fifth lower than that toward Leu-pNA. D198I F221W SSAP also was observed to have an approximately 50-fold lower activity toward cystinyl-OMe than toward cystinyl-pNA. These decreases in activity are quite similar to those observed with the wild type (the ratios of activity on -pNA to that on methyl ester derivatives are 0.3 and 0.03 for Leu derivatives and cystinyl derivatives, respectively) (Table 3). These similar ratios of activity suggest that there are no effects of the D198F F221I and D198I F221W mutations on the binding of the flanking moiety of substrates. In contrast, the wild type has a 1.3-fold-higher activity toward Arg-OMe than toward Arg-pNA, whereas D198P F221E SSAP has fivefold-lower activity toward Arg-OMe than toward Arg-pNA. This result suggests that the D198P F221E double mutation affects not only the binding of the Arg side chain but also the binding of the flanking moiety.
There are many reports on techniques of altering the substrate specificity of peptidases, such as subtilisin (21), chymotrypsin (25), and methionine aminopeptidase (27). In this study, we also demonstrated a method of changing the substrate preference of aminopeptidase by introducing an environmental change around the bound substrate. We speculate that the reconstruction of enzymes with a wide range of substrate preferences for use in the synthesis of chiral amino acids is possible by introducing mutations of residues associated with the environment around the bound substrate.
Besides their use in synthesis of chiral amino acids, aminopeptidases have been demonstrated to be useful in the preparation of debittered protein hydrolysates (17) and the synthesis of dipeptides (6). In this class of enzymes, we consider the possibility that there is a region associated with the recognition of the flanking moiety of the substrate. Thus, further studies of the recognition of the penultimate residue by SSAP are needed to modify SSAP for use in industries.
Acknowledgments
This research was supported in part by grants from the Noda Institute for Scientific Research.
Footnotes
Published ahead of print on 6 October 2006.
REFERENCES
- 1.Arima, J., M. Iwabuchi, and T. Hatanaka. 2004. Gene cloning and overproduction of an aminopeptidase from Streptomyces septatus TH-2, and comparison with a calcium-activated enzyme from Streptomyces griseus. Biochem. Biophys. Res. Commun. 317:531-538. [DOI] [PubMed] [Google Scholar]
- 2.Arima, J., Y. Uesugi, M. Iwabuchi, and T. Hatanaka. 2005. Alteration of leucine aminopeptidase from Streptomyces septatus TH-2 to phenylalanine aminopeptidase by site-directed mutagenesis. Appl. Environ. Microbiol. 71:7229-7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arima, J., Y. Uesugi, M. Iwabuchi, and T. Hatanaka. 2006. Study on peptide hydrolysis by aminopeptidases from Streptomyces griseus, Streptomyces septatus, and Aeromonas proteolytica. Appl. Microbiol. Biotechnol. 70:541-547. [DOI] [PubMed] [Google Scholar]
- 4.Arima, J., Y. Uesugi, M. Uraji, S. Yatsushiro, S. Tsuboi, M. Iwabuchi, and T. Hatanaka. 2006. Modulation of Streptomyces leucine aminopeptidase by calcium: identification and functional analysis of key residues in activation and stabilization by calcium. J. Biol. Chem. 281:5885-5894. [DOI] [PubMed] [Google Scholar]
- 5.Arima, J., Y. Uesugi, M. Uraji, M. Iwabuchi, and T. Hatanaka. 2006. The role of Glu196 in the environment around the substrate binding site of leucine aminopeptidase from Streptomyces griseus. FEBS Lett. 580:912-917. [DOI] [PubMed] [Google Scholar]
- 6.Arima, J., Y. Uesugi, M. Uraji, M. Iwabuchi, and T. Hatanaka. 2006. Dipeptide synthesis by aminopeptidase from Streptomyces septatus TH-2 and its application to synthesis of biologically active peptides. Appl. Environ. Microbiol. 72:4225-4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bienvenue, D. L., R. S. Mathew, D. Ringe, and R. C. Holz. 2002. The aminopeptidase from Aeromonas proteolytica can function as an esterase. J. Biol. Inorg. Chem. 7:129-135. [DOI] [PubMed] [Google Scholar]
- 8.Chevrier, B., H. D'Orchymont, C. Schalk, C. Tarnus, and D. Moras. 1996. The structure of the Aeromonas proteolytica aminopeptidase complexed with a hydroxamate inhibitor—involvement in catalysis of Glu151 and two zinc ions of the co-catalytic unit. Eur. J. Biochem. 237:393-398. [DOI] [PubMed] [Google Scholar]
- 9.Fundoiano-Hershcovitz, Y., L. Rabinovitch, Y. Langut, V. Reiland, G. Shoham, and Y. Shoham. 2004. Identification of the catalytic residues in the double-zinc aminopeptidase from Streptomyces griseus. FEBS Lett. 571:92-196. [DOI] [PubMed] [Google Scholar]
- 10.Gilboa, R., H. M. Greenblatt, M. Perach, A. Spungin-Bialik, U. Lessel, G. Wohlfahrt, D. Schomburg, S. Blumberg, and G. Shoham. 2000. Interactions of Streptomyces griseus aminopeptidase with a methionine product analogue: a structural study at 1.53 Å resolution. Acta Crystallogr. D Biol. Crystallogr. 56:551-558. [DOI] [PubMed] [Google Scholar]
- 11.Gilboa, R., A. Spungin-Bialik, G. Wohlfahrt, D. Schomburg, S. Blumberg, and G. Shoham. 2001. Interactions of Streptomyces griseus aminopeptidase with amino acid reaction products and their implications toward a catalytic mechanism. Proteins 44:490-504. [DOI] [PubMed] [Google Scholar]
- 12.Greenblatt, H. M., O. Almog, B. Maras, A. Spungin-Bialik, D. Barra, S. Blumberg, and G. Shoham. 1997. Streptomyces griseus aminopeptidase: X-ray crystallographic structure at 1.75 Å resolution. J. Mol. Biol. 265:620-636. [DOI] [PubMed] [Google Scholar]
- 13.Holz, R. C., K. P. Bzymek, and S. I. Swierczek. 2003. Co-catalytic metallopeptidases as pharmaceutical targets. Curr. Opin. Chem. Biol. 7:197-206. [DOI] [PubMed] [Google Scholar]
- 14.Lowther, W. T., and B. W. Matthews. 2002. Metalloaminopeptidases: common functional themes in disparate structural surroundings. Chem. Rev. 102:4581-4607. [DOI] [PubMed] [Google Scholar]
- 15.Mahadevan, D., and J. W. Saldanha. 1999. The extracellular regions of PSMA and the transferrin receptor contain an aminopeptidase domain: implications for drug design. Protein Sci. 8:2546-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishima, N., K. Mizumoto, Y. Iwasaki, H. Nakano, and T. Yamane. 1997. Insertion of stabilizing loci in vectors of T7 RNA polymerase-mediated Escherichia coli expression systems: a case study on the plasmids involving foreign phospholipase D gene. Biotechnol. Prog. 13:864-868. [DOI] [PubMed] [Google Scholar]
- 17.Nishiwaki, T., S. Yoshimizu, M. Furuta, and K. Hayashi. 2002. Debittering of enzymatic hydrolysates using an aminopeptidase from the edible basidiomycete Grifola frondosa. J. Biosci. Bioeng. 93:60-63. [PubMed] [Google Scholar]
- 18.Prescott, J. M., and S. H. Wilkes. 1976. Aeromonas aminopeptidase. Methods Enzymol. 45:530-543. [DOI] [PubMed] [Google Scholar]
- 19.Reiland, V., R. Gilboa, A. Spungin-Bialik, D. Schomburg, Y. Shoham, S. Blumberg, and G. Shoham. 2004. Binding of inhibitory aromatic amino acids to Streptomyces griseus aminopeptidase. Acta Crystallogr. D Biol. Crystallogr. 60:1738-1746. [DOI] [PubMed] [Google Scholar]
- 20.Sanderink, G. J., Y. Artur, and G. Siest. 1988. Human aminopeptidases: a review of the literature. J. Clin. Chem. Clin. Biochem. 26:795-807. [DOI] [PubMed] [Google Scholar]
- 21.Takagi, H., and M. Takahashi. 2003. A new approach for alteration of protease functions: pro-sequence engineering. Appl. Microbiol. Biotechnol. 63:1-9. [DOI] [PubMed] [Google Scholar]
- 22.Taylor, A. 1993. Aminopeptidases: structure and function. FASEB J. 7:290-298. [DOI] [PubMed] [Google Scholar]
- 23.Taylor, A. 1993. Aminopeptidase: towards a mechanism of action. Trends Biochem. Sci. 18:167-172. [PubMed] [Google Scholar]
- 24.Taylor, A. 1996. Aminopeptidases, p. 1-20. Landes Bioscience Publishers, Austin, Texas.
- 25.Venekei, I., L. Szilagyi, L. Graf, and W. J. Rutter. 1996. Attempts to convert chymotrypsin to trypsin. FEBS Lett. 379:143-147. [PubMed] [Google Scholar]
- 26.Wagner, F. W., S. H. Wilkes, and J. M. Prescott. 1972. Specificity of Aeromonas aminopeptidase toward amino acid amides and dipeptides. J. Biol. Chem. 247:1208-1210. [PubMed] [Google Scholar]
- 27.Walker, K. W., and R. A. Bradshaw. 1999. Yeast methionine aminopeptidase. I. Alteration of substrate specificity by site-directed mutagenesis. J. Biol. Chem. 274:13403-13409. [DOI] [PubMed] [Google Scholar]