Abstract
Pyruvate carboxylase (PYC) is an ecologically, medically, and industrially important enzyme. It is widespread in all three domains of life, the archaea, bacteria, and eukarya. PYC catalyzes ATP-dependent carboxylation of pyruvate to oxaloacetate. Detailed structure-function studies of this enzyme have been hampered due to the unavailability of a facile recombinant overexpression system. Except for the α4 enzyme from a thermophilic Bacillus species, Escherichia coli has been unsuitable for overexpression of PYCs. We show that a Pseudomonas aeruginosa strain carrying the T7 polymerase gene can serve as a host for the overexpression of Mycobacterium smegmatis α4 PYC and Pseudomonas aeruginosa α4β4 PYC under the control of the T7 promoter from a broad-host-range conjugative plasmid. Overexpression occurred both in aerobic (LB medium) and nitrate-respiring anaerobic (LB medium plus glucose and nitrate) cultures. The latter system presented a simpler option because it involved room temperature cultures in stationary screw-cap bottles. We also developed a P. aeruginosa Δpyc strain that allowed the expression of recombinant PYCs in the absence of the native enzyme. Since P. aeruginosa can be transformed genetically and lysed for cell extract preparation rather easily, our system will facilitate site-directed mutagenesis, kinetics, X-ray crystallographic, and nuclear magnetic resonance-based structure-function analysis of PYCs. During this work we also determined that, contrary to a previous report (C. K. Stover et al., Nature 406:959-964, 2000), the open reading frame (ORF) PA1400 does not encode a PYC in P. aeruginosa. The α4β4 PYC of this organism was encoded by the ORFs PA5436 and PA5435.
Pyruvate carboxylase (PYC) synthesizes oxaloacetate from pyruvate (19, 45) (Fig. 1) and serves gluconeogenic, glycerogenic, and anaplerotic roles, which are often vital for the survival of a cell (2). This ecologically, medically, and industrially important enzyme (4, 8, 19, 30, 31, 33, 36, 37) is widespread (19, 31, 33) and found in organisms belonging to all three domains of life, the archaea, bacteria, and eukarya (19, 31, 33). All archaeal and certain bacterial PYCs are the α4β4 type, and each is composed of the ∼54-kDa PYCA (biotin carboxylase subunit) and ∼65-kDa PYCB (carboxyltransferase plus biotin carboxyl carrier subunit) (19, 25, 33). Most bacterial and all eukaryotic PYCs are the α4 type (19), and in each, a 110- to 130-kDa subunit carries both the PYCA and PYCB domains (19, 25, 33, 40). Defects in PYC have been implicated in human diseases, such as lactic acidemia (19). This enzyme also plays an important role in the development of type 2 diabetes (19, 30) and is involved in the establishment of dormancy by Mycobacterium tuberculosis in human organs (22, 31). PYC determines the yield in a commercial amino acid production process (36, 37) and CO2 fixation rates in Rhizobium species (8). Consequently, PYC has been studied extensively (1, 19). However, until recently, the tools for manipulating the primary structure were not available for this important enzyme. Escherichia coli does not contain a PYC and often has been shown to be unsuitable for the overexpression of this enzyme (15, 18). There have been reports of the expression of PYC in E. coli for the purpose of metabolic engineering (10, 26, 28, 47), but in these instances the enzyme was expressed only in minor amounts. Recombinant forms of human PYC have been expressed in a human cell line (15, 18), and a homologous system for Saccharomyces cerevisiae enzyme has also been developed (2). However, these hosts do not offer the speed and ease that are characteristic of a bacterial system. Very recently, an α4-type PYC from a thermophilic Bacillus species has been overexpressed in E. coli with activity (44), and its primary structure has been manipulated (44, 50). This is a rare exception. The α4-PYC of Corynebacterium glutamicum has been overexpressed in the same organism (20, 35). However, this organism has a rather rigid cell wall (12), and consequently, extract preparation requires a harsh cell lysis method (46). Often extracts of PYC-expressing C. glutamicum cells prepared by this method do not contain significant PYC activity (46). Also, transformation of this organism is relatively inefficient (24). We report in this paper that an α4-type and an α4β4-type PYC, for which E. coli is not a suitable host, can be overexpressed recombinantly in Pseudomonas aeruginosa. During this work we showed that contrary to a previous report (42), the open reading frame (ORF) PA1400 does not encode a PYC in P. aeruginosa. We identified the genes for PYC in this organism.
FIG. 1.
Pyruvate carboxylase reaction. OAA, oxaloacetate.
MATERIALS AND METHODS
Bacterial strains, plasmids, primers, and culture conditions.
The strains, plasmids, and oligonucleotide primers used in this study are listed in Table 1. Unless mentioned otherwise, Luria-Bertani (LB) liquid medium and TYE solid medium supplemented with appropriate antibiotics (39, 48) were used for the aerobic cultivation of Escherichia coli and Pseudomonas aeruginosa strains. For selecting plasmid-bearing strains of E. coli and P. aeruginosa, respectively, ampicillin (100 μg/ml) and carbenicillin (500 μg/ml) were used. Other specialized media and selection conditions are described below in Results.
TABLE 1.
Bacterial strains, plasmids, and primers used in this study
| Bacterial strain, plasmid, or primer | Description or primer sequence | Reference(s) or primer restriction site |
|---|---|---|
| Bacterial strains | ||
| E. coli DH5α | supE44 hsdR17 recA endA1 gyrA96 thi-1 relA1 | 11 |
| E. coli SM10 | thi-1 thr leu tonA lacY supE recA::RP4-2-Tc::Mu (Kmr) | 7 |
| E. coli ALS786 | F− λ−ilvG rfb-50 rph-1 ldh::Kn | 10, 27 |
| E. coli BL21(DE3) | F−ompT gal dcm lon hsdSB (rB− mB−) λ(DE3) | 43 |
| P. aeruginosa PAO1 | Wild type | 16 |
| P. aeruginosa ADD1976 | Tcr Cbs mini-D180 T7 polymerase gene on the chromosome | 3 |
| P. aeruginosa BM301 | P. aeruginosa ADD1976 ΔpycAB::accC1/gfp | This work |
| P. aeruginosa BM302 | P. aeruginosa ADD1976 Δpa1400::accC1/gfp | This work |
| Plasmids | ||
| pEX18Ap | Vector for construction of deletion strains in P. aeruginosa | 13 |
| pEX18ApSacIminus | pEX18Ap digested with SacI, treated with T4 polymerase, and recircularized | This work |
| pPS858 | Source of aacC1 gfp cassette | 13 |
| pKBJK1 | P. aeruginosa pycAB upstream and downstream regions digested with BamHI/SacI and SacI/HindIII, respectively; cloned into pEX18ApSacIminus cut with BamHI/HindIII | This work |
| pKBJK2 | aacC1 gfp cassette excised from pPS858 with SacI and cloned into pKBJK1 digested with SacI | This work |
| pEP1400a | P. aeruginosa PA1400 upstream and downstream regions digested with HindIII/SacI and SacI/BamHI, respectively; cloned into pEX18ApSacIminus cut with BamHI/HindIII | This work |
| EP1400 | aacC1 gfp cassette excised from pPS858 with SacI and cloned into pEP1400a | This work |
| pET19b | T7-dependent expression vector for E. coli | Novagen |
| pKS27 | Expression vector for P. aeruginosa | 34 |
| pHL31 | NdeI/BamHI-cut P. aeruginosa pycAB cloned into NdeI/BamHI-digested pET19b | This work |
| pHL36 | P. aeruginosa pycAB excised from pHL31 with XbaI/BamHI and cloned into XbaI/BamHI-digested pKS27 | This work |
| pHL51 | NdeI/XhoI-cut M. smegmatis pyc cloned into NdeI/XhoI-digested pET19b | This work |
| pHL56 | M. smegmatis pyc excised from pHL51 with XbaI/XhoI and cloned into XbaI/XhoI-digested pKS27 | This work |
| pTrc99A-pyc | bla lacIq trc ColE1 R. etli pyc | 10 |
| Primersa | ||
| PaPYCABKO/UF | GCTCGCGGATCCAGCCAGTCGGCCATGGGAGACGCGAG | BamHI |
| PaPYCABKO/UR | CACGGAGCTCTCCCTCTTCGGGGTTGGGAACCTGGTACGGTCG | SacI |
| PaPYCABKO/DF | GGCGAGCTCCTGATCGAGATGACTGACTGACCGCGACAGACGGTTAAAGTCCCTG | SacI, three stop codons in three frames |
| PaPYCABKO/DR | CGCCAAGCTTCTGGGCTTCACCTACGAAGGCACCTTCC | HindIII |
| PaPYC/UF | GGCTGCAAGCTTCGTCGTCGGCCTGGTGCGACCAGTAGAGATTC | HindIII |
| PaPYC/UR | CACGGCAGGTTCTCCGGGGAGCTCGTCAGGCGGCCGGCGAAAG | SacI |
| PaPYC/DF | CGTTCGAGCTCACTAGGTAGATGATCGGCCCGCAGTGCCCTGGCGCCTCAGGACG | SacI, one stop codon |
| PaPYC/DR | CGCTCAAACATTCCCGGAGGGATCCATGAATACCC | BamHI |
| PaPYC-VP/1F | CTGGAACAGGTCGGTGCTGGTCTTCAGCAGGGCTTC | |
| PaPYC-VP/2R | CAGCAGCATGCAGAACCAGGTGAGCAGGAAC | |
| PaPYCAB1F | GAGGGAGACCCATATGATCAA GAAGATCCTGATCG | NdeI |
| PaPYCAB2R | CCCAGGGACTTTAACCGTGGATCCCGGTCAGCCCGC | BamHI |
| MsmPYC1F | GGAAATAGACACAGCGGTTAGGGTTACATATGATCTCC | NdeI |
| MsmPYC2R | GCCCGCGATGACTCGAGTCAGCTGACCACCACCAGCA | XhoI |
Restriction sites and stop codons are shown in bold type and underlined.
DNA methods.
Generally, all DNA manipulations were performed by standard methods (39). Chromosomal DNA from Mycobacterium smegmatis mc2155 was isolated as described previously (31). P. aeruginosa chromosomal DNA was purified according to a method that has been described for E. coli (39). Each PCR was carried out by using the PfuTurbo DNA polymerase from Stratagene (La Jolla, CA). The DNA sequence for a clone derived via PCR was verified by determining the sequences for both strands. E. coli DH5α (11) was used for the construction of all vectors. The DNA hybridization experiments were performed according to the manufacturer's protocol (Roche, Mannheim, Germany). Positively charged nylon membranes (MagnaProbe nylon membrane; Fisher Scientific, Pittsburgh, PA) were used for the transfers. The blotted DNA samples were probed with digoxigenin (DIG)-labeled probes. These probes were generated by random priming of the appropriate DNA fragments with the High-Prime-DIG labeling kit (Roche). The temperatures for prehybridization, hybridization, and posthybridization washes were as indicated (see the legends to Fig. 2B and 3B). The hybridizing bands were detected by using alkaline phosphatase-conjugated anti-DIG antibody and the color substrates nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate.
FIG. 2.
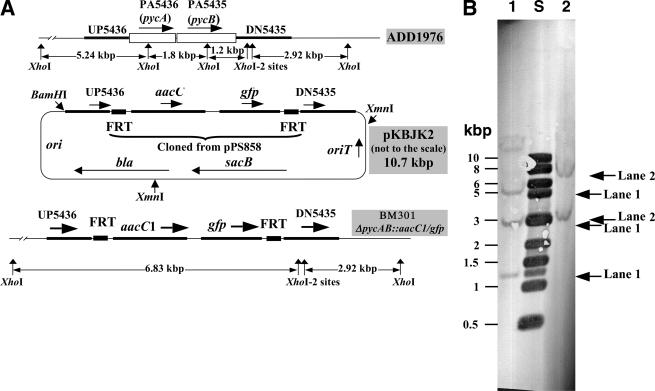
Construction of P. aeruginosa ΔpycAB::aacC1/gfp. (A) Schematic representations of the wild-type (ADD1976) chromosome, a suicide vector (pKBJK2; based on pEX18Ap and carries a 1.36-kbp region upstream of PA5436 [UP5436], a 1.69-kbp region downstream of PA5435 [DN5435], and a 1.8-kbp aacC1/gfp cassette flanked with Flp recombinase target site [FRT]), and the chromosome of BM301 (Δpa1400::aacC1/gfp derivative of strain ADD1976). The schematic representations are not drawn to scale. aacC1, gentamicin resistance gene; gfp, green fluorescent protein gene; ori, pMB1-based origin of DNA replication; oriT, origin for conjugation-mediated plasmid transfer; sacB, Bacillus subtilis levansucrase-encoding gene; bla, β-lactamase gene; pycA and pycB, genes for the PYC subunits. (B) Verification of the P. aeruginosa BM301 genotype via DNA hybridization. The temperatures for prehybridization, hybridization, and posthybridization washes were 37, 47, and 65°C, respectively. The arrows point to the hybridizing bands in the indicated lanes. Lane S, molecular mass standards. XhoI-digested chromosomal DNA from strain ADD1976 (wild type) (lane 1) and BM301 (lane 2) were analyzed. The probe was DIG-labeled XmnI- and BamHI-digested pKBJK2 (a mixture of three DNA fragments) (see Fig. 2A).
FIG. 3.
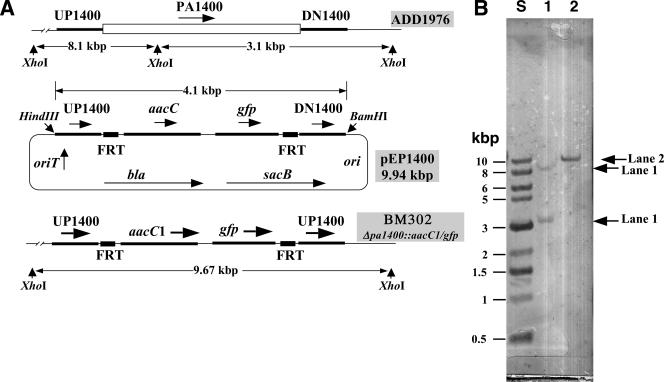
Construction of the P. aeruginosa BM302 Δpa1400::aacC1/gfp strain. (A) Schematic representations of the wild-type (ADD1976) chromosome, a suicide vector (pEP1400; based on pEX18Ap and carries a 1.08-kbp region upstream of PA1400 [UP1400] and 1.22-kbp region downstream of PA1400 [DN1400]), and the chromosome of BM302 (Δpa1400::aacC1/gfp derivative of strain ADD1976). Other details are given in the legend to Fig. 2A. The schematic representations are not drawn to scale. (B) Verification of P. aeruginosa BM302 genotype via DNA hybridization. The temperatures for prehybridization, hybridization, and posthybridization washes were 37, 50, and 65°C, respectively. The arrows point to the hybridizing bands. Lane S, molecular mass standards. XhoI-digested chromosomal DNA of strain ADD1976 (wild type) (lane 1) and BM302 (lane 2) were analyzed with the 4.1-kbp BamHI-HindIII fragment of pEP1400 as the insert probe (see panel A for the schematic representation). The vector probe did not hybridize to either the ADD1976 or BM302 chromosomal DNA (not shown).
Construction of a pyruvate carboxylase deletion strain of Pseudomonas aeruginosa.
The pyruvate carboxylase deletion strain of Pseudomonas aeruginosa was obtained by deleting the pycAB (PA5436 and PA5435) coding sequence from P. aeruginosa ADD1976 (3) by use of a sacB-based counterselection method (13). The pEX18Ap plasmid was used for the construction of the suicide vector (13). The SacI site of this plasmid was removed by digesting it with SacI and then treating the digested DNA with T4 polymerase and deoxynucleoside triphosphates. Ligation of the resulting blunt end product provided the plasmid pEX18ApSacIminus. The upstream region of the pycAB coding sequence (UP5436; Fig. 2A) was amplified from P. aeruginosa PAO1 chromosomal DNA by use of the PaPYCABKO/UF and PaPYCABKO/UR primers (Table 1). Similarly, the pycAB downstream region (DN5435; Fig. 2A) was amplified by use of the PaPYCABKO/DF and PaPYCABKO/DR primers (Table 1). The BamHI- and SacI-digested UP5436 element, the SacI- and HindIII-digested DN5435 element, the 1.8-kbp SacI fragment of plasmid pPS858 carrying an aacC1/gfp cassette (13), and BamHI- and HindIII-digested pEX18ApSacIminus were ligated to obtain the suicide plasmid pKBJK2 (Fig. 2A). The suicide plasmid was delivered into P. aeruginosa ADD1976 via conjugation from E. coli SM10 on LB agar (7, 13, 29), and from the mating mixture the merodiploid exconjugants were selected on Vogel-Bonner minimal medium (13) containing citrate (0.3%), gentamicin (150 μg/ml), and carbenicillin (500 μg/ml). One of the merodiploid strains was propagated in LB medium without any selection. An aliquot of this culture was plated on LB agar containing 5% sucrose and 150 μg/ml gentamicin (a counterselection medium [13]) to obtain the strain P. aeruginosa BM301 ΔpycAB::aacC1/gfp (Fig. 2A).
Deletion of ORF PA1400 from the P. aeruginosa chromosome.
The method of deleting the ORF PA1400 from the P. aeruginosa chromosome was the same as that described above for the pyruvate carboxylase genes. The upstream and downstream regions of PA1400 (Fig. 3A), amplified by use of the primer pairs Pa1400KO/UF and Pa1400KO/UR and Pa1400KO/DF and Pa1400KO/DR, respectively (Table 1), were used for the construction of the suicide vector pEP1400 (Fig. 3A). This vector was used to construct P. aeruginosa BM302 Δpa1400::aacC1/gfp (Fig. 3A).
Generation of overexpression constructs for P. aeruginosa α4β4-type pyruvate carboxylase.
The pycAB coding sequence from the P. aeruginosa chromosome was PCR amplified by use of the primers PaPYCAB1F and PaPYCAB2R (Table 1) and cloned into the NdeI and BamHI sites of pET19b (Novagen, Inc., Madison, WI) to obtain the pHL36 plasmid. In pHL36, the pycAB coding sequence was fused in frame to the polyhistidine sequence of the vector; the pycA-pycB intergenic region in the clone was the same as that present on the P. aeruginosa chromosome. pHL36 was used for the expression studies in E. coli.
For the expression of the recombinant enzyme in P. aeruginosa, a construct based on pKS27 (34) was developed. pKS27 is a broad-host-range vector that can be stably maintained in both E. coli and P. aeruginosa (34, 49). This vector contains the T7 promoter, and this element is followed by the multicloning site (a XbaI and BspEI fragment) that was derived from pET27b(+) (Novagen) (34). The 3.2-kb XbaI-BamHI fragment from pHL36 carrying the pycAB coding sequence, the in-frame 5′ polyhistidine-coding sequence, and a ribosome binding site was cloned into the XbaI- and BamHI-digested pKS27 to obtain pHL53. In pHL53, the cloned fragment was placed under the control of the vector-borne T7 promoter.
The pycAB constructs in pHL36 and pHL53 were expected to coexpress PYCA and PYCB polypeptides and to generate an α4β4 enzyme where the PYCA subunit would be in a His10-tagged form.
Generation of overexpression constructs for Mycobacterium smegmatis α4-type pyruvate carboxylase.
The method of generating overexpression constructs for Mycobacterium smegmatis α4-type pyruvate carboxylase was similar to that described for P. aeruginosa PYC. M. smegmatis PYC is a homotetramer, and the 121-kDa subunits are encoded by the pyc gene (31). By use of the primers MsmPYCB1F and MsmPYCB2R (Table 1), the pyc coding sequence was amplified from M. smegmatis chromosomal DNA and cloned into NdeI- and XhoI-digested pET19b to generate pHL31. Subcloning of the 3.4-kbp XbaI-XhoI fragment from pHL31 carrying the pyc gene, the in-frame 5′ polyhistidine-encoding sequence, and a ribosome binding site into pKS27 (34) provided pHL51. pHL31 and pHL51 were used for the expression of His10-tagged PYC protein in E. coli and P. aeruginosa, respectively.
Overexpression of recombinant pyruvate carboxylases in E. coli and P. aeruginosa under aerobic conditions.
E. coli BL21(DE3)(pHL31), E. coli BL21(DE3)(pHL36), and E. coli ALS786(pTrc99A-pyc) were grown under vigorous agitation in LB medium supplemented with ampicillin (100 μg/ml). When the optical density at 600 nm of the culture reached approximately 0.5 (as measured using a Lambda model 25 UV and visible spectrometer; Perkin-Elmer Instruments, Shelton, Connecticut), PYC expression was induced by the addition of isopropyl-1-thio-β-d-galactopyranoside (IPTG) to a final concentration of 1 mM. Cultivation was continued for an additional 3 h. Then the cells were harvested by centrifugation at 10,000 × g at 4°C and stored at −20°C. Unless mentioned otherwise, the same method was used for expression of PYCs in P. aeruginosa BM301(pHL51) and P. aeruginosa BM301(pHL53), except that carbenicillin (250 μg/ml) replaced ampicillin.
Overexpression of recombinant pyruvate carboxylases in P. aeruginosa BM301 under anaerobic conditions.
The medium used to overexpress recombinant pyruvate carboxylases in P. aeruginosa BM301 under anaerobic conditions contained 1% tryptone, 0.5% yeast extract, 1% KNO3, and 0.5% glucose. Glucose was added to sterile and cooled medium from a sterile stock. The complete sterile medium was distributed into sterile 1,000-ml screw-cap Pyrex storage bottles with polypropylene caps (catalog number 1395-1L, Corning Inc., Acton, MA) up to a level of 1,000 ml and was inoculated with 1 ml of overnight culture of the desired P. aeruginosa strain. Then the bottles were closed with loosely fitted plastic caps and incubated at room temperature (∼25°C). For certain experiments, the medium was supplemented with carbenicillin (250 μg/ml) before inoculation, and the cultures were induced with IPTG as described above.
Purification of recombinant P. aeruginosa α4β4-type pyruvate carboxylase.
Frozen P. aeruginosa BM301(pHL53) cells (0.7 g) containing overexpressed PYC protein were resuspended in an equal volume of 100 mM potassium phosphate buffer, pH 8. The cells in the suspension were disrupted by four passages through a French pressure cell at 1,360 atm. The broken cell slurry was centrifuged at 20,000 × g for 60 min at 4°C. The resulting supernatant was recentrifuged at 100,000 × g for 60 min at 4°C. The supernatant from this last centrifugation step was diluted with an equal volume of a solution containing 200 mM NaCl and 20 mM imidazole. This diluted supernatant was loaded onto a column of 2 ml Ni-nitrilotriacetic acid (Ni-NTA) Superflow agarose (QIAGEN Inc., Valencia, CA) that was preequilibrated with an equilibration buffer containing 50 mM potassium phosphate buffer, pH 7, 100 mM NaCl, and 10 mM imidazole. Then, the column was washed with 10 ml of equilibration buffer to remove unbound material. Finally, the bound proteins were eluted with 5 ml each of the 50, 100, 150, 200, 300, 400, and 500 mM imidazole solutions, which also contained 50 mM potassium phosphate buffer, pH 7, and 100 mM NaCl. PYC eluted at imidazole concentrations of 150 to 500 mM. The fractions at 200 and 300 mM imidazole contained most of the eluted PYC protein, and these were pooled and stored at 4°C. At each step, liquid was applied to the column under a gravity flow.
Purification of recombinant M. smegmatis α4-type pyruvate carboxylase.
The recombinant M. smegmatis α4-type pyruvate carboxylase was purified to homogeneity through two successive chromatography steps. Fifteen grams of frozen P. aeruginosa BM301(pHL51) cells containing overexpressed recombinant PYC were resuspended in 15 ml of 100 mM HEPES-KOH buffer, pH 8, 600 mM KCl, and 20 mM imidazole. The cell suspension was diluted to a final volume of 60 ml with buffer B (50 mM HEPES-KOH buffer, pH 8, 300 mM KCl, and 10 mM imidazole). Then the cells were disrupted by three passages through a French pressure cell at 1,360 atm, and the broken cell slurry was clarified through two centrifugation steps, one at 35,000 × g for 30 min at 4°C and the other at 100,000 × g for 60 min at 4°C. The resulting supernatant was fractionated on a 5 ml Ni-NTA agarose column that was preequilibrated with buffer B as follows. The cell extract supernatant was applied to the column. The column was washed with 50 ml of buffer B and 10 ml each of 0.5 M, 1 M, and 2 M KCl prepared in the same buffer. The KCl concentration in the column was lowered by washing it with 15 ml buffer B. Then the column was eluted in steps with 10 ml each of 50, 100, 150, 200, and 250 mM imidazole in 50 mM HEPES-KOH buffer, pH 8, containing 300 mM KCl. The 100, 150, and 200 mM imidazole fractions contained PYC activity and were pooled. The enzyme in this pool was concentrated on a Centricon 30 filter (30-kDa cutoff; Amicon, Beverly, MA). The retentate was placed in buffer A (50 mM Tris-HCl buffer, pH 8, 1 M KCl, 5 mM MgCl2, 2 mM dithiothreitol) by dilution with this buffer and further filtration. The final retentate was applied to a 15-ml avidin-Sepharose column that was preequilibrated with 150 ml buffer A. The column was washed with the following: 25 ml of buffer A without KCl (low-salt buffer), 165 ml buffer A, and 115 ml low-salt buffer. The bound enzyme was eluted with 75 ml of 1 mM d-biotin in low-salt buffer.
Gel electrophoresis and pyruvate carboxylase assay.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with cell lysates and purified proteins were performed by the method of Laemmli (21). The gels were stained with Coomassie blue R-250. Pyruvate carboxylase assays were conducted as described previously (33), but with some modifications. The assay mixture contained 50 mM HEPES-NaOH buffer, pH 8, 8 mM MgCl2, 20 mM KHCO3, 8 mM ATP, 20 mM pyruvate, 0.2 mM NADH, 50 μM acetyl coenzyme A (acetyl-CoA) (for the M. smegmatis and Rhizobium etli enzymes), and 2 U malate dehydrogenase from Thermus flavus per ml. The assay was conducted at a temperature of 37°C.
RESULTS AND DISCUSSION
Identification of the pyruvate carboxylase genes in Pseudomonas aeruginosa PAO1 genome.
Pseudomonas aeruginosa possesses an α4β4-type PYC (38, 41). Accordingly, it is expected to carry a pycA gene (biotin carboxylase) and a pycB gene (carboxyltransferase plus biotin carrier) (33). However, the annotation of the P. aeruginosa genome sequence did not document the presence of these genes in this organism (42). Rather it suggested that the ORF PA1400 encodes a putative 117-kDa PYC polypeptide, which would indicate the presence of an α4-type PYC in this organism (42). On the basis of the available information on PYC structures (19, 25, 33, 40), we believed that PA1400 did not encode a PYC. This ORF showed the following domain arrangement: NH2 terminus-biotin carboxylase-biotin carrier-carboxyltransferase-COO− terminus. The corresponding pattern for an α4-PYC is NH2 terminus-biotin carboxylase-carboxyltransferase-biotin carrier-COO− terminus (25, 33). Therefore, we examined the genome sequence further. We found that the ORFs PA5436 and PA5435, which are separated by 15 bp and form an operon-like arrangement, had the potential of encoding the PYCA and PYCB polypeptides, respectively. In the database, these ORFs have been annotated as a biotin carboxylase and oxaloacetate decarboxylase α-subunit, respectively (42).
To test our hypotheses, we carried out deletion mutagenesis. For the reasons stated in the following section, we chose to work with P. aeruginosa ADD1976 (3), a derivative of P. aeruginosa PAO1 (16). The removal of the PA5436 and PA5435 coding sequences and the intergenic region from the chromosome of ADD1976 provided strain BM301 (Fig. 2A). Similarly, BM302, a strain lacking PA1400, was obtained (Fig. 3A). The mutant genotypes were confirmed via DNA hybridization or Southern analysis (Fig. 2B and 3B); the vector backbone did not integrate into the chromosomes of these strains. The PYC specific activities in the cell extracts of strains ADD1976, BM301, and BM302 were 0.025, 0.001, and 0.024 μmol/min/mg of protein unit, respectively. Therefore, deletion of PA1400 did not eliminate the PYC activity in the cell, whereas a strain lacking PA5436 and PA5435 was devoid of this activity. From these results, we concluded that in P. aeruginosa, PA5436 and PA5435 encoded the PYCA and PYCB subunits of an α4β4-type PYC, respectively, and PA1400 did not represent a PYC. The data presented in the following section provide further support for this conclusion.
Overexpression trials for P. aeruginosa, M. smegmatis, and Rhizobium etli PYCs in E. coli.
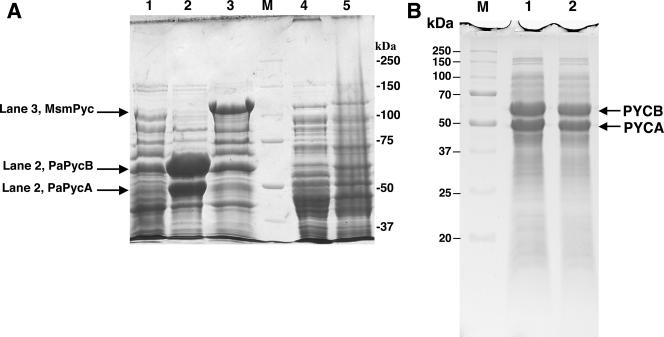
For P. aeruginosa and M. smegmatis enzymes, we used E. coli BL21(DE3) as the host, and the overexpression constructs were based on pET19b (43). This combination allows expression of a cloned gene from the highly active T7 promoter. We also wanted to test whether one of the previously described E. coli-based PYC expression systems (10, 26, 28, 47), which were used for physiological studies, overproduces a PYC. For this purpose, we chose the pTrc99A-pyc plasmid, an overexpression vector for R. etli PYC and E. coli ALS786 (Table 1) (10). SDS-PAGE analyses of cell lysates showed that E. coli BL21(DE3) did not overproduce M. smegmatis PYC from pHL31 or P. aeruginosa PYC from pHL36 upon IPTG induction (data not shown). A similar observation was made for the expression of R. etli PYCs in E. coli ALS786(pTrc99A-pyc) (Fig. 4A, lane 5).
FIG. 4.
SDS-PAGE analysis for the expression of pyruvate carboxylase in E. coli and Pseudomonas aeruginosa. SDS-PAGE was performed with a 7.5% polyacrylamide gel. Cells that were induced with IPTG were examined. Each cell extract supernatant was prepared by passing a cell suspension in 100 mM HEPES buffer, pH 8, containing 10 mM MgCl2 through a French pressure cell at 1,360 atm and centrifuging the resulting lysate at 20,000 × g for 1 h. Lane M, molecular mass standards. The molecular masses (in kilodaltons) for the standards are shown to the right (A) or left (B) of the gel. (A) Cells analyzed (amount of cell extract protein) are as follows: lane 1, P. aeruginosa BM301 (control) (82 μg); lane 2, P. aeruginosa BM301(pHL53) expressing P. aeruginosa α4β4-PYC with 52 kDa for PYCA and 66 kDa for PYCB subunits (79 μg); lane 3, P. aeruginosa BM301(pHL51) expressing M. smegmatis α4-PYC with 121-kDa subunits (70 μg); lane 4, E. coli ALS786 (80 μg); lane 5, E. coli ALS786(pTrc99A-pyc) (81 μg). MSMPyc, M. smegmatis Pyc; PaPycB and PaPycA, P. aeruginosa PycB and PycA, respectively. (B) Cell lysates of P. aeruginosa BM301(pHL53) grown anaerobically with (lane 1, 60 μg protein) and without (lane 2, 60 μg protein) carbenicillin selection. Addition of IPTG did not improve the PYC expression level (data not shown). Each cell lysate was prepared as follows: 100 μl culture with an optical density at 600 nm of 2 units was centrifuged at 14,000 × g for 2 min, and the resulting pellet was resuspended in 100 μl of 25 mM potassium phosphate buffer, pH 7.2. Then the suspension was mixed with an equal volume of a denaturing solution (60 mM Tris-HCl buffer, pH 6.8, 2% SDS, 5% β-mercaptoethanol, 10% glycerol [vol/vol], and 0.01% bromophenol blue) and boiled for 10 min. This denatured and solubilized cell lysate was then analyzed.
Overexpression of P. aeruginosa and M. smegmatis PYCs in P. aeruginosa.
We chose P. aeruginosa as the expression host for the following reasons. It possesses a PYC and therefore was more likely to allow the expression of a PYC to a high cellular level. It is genetically tractable. Also, the strains of P. aeruginosa that carry T7 polymerase on the chromosome and allow expression of cloned genes from the highly active T7 promoter are available (3, 14). As shown below, this system allowed us to overexpress recombinant PYCs.
P. aeruginosa ADD1976 was the starting point for the construction of our expression host. This strain carries an inducible T7 polymerase gene on the chromosome (49). We wanted to ensure that the recombinant enzyme could be purified to homogeneity without a contamination of the host enzyme. This is especially important if the enzyme has to be produced without an engineered affinity tag. For this reason, the pyruvate carboxylase genes from the chromosome of P. aeruginosa ADD1976 were deleted (see previous section). The resulting strain (BM301 ΔpycAB::acc1/gfp) was used as the expression host (Table 1 and Fig. 2). The expression vectors were based on pKS27, a broad-host-range plasmid that replicates both in E. coli and P. aeruginosa and carries the T7 promoter (34, 49). In each case, the pyc coding sequence, along with a His10 sequence and a ribosome binding site, was cloned from the corresponding pET19-based construct (pHL31 or pHL36) into pKS27. In the resulting plasmids, pHL51 and pHL53, the pyc genes were placed under the control of the vector-borne T7 promoter. The data in Fig. 4A show that from pHL51 and pHL53, respectively, M. smegmatis and P. aeruginosa PYCs were successfully overexpressed in strain BM301 (lanes 3 and 2). We also determined the PYC activities in these cell extracts and that of E. coli ALS786(pTrc99A-pyc) and found the following values (μmol of oxaloacetate formed/mg of protein/min): P. aeruginosa BM301, 0.001; P. aeruginosa BM301(pHL51), 0.349; P. aeruginosa BM301(pHL53), 0.778; E. coli ALS786, 0; and E. coli ALS786(pTrc99A-pyc), 0.033. Therefore, a T7 promoter-based expression vector with a P. aeruginosa Δpyc strain (BM301) as the expression host provided a much higher expression level of a PYC protein than a E. coli strain can provide. The PYCs were found in the supernatant fraction of the cell extracts (Fig. 4A), indicating that these proteins were expressed in soluble forms. In P. aeruginosa BM301, both M. smegmatis and P. aeruginosa PYCs were overexpressed even in the absence of carbenicillin selection and IPTG induction P. aeruginosa (data not shown). It was also possible to overproduce P. aeruginosa α4β4-PYC in P. aeruginosa BM301(pHL53) when the culture was raised anaerobically with nitrate as the electron acceptor and with or without IPTG induction and carbenicillin selection; some of these results are shown in Fig. 4B. The latter system was the simplest and least expensive option, for it did not require a shaker or a shaking flask, only a screw-cap storage bottle. Also, it did not involve the use of IPTG or carbenicillin, which are expensive. M. smegmatis PYC could not be overexpressed in an anaerobic culture of P. aeruginosa BM301(pHL51); the reason for this observation is unknown.
Purification of recombinant P. aeruginosa and M. smegmatis PYCs.
Recombinant P. aeruginosa α4β4-type enzyme was purified to homogeneity from clarified extract of aerobically grown P. aeruginosa BM301(pHL53) cells via Ni-NTA chromatography (Fig. 5A). The purified enzyme had a specific activity of 5.7 μmol/min/mg. The yield was 4.5 mg PYC protein per g (wet weight) of cell pellet. M. smegmatis PYC was purified to homogeneity from IPTG-induced P. aeruginosa BM301(pHL51) cells via a two-step method, where a fractionation over a Ni-NTA agarose column preceded avidin-Sepharose chromatography (Fig. 5B); the specific activity of the purified enzyme was 19.43 μmol/min/mg, and the yield was 1.5 mg PYC protein per g (wet weight) of cell pellet. A homogeneous preparation of recombinant P. aeruginosa PYC was also obtained from anaerobically grown P. aeruginosa BM301(pHL53) cells (data not shown). Recombinant M. smegmatis PYC was absolutely dependent on acetyl-CoA for activity, and P. aeruginosa enzyme was fully active without this effector. This observation was consistent with those described for these enzymes isolated from the original host (6, 32, 38).
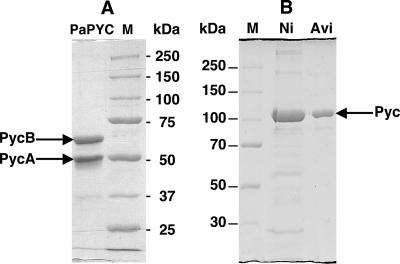
FIG. 5.
SDS-PAGE analysis of purified pyruvate carboxylase preparations. Gels with 7.5% polyacrylamide concentration were used. Lanes M, molecular mass standards. The molecular masses (in kilodaltons) for the standards are shown to the right (A) or left (B) of each gel. (A) Ni-NTA chromatography-purified recombinant P. aeruginosa α4β4 PYC from P. aeruginosa BM301(pHL53) (2.3 μg protein). PaPYC, P. aeruginosa PYC. (B) Purified recombinant M. smegmatis α4-PYC from P. aeruginosa BM301(pHL51). Lanes: Ni, enzyme from Ni-NTA chromatography (10 μg protein); Avi, enzyme from avidin-Sepharose chromatography (3 μg protein).
Lack of PYC overexpression in E. coli.
The reason for a lack of overexpression of a PYC in E. coli is currently unknown. On the basis of the available information, one could speculate that the expression of PYC in E. coli is regulated via the acetyl-CoA carboxylase (Acc) system. Acc, composed of AccA, AccB, AccC, and AccD polypeptides, is an essential enzyme for membrane lipid synthesis in E. coli (9). Structurally, PYC and E. coli Acc are highly homologous, and both have the same functional modules (33). The N-terminal part of the PYCB domain of an α4-PYC or the PYCB subunit of an α4β4-PYC acts as a carboxyl transferase, and in E. coli Acc, this function is provided by the AccA and AccD subunits (33). The C-terminal part of the PYCB domain or subunit is homologous to the biotin carboxyl carrier protein of AccB (33). Finally, the PYCA domain or subunit of a PYC is equivalent to the biotin carboxylase or the AccC of E. coli (33). It has been shown that in E. coli, AccB autoregulates the transcription of the accBC operon (17). The result is that the presence of 50 or more copies of accBC operon provides only a two- to threefold increase in the cellular levels of AccB and AccC (23). It is possible that the expression of a PYC from the T7 promoter tends to interfere with the E. coli Acc synthesis, and via an unknown mechanism, the cell counters this negative effect by affecting the expression of the cloned gene.
Conclusion.
We identified the ORFs (PA5436 and PA5435) that encode the P. aeruginosa PYC. We showed that contrary to a previous report (42), the ORF PA1400 does not encode a PYC. P. aeruginosa was found to be a suitable host for the overexpression of an α4- and an α4β4-PYC that could not be overproduced in E. coli. Therefore, plasmids pHL51 and pHL53 will allow easy manipulation of the structures of two types of PYCs, and the use of P. aeruginosa BM301 ΔpycAB::aacC1/gfp will ensure that the purified recombinant enzymes will be free of the host enzyme. P. aeruginosa is readily transformed with replicable plasmid DNA generated in E. coli either via electrotransformation (5) or conjugation with E. coli (13). The organism is also easily lysed by use of a French pressure cell or sonicator, which allows facile preparation of cell extracts. Therefore, the system described in this report will facilitate site-directed mutagenesis, kinetics, X-ray crystallographic, and nuclear magnetic resonance-based structure-function analysis of an important enzyme.
Acknowledgments
We thank Kristin L. Boswell for assistance in the construction of pEX18ApSacIminus. We thank Herbert P. Schweizer for the gifts of pEX18Ap, pPS858, and E. coli SM10; Elliot Altman for E. coli ALS786 and pTrc99A-pyc; and Hiten Patel for pKS27 and P. aeruginosa ADD1976. We thank an anonymous reviewer for suggesting that we test whether a previously published E. coli strain that carries a pyc gene on a plasmid and has been used in physiological studies overexpresses PYC.
This work was supported by start-up funds to B.M. from the Virginia Bioinformatics Institute (VBI) and the Institute for Biomedical and Public Health Sciences (IBPHS) of the Virginia Polytechnic Institute and State University.
Footnotes
Published ahead of print on 22 September 2006.
REFERENCES
- 1.Attwood, P. V., and M. A. Geeves. 2002. Changes in catalytic activity and association state of pyruvate carboxylase which are dependent on enzyme concentration. Arch. Biochem. Biophys. 401:63-72. [DOI] [PubMed] [Google Scholar]
- 2.Branson, J. P., M. Nezic, J. C. Wallace, and P. V. Attwood. 2002. Kinetic characterization of yeast pyruvate carboxylase isozyme pyc1. Biochemistry 41:4459-4466. [DOI] [PubMed] [Google Scholar]
- 3.Brunschwig, E., and A. Darzins. 1992. A two-component T7 system for the overexpression of genes in Pseudomonas aeruginosa. Gene 111:35-41. [DOI] [PubMed] [Google Scholar]
- 4.Bushell, M. E., and A. T. Bull. 1981. Anaplerotic metabolism of Aspergillus nidulans and its effect on biomass synthesis in carbon limited chemostats. Arch. Microbiol. 128:282-287. [DOI] [PubMed] [Google Scholar]
- 5.Choi, K. H., A. Kumar, and H. P. Schweizer. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J. Microbiol. Methods 64:391-397. [DOI] [PubMed] [Google Scholar]
- 6.Cohen, N. D., J. A. Duc, H. Beegen, and M. F. Utter. 1979. Quaternary structure of pyruvate carboxylase from Pseudomonas citronellolis. J. Biol. Chem. 254:9262-9269. [PubMed] [Google Scholar]
- 7.de Lorenzo, V., and K. N. Timmis. 1994. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 235:386-405. [DOI] [PubMed] [Google Scholar]
- 8.Dunn, M. F., G. Araiza, M. A. Cevallos, and J. Mora. 1997. Regulation of pyruvate carboxylase in Rhizobium etli. FEMS Microbiol. Lett. 157:301-306. [DOI] [PubMed] [Google Scholar]
- 9.Fall, R. R., A. M. Nervi, A. W. Alberts, and P. R. Vagelos. 1971. Acetyl CoA carboxylase: isolation and characterization of native biotin carboxyl carrier protein. Proc. Natl. Acad. Sci. USA 68:1512-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gokarn, R. R., J. D. Evans, J. R. Walker, S. A. Martin, M. A. Eiteman, and E. Altman. 2001. The physiological effects and metabolic alterations caused by the expression of Rhizobium etli pyruvate carboxylase in Escherichia coli. Appl. Microbiol. Biotechnol. 56:188-195. [DOI] [PubMed] [Google Scholar]
- 11.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 12.Hirasawa, T., M. Wachi, and K. Nagai. 2000. A mutation in the Corynebacterium glutamicum ltsA gene causes susceptibility to lysozyme, temperature-sensitive growth, and l-glutamate production. J. Bacteriol. 182:2696-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 14.Hoang, T. T., A. J. Kutchma, A. Becher, and H. P. Schweizer. 2000. Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid 43:59-72. [DOI] [PubMed] [Google Scholar]
- 15.Hobbs, S., S. Jitrapakdee, and J. C. Wallace. 1998. Development of a bicistronic vector driven by the human polypeptide chain elongation factor 1alpha promoter for creation of stable mammalian cell lines that express very high levels of recombinant proteins. Biochem. Biophys. Res. Commun. 252:368-372. [DOI] [PubMed] [Google Scholar]
- 16.Holloway, B. W. 1955. Genetic recombination in Pseudomonas aeruginosa. J. Gen. Microbiol. 13:572-581. [DOI] [PubMed] [Google Scholar]
- 17.James, E. S., and J. E. Cronan. 2004. Expression of two Escherichia coli acetyl-CoA carboxylase subunits is autoregulated. J. Biol. Chem. 279:2520-2527. [DOI] [PubMed] [Google Scholar]
- 18.Jitrapakdee, S., M. E. Walker, and J. C. Wallace. 1999. Functional expression, purification, and characterization of recombinant human pyruvate carboxylase. Biochem. Biophys. Res. Commun. 266:512-517. [DOI] [PubMed] [Google Scholar]
- 19.Jitrapakdee, S., and J. C. Wallace. 1999. Structure, function and regulation of pyruvate carboxylase. Biochem. J. 340:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koffas, M. A., G. Y. Jung, J. C. Aon, and G. Stephanopoulos. 2002. Effect of pyruvate carboxylase overexpression on the physiology of Corynebacterium glutamicum. Appl. Environ. Microbiol. 68:5422-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 22.Lamichhane, G., M. Zignol, N. J. Blades, D. E. Geiman, A. Dougherty, J. Grosset, K. W. Broman, and W. R. Bishai. 2003. A postgenomic method for predicting essential genes at subsaturation levels of mutagenesis: application to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 100:7213-7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, S. J., and J. E. Cronan, Jr. 1993. Growth rate regulation of Escherichia coli acetyl coenzyme A carboxylase, which catalyzes the first committed step of lipid biosynthesis. J. Bacteriol. 175:332-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liebl, W., A. Bayerl, B. Schein, U. Stillner, and K. H. Schleifer. 1989. High efficiency electroporation of intact Corynebacterium glutamicum cells. FEMS Microbiol. Lett. 53:299-303. [DOI] [PubMed] [Google Scholar]
- 25.Lim, F., C. P. Morris, F. Occhiodoro, and J. C. Wallace. 1988. Sequence and domain structure of yeast pyruvate carboxylase. J. Biol. Chem. 263:11493-11497. [PubMed] [Google Scholar]
- 26.Lin, H., K. Y. San, and G. N. Bennett. 2005. Effect of Sorghum vulgare phosphoenolpyruvate carboxylase and Lactococcus lactis pyruvate carboxylase coexpression on succinate production in mutant strains of Escherichia coli. Appl. Microbiol. Biotechnol. 67:515-523. [DOI] [PubMed] [Google Scholar]
- 27.Liu, D., and P. R. Reeves. 1994. Escherichia coli K12 regains its O antigen. Microbiology 140:49-57. [DOI] [PubMed] [Google Scholar]
- 28.March, J. C., M. A. Eiteman, and E. Altman. 2002. Expression of an anaplerotic enzyme, pyruvate carboxylase, improves recombinant protein production in Escherichia coli. Appl. Environ. Microbiol. 68:5620-5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metcalf, W. W., and R. S. Wolfe. 1998. Molecular genetic analysis of phosphite and hypophosphite oxidation by Pseudomonas stutzeri WM88. J. Bacteriol. 180:5547-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moller, D. E. 2001. New drug targets for type 2 diabetes and the metabolic syndrome. Nature 414:821-827. [DOI] [PubMed] [Google Scholar]
- 31.Mukhopadhyay, B., E. M. Concar, and R. S. Wolfe. 2001. A GTP-dependent vertebrate-type phosphoenolpyruvate carboxykinase from Mycobacterium smegmatis. J. Biol. Chem. 276:16137-16145. [DOI] [PubMed] [Google Scholar]
- 32.Mukhopadhyay, B., and E. Purwantini. 2000. Pyruvate carboxylase from Mycobacterium smegmatis: stabilization, rapid purification, molecular and biochemical characterization and regulation of the cellular level. Biochim. Biophys. Acta 1475:191-206. [DOI] [PubMed] [Google Scholar]
- 33.Mukhopadhyay, B., S. F. Stoddard, and R. S. Wolfe. 1998. Purification, regulation, and molecular and biochemical characterization of pyruvate carboxylase from Methanobacterium thermoautotrophicum strain deltaH. J. Biol. Chem. 273:5155-5166. [DOI] [PubMed] [Google Scholar]
- 34.Patel, H. M., J. Tao, and C. T. Walsh. 2003. Epimerization of an L-cysteinyl to a D-cysteinyl residue during thiazoline ring formation in siderophore chain elongation by pyochelin synthetase from Pseudomonas aeruginosa. Biochemistry 42:10514-10527. [DOI] [PubMed] [Google Scholar]
- 35.Peters-Wendisch, P. G., C. Kreutzer, J. Kalinowski, M. Patek, H. Sahm, and B. J. Eikmanns. 1998. Pyruvate carboxylase from Corynebacterium glutamicum: characterization, expression and inactivation of the pyc gene. Microbiology 144:915-927. [DOI] [PubMed] [Google Scholar]
- 36.Peters-Wendisch, P. G., B. Schiel, V. F. Wendisch, E. Katsoulidis, B. Mockel, H. Sahm, and B. J. Eikmanns. 2001. Pyruvate carboxylase is a major bottleneck for glutamate and lysine production by Corynebacterium glutamicum. J. Mol. Microbiol. Biotechnol. 3:295-300. [PubMed] [Google Scholar]
- 37.Pfefferle, W., B. Mockel, B. Bathe, and A. Marx. 2003. Biotechnological manufacture of lysine. Adv. Biochem. Eng. Biotechnol. 79:59-112. [DOI] [PubMed] [Google Scholar]
- 38.Purcell, A. W., and J. C. Wallace. 1996. The accessibility of the prosthetic group biotin during monomeric avidin affinity chromatography. Anal. Biochem. 238:213-216. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook, J., E. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, New York, N.Y.
- 40.Samols, D., C. G. Thornton, V. L. Murtif, G. K. Kumar, F. C. Haase, and H. G. Wood. 1988. Evolutionary conservation among biotin enzymes. J. Biol. Chem. 263:6461-6464. [PubMed] [Google Scholar]
- 41.Seubert, W., and U. Remberger. 1961. Purification and mechanism of action of pyruvate carboxylase from Pseudomonas citronellolis. Biochem. Z. 334:401-414. (In German.) [PubMed] [Google Scholar]
- 42.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 43.Studier, F. W., A. H. Rosenberg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185:60-89. [DOI] [PubMed] [Google Scholar]
- 44.Sueda, S., M. N. Islam, and H. Kondo. 2004. Protein engineering of pyruvate carboxylase: investigation on the function of acetyl-CoA and the quaternary structure. Eur. J. Biochem. 271:1391-1400. [DOI] [PubMed] [Google Scholar]
- 45.Utter, M. F., and D. B. Keech. 1960. Formation of oxaloacetate from pyruvate and carbon dioxide. J. Biol. Chem. 235:PC17-PC18. [PubMed] [Google Scholar]
- 46.Uy, D., S. Delaunay, J. Engasser, and J. Goergen. 1999. A method for the determination of pyruvate carboxylase activity during the glutamic acid fermentation with Corynebacterium glutamicum. J. Microbiol. Methods 39:91-96. [DOI] [PubMed] [Google Scholar]
- 47.Vemuri, G. N., T. A. Minning, E. Altman, and M. A. Eiteman. 2005. Physiological response of central metabolism in Escherichia coli to deletion of pyruvate oxidase and introduction of heterologous pyruvate carboxylase. Biotechnol. Bioeng. 90:64-76. [DOI] [PubMed] [Google Scholar]
- 48.Wanner, B. L. 1994. Gene expression in bacteria using TnphoA and TnphoA′p elements to make and switch phoA gene, lacZ (op), and lacZ (pr) fusions. Methods Mol. Genet. 3:291-310. [Google Scholar]
- 49.Watson, A. A., R. A. Alm, and J. S. Mattick. 1996. Construction of improved vectors for protein production in Pseudomonas aeruginosa Gene 172:163-164. [DOI] [PubMed] [Google Scholar]
- 50.Yong-Biao, J., M. N. Islam, S. Sueda, and H. Kondo. 2004. Identification of the catalytic residues involved in the carboxyl transfer of pyruvate carboxylase. Biochemistry 43:5912-5920. [DOI] [PubMed] [Google Scholar]